Abstract
As an alternative to the traditional nickel-based filler metals, new-type iron-based filler metal has become a development trend for stainless steel brazing in exhaust gas recirculation (EGR) cooler fabrication, aiming at decreasing brazing temperature and obtaining higher joint strength with minimal erosion as well as better corrosion resistance. In particular, achieving the satisfying properties with inexpensive raw materials is desirable for broad industrial applications at an economic cost. A new type of iron-based filler metal was designed and developed for SUS304 stainless steel brazing. The effect of B and Mo content on interface microstructure, lap-joint shear strength, microhardness, and corrosion resistance of brazed seam was investigated. The optimum brazing parameters were achieved at 1050 °C-20 min. Both brazing temperature and holding time are critical factors for controlling the interface microstructure and hence the mechanical properties of the brazed joints. The interface microstructure and erosion of T-joints were observed and analyzed. Additionally, results from the evaluation on wettability, shear strength, and corrosion resistance have been benchmarked against commercial iron-based and nickel-based filler metals. Finally, tensile strength tests of brazed joint with developed iron-based filler metal in a form of paste and amorphous foil were conducted at both room temperature and high temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Exhaust gas recirculation (EGR) cooler system can effectively meet the requests of automobile off-gas purification treatment. Currently, nickel-based and pure copper brazing filler metals are mostly used for fabricating EGR coolers which are made from stainless steel to withstand high service temperatures and corrosive environments [1,2,3]. When the brazing temperature is required to be low, BNi2 filler metal is the first choice because its brazing temperature is 1050 °C. However, the presence of boron might cause embrittlement of the base metals, and the corrosion resistance of BNi2 is low. Boron-free nickel-based filler metal such as BNi5 has good corrosion resistance but brazes at rather high temperature (1150–1200 °C). HBNi613 is specially developed for the new generation of EGR cooler with a fairly low brazing temperature (1050–1100 °C). Generally, the disadvantage with all nickel-based filler metals is the high content (minimum 60%) of expensive nickel, which increases the production cost of heat exchangers. Moreover, pure copper or copper-based filler metals have a good ductility but often exhibit a poor corrosion resistance, easy causing corrosion failure of the engine [4,5,6,7].
To overcome the disadvantage of high-cost nickel-based filler metals and meet the challenge of corrosion resistance, choosing iron instead of nickel as the base element, which is considered to reduce the costs up to 30%, has become the new development trend in brazing filler metals for EGR coolers [8,9,10]. Previous efforts focused on the boron-free iron-based filler metals such as typical BrazeLet F300 (Fe24Cr20Ni5Si7P) from Höganäs (Sweden) and Amdry805 (Fe29Cr18Ni7Si6P) from Sulzer (Switzerland) Inc. The brazing temperatures of these two filler metals are 1100 °C and 1176 °C respectively. BrazeLet® F300 is a commercial iron-based brazing filler metal and has excellent wetting properties and low brazing temperature compared with other nickel-based filler metals and creates a uniform joint with high strength and high corrosion resistance and oxidation resistance. Niferobraz® 9080 (Fe29.5Cr20Ni7Si6P) newly developed by Wall Colmonoy (USA) was used to braze SUS 304 steel at 1100 °C and was proved good corrosion resistance due to its high Cr content [11]. Some patents reported the new design of iron-based filler metals with the addition of Cu, Mo, Ti, or rare earth elements in order to increase corrosion resistance or obtain joins with high ductility [6, 8, 12]. But, these filler metals also have high brazing temperatures ranging from 1110 to 1160 °C. However, considering the effect of grain growth of stainless steel on the ductility and hardness at high temperatures, the maximum brazing temperature is 1095 °C, according to ASM speciality handbook Stainless Steel. On the other hand, the rate and depth of erosion can increase by increasing the brazing temperature. Therefore, if brazing temperature is too high, iron-based filler metals have a tendency to erode stainless steel more than traditional nickel-based alloy [13]. Due to excess erosion/dissolution of solid substrate in molten filler, iron might react with nickel to generate FeNi3 compounds in brazed joints, which deteriorate the parent material properties and decrease the joint strength.
Therefore, a new-type iron-based filler metal with melting point depressant elements is preferred to solve the above temperature-property trade-off problem.
With the aim to decrease brazing temperature, ensure good wetting of the base metals, and increase joint strength with minimal erosion and better corrosion resistance, as well as satisfy the requirement of large gap brazing, this study presents the investigation of a new-type iron-based filler metal with the modified composition of different elements. The highlight of the research is the effect of boron and molybdenum on the microstructure, mechanical properties, and corrosion behavior of brazed 304 stainless steel joints. The effects of brazing parameters including temperature and holding time on the mechanical properties were discussed. The properties of developed iron-based filler metal were also compared with typical commercial iron-based and nickel-based filler metals.
2 Experimental procedures
2.1 Materials
The base materials used in the investigation were 304 austenite stainless steel. Regarding the design of iron-based filler metal, it can be beneficial if the main composition is similar to that of the stainless steel to be brazed. Fe is no doubt the base component but the balance of the other constitutional elements should also be adjusted. Nickel not only enhances the oxidation resistance of the filler alloy but also increases the strength of the brazed joint. But to meet the requirement of cost saving, the nickel content is maintained at 20 wt.%. But if this content exceeds 35 wt.%, it may cause a decrease in the joint strength. Chromium content above 11 wt.% is required for the providing the corrosion resistance characteristics [1]. In the current research, the chromium content is limited to 12 wt.%. Fundamentally, boron, silicon, and phosphorus are effective melting point depressants to reduce the melting point of the brazing filler metals, which has the same effect as for nickel-based filler metals [4]. Indeed, the contents of silicon and phosphorus above 10 wt.% each are not favorable owing to the high risk for brittle phase formation. Therefore, it is recommended to keep the phosphorus content between 4 and 8 wt.% and silicon between 2 and 6 wt.%. The Fe-B binary system has a minimum melting point of 1174 °C with approx. 4 wt.% boron. However, the boron increases the risk of embrittlement of the brazed joints because boron atoms appear to diffuse into the lattice of the base metals, resulting in brittle precipitations of CrB phase. In order to further ensure the synergistic effect of Si, P, and B to the melting point depression, it is preferable to adjust the precise addition of boron. Furthermore, copper has been proved to reduce the diffusion of silicon and phosphorous into the base metals as well as produce a positive effect on the corrosion resistance when only 2 wt.% copper is added. But when the amount of Cu exceeds 5 wt.%, the strength of the filler metals will decrease [1]. Thus the copper content is set up at 3–5 wt.% in the research. Molybdenum exhibits improved wettability and enhanced joint strength. Especially, it can react with carbon in stainless steel to form a stable Mo2C phase at 800 °C that inhibits the aggregation of cementite and thus improve the corrosion resistance. It has also been reported that the additions of molybdenum were effective in reducing erosion. But when the amount of Mo exceeds 5 wt.%, the balance of the solidus temperature of the filler alloy will be disrupted because pure molybdenum has a high melting point of 2610 °C; thus, Mo is limited to 5 wt.% or less.
For the above-mentioned reason, the experimental iron-based filler metals are shown in Table 1. Two groups of filler metals which contain eight chemical elements, i.e., Fe, Ni, Cr, Cu, Si, P, B, and Mo were designed. The contents of nickel, chromium, copper, silicon, and phosphorus elements were kept unchanged at 20, 12, 3, 4, and 7 (in wt.%) respectively. In group 1#, when the Mo content is maintained at 3 wt.%, the B content increases from 0 to 1 wt.% gradually. In group 2#, when the B content is maintained at 0.25 wt.%, the Mo element increases from 0.5 to 4 wt.%. The mass balance is obtained by adjusting the contents of iron accordingly.
In addition, there are two groups of components designed to test the melting characteristics of iron-based filler metal. The composition is shown in Table 2.
The test uses a purity of 99.9% (mass fraction), 300 mesh size of iron powder, nickel powder, chromium powder, copper powder, silicon powder, molybdenum powder, phosphorus iron powder, and boron iron powder as raw materials to prepare filler metal. The filler metals were prepared in the form of mixed powder for performance test, i.e., wetting and melting point characterization, whereas, during brazing process, they were prepared and used in the form of paste and amorphous brazing foil (ABF) when placed at the gap between the base materials to be joined. Thus, the individual effect of boron and molybdenum on the microstructure and corresponding mechanical properties of brazed joints will be investigated.
2.2 Experimental procedures
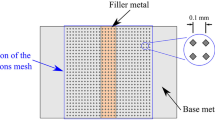
For prepared filler metals in a paste form, a single-lap joint and T-shaped joint were designed for capillary brazing. The dimensions of SUS304 stainless steel specimens for lap-joints and T-joints were 100 × 10 × 3 mm and 50 × 10 × 2 mm respectively. Figure 1 shows the schematic illustration of lap-joints with a clearance of 100 μm. Moreover, in order to investigate the high-temperature tensile properties of brazed joints, a butt-joint was designed with the dimensions of Φ16 × 110 mm and the clearance of 50 μm was chosen.
The brazing experiments were carried out in VDW-30 type vacuum furnace with the vacuum level up to 10−3 mbar. The brazing temperature was chosen after measuring the melting point of the prepared filler metals. The specimens were held for 10–50 min at brazing temperature and then were slowly cooled to room temperature in the furnace.
The melting point of prepared filler metals was measured using the STA 449C, Netzsch Differential Scanning calorimeter (DSC). A sample of the filler powder (approx. 2 g) was taken and put directly in a crucible. Under a nitrogen atmosphere, it was heated to 1200 °C with a heating rate of 40 °C/min.
The interface microstructure of brazed joints was characterized by an optical microscope (OM) and scanning electron microscopy (SEM, HITACHIS-3400N) equipped with an energy dispersive spectrometer (EDS).
Three shear strength samples were carried out by a MTS810 Universal Testing Machine at a constant speed of 0.5 mm/min at room temperature. In accordance with AWS C/3.2:2008 and ISO783-1999 (the standard method for tensile testing at elevated temperature), the brazed butt-joints were machined for standard tensile specimen preparation. The general specimen design is shown in Fig. 2. Then the samples were then subjected to uniaxial tensile testing using a MTS810 Universal Testing Machine with the temperature controlled furnace. The crosshead speeds were 0.1 mm/min at room temperature and 0.01 mm/min at 600 °C respectively. Furthermore, in order to investigate the performance of the reaction products in the joints, the average microhardness of brazed joint interface was measured by HXD-100 type micro-Vickers Hardness tester under a 100-N load at room temperature.
Immersion test was performed to investigate the corrosion behavior of the brazed joints. Specimens were cut from the brazed lap-joints into small blocks (6 × 5 × 5 mm) and immersed in a solution of 10% H2SO4 for 48 h (2 days) and 168 h (7 days) to measure the weight loss. Before and after immersion, the specimens were cleaned in ethyl alcohol using ultrasonic equipment before the weight measurement.
3 Results and discussions
3.1 Melting characterization and brazing temperature determination
DSC thermal measurement instruments were used to measure the difference in temperature between a sample and a reference sample during the heating or cooling process. DSC test results of the above iron-based alloys can be seen in Table 2. The melting characteristics were different from the previous reported iron-based filler metals [1, 3, 4, 6, 8, 11, 14]. According to the DSC test, it can be determined that the recommended brazing temperature could be reduced to 1050 °C.
As shown in Fig. 3, there are two peaks in the DSC curve of filler metal a, from which it can be inferred that there are more than one eutectic structure or both eutectic and non-eutectic structures in the microstructure of filler metal a. This is because filler metal a does not contain element B, resulting in a different crystal phase, and the phase transition temperature is different. When the temperature rises, the eutectic phase with a low melting temperature melts first, and the compound with a high melting temperature phase melts, so the difference is poor. There are two peaks in the thermal analysis results. Obviously, there is a multi-peak phenomenon in brazing material a. It is unfavorable to the brazing seam filling process. Because the filler metal a has two melting temperature range values, the melting range of the entire brazing alloy is too wide, which is not conducive to the rapid spread of filler metal during brazing process which indicates that the design of the filler metal composition is unreasonable. The DSC curve of filler metal b has only one peak, indicating that almost all of them are uniform, single eutectic structures, and the melting temperature range of filler metal b is narrow and the melting temperature is relatively low; therefore, the filler metal has good fluidity and is beneficial to the filling process. In this test temperature range, the addition of the elements Mo and B did not cause the DSC curve to be multimodal, but instead narrowed the melting temperature range of the filler metal alloy. The narrow melting range was conducive to the rapid melting of the filler metal on the base metal. Then spread out quickly, No. 4 was nearly identical, while filler metal b has the narrower melting range and the lower liquids temperature. As a consequence, brazing can be performed at a remarkable lower temperature of 1050 °C.
3.2 Effect of B and Mo on microstructure and mechanical property of brazed joints
The brazing experiments were carried out at 1050 °C with 1# and 2# group filler metals prepared according to Table 1. Figure 4 shows the microstructure of brazed joints with variations on element B, while Mo content was kept constant at 3 wt.%. A significant difference was observed in the interface microstructure when the B element content increased gradually. In Fig. 4a, when the filler metal contains no B element, there were obvious local areas depleted of filler. It is considered that the brazing temperature did not reach the melting temperature of this filler metal, so the filler did not totally melt and thoroughly wet the surface to be jointed. The brazing seam is neat and uniform, see Fig. 4b, when B content is 0.25 wt.%. The magnified microstructure of Fig. 4b is shown in Fig. 4c. The dark areas in the middle of the seam illustrate the appearance of hard and brittle phases, which has been confirmed to be FeCrNiP precipitates by EDS analysis, while the gray areas in the jagged texture clarify the appearance of ductile phases. While the black hard brittle phase is surrounded by the gray ductile phase, it prevents microcracks from spreading. However, increasing B content (0.75 wt.%) accelerates the formation of black brittle phases in the branch texture, which probably promotes cracks extension along the brazing seam due to non-uniform joint microstructure, as shown in Fig. 4d.
Figure 5 illustrates the room temperature shear strength of the brazed joints. The results indicated that B contents influenced the shear strength obviously. The maximum shear strength was 71.2 MPa when the B content is 0.25 wt.%. The shear strength of the brazing joint is relatively lower when B content is zero, which can be attributed to the unmelted filler metal at the fixed brazing temperature. With the increase of B content, the flow of filler metal was improved. But due to the high diffusivity of boron atoms into the base material, hard and brittle CrxBy (CrB or CrB3) phases is easy to precipitate and accumulate at the joint interface, which might promote micro-crack formation and propagation at the interface during the shear test. Therefore, it appears to have a detrimental effect on the joint strength when B content increase to 1 wt.%.
As is demonstrated in the Vickers microhardness results of brazing seam center (Fig. 5), the hardness value shows a gradual upward trend as the B content increases. When B content is 1 wt.%, it reaches the maximum hardness values of 630 HV. The increase of FeCrNiP and CrxBy hard and brittle phase contents is the main reason that the hardness value elevates in the center of brazed seam.
Figure 5 also represents the dependence of weight loss of brazed joints on the B content. The results indicate that corrosion resistance is the worst when the filler metal is boron-free, the weight loss reached almost 0.6 g after 1-week (168-h) immersion test. With the increasing B content, the weight loss decreases first, reaches a minimum value, and then increases. When B content is 0.25 wt.%, the brazed joint showed the highest corrosion resistance.
Figure 6 depicts the microstructure of brazed joints with variations on element Mo, while the B content was kept constant at 0.25 wt.%. The interface microstructure was uniform and compact. In Fig. 6a, when the filler metal contains 0.5 wt.% Mo element, the brazing seam is composed of a solid solution and intersecting phases appearing in the form of herringbone structure, thus it is difficult to distinguish the black phases from the gray phases. Additionally, when Mo content is 3 wt.%, in Fig. 6b, several gray intermetallic compounds accumulate in the brazing seam region. Fig. 6c presents the magnified micrograph of the interface area. The EDS chemical compositions of each spot in Fig. 6c are listed in Table 3. Figure 7 shows the XRD phase analysis results for the cross-section of brazed joints with 3 wt.% Mo content. According to EDS and XRD results, the large amount of herringbone-like gray phase mainly consisted of Fe, Cr, and Ni, which is identified to be ductile FeCrNi solid solution. While the black phase that formed in the vicinity of gray phase was determined to be brittle FeCrNiP phase, the presence of the small and spherical phases in brazing seam and in a region near the substrate was confirmed to be dispersed molybdenum carbide (Mo2C) by EDS and XRD analysis, which is produced by the strong interaction between Mo and carbon in the stainless steel substrate. It is important to note that these fine Mo2C precipitates might result in the formation of a fine and homogeneous microstructure in the joint and thus lead to higher dispersion strengthening as well as improve thermal stability of joints. Moreover, some island-like structures in brazing seam, which contained of Mo, P, and Si, could be determined to be MoSiP phase. In brief, intensive interaction including dissolution, reaction, and interdiffusion occurred at the interface during brazing, Mo forms a compound together with mainly Si and P and further reacts with carbon to form a small fraction of strengthening precipitates like Mo2C, which corresponds with Ref. [1].
Furthermore, the effect of Mo for further improving the joints strength and the corrosion resistance was found. Figure 8 illustrates the shear strength of the brazed joints. The results indicated that Mo content indeed influenced the shear strength. The shear strength of the joints gradually increased with Mo content within a range of 0.5-3 wt.% until the maximum shear strength of 71.2 MPa was reached and then decreased dramatically at a content of 4 wt.%. The change of shear strength was well dependent on the microstructure evolution of the joints. The addition of Mo results in fine seam microstructures as well as homogeneous distribution of Mo2C precipitates, which contributes to enhance the mechanical property of brazed joints. In the case that Mo content increases to 4 wt.%, the balance of the melting temperature in the filler metals is disrupted and the liquidus temperature may exceed the brazing temperature, which influences wetting and spreading of molten filler, thereby leading to an obvious decline in performance of the joint. Correspondingly, an increase in the Vickers microhardness with Mo element was obtained due to the formation of a hard brittle phase in the center of brazing seam, as shown in Fig. 8. When Mo content is 3 wt.%, it reaches the maximum hardness values of 577 HV.
Figure 8 also represents the dependence of weight loss of brazed joints on the Mo content. The results indicate that corrosion resistance is the worst when the filler metal contains 0.5 wt.% Mo. After the 1-week (168-h) immersion test, with the increasing Mo content, the weight loss decreases first, reaches a minimum value, and then increases. When Mo content is 3 wt.%, the brazed joint showed the highest corrosion resistance. It is believed that the presence of Mo in filler metal enhances their corrosion resistance but the amount of Mo content should be limited to 4 wt.% or less, preferably to 0.5 to 4 wt.%.
3.3 Effect of brazing temperature and holding time on the microstructure and property of joints
Based on the above investigations on the melting characterization and the influence of B and Mo content on the joint properties, the suitable composition of iron-based filler metal for brazing test, i.e., Fe20Ni12Cr3Cu4Si7P0.25B3Mo was developed and prepared. In order to obtain optimal brazing parameters, the effect of brazing temperature and holding time was studied respectively. The employed brazing temperatures were set up at 1030, 1040, 1050, 1060, and 1070 °C for 30 min, and the holding times used were 10, 20, 30, 40, and 50 min at 1050 °C.
Figure 9 shows the average shear strength of the joints brazed at different brazing temperatures for a constant holding time of 30 min. SEM images of the microstructures of the brazed joints are shown in Fig. 13. It can be seen that the shear strength increases when the brazing temperature increases from 1030 to 1050 °C but decreases as the brazing temperature increases to 1070 °C. The maximum shear strength of the joints was 75.7 MPa at 1050 °C. When the temperature is lower, the filler metal was not fully melted, thus the wettability and filling properties of the filler metal are relatively poor at 1030 °C, which is not sufficient to fill the gap. Therefore, brazing defects like an unfilled brazing gap were observed, as shown in Fig. 10a, which result in the lowest shear strength of 41.3 MPa. Increasing temperature can improve the wetting and flow of filler metal, leading to interaction with substrates as well as accelerate mutual diffusion of elements across the interface, which influences the high strength of the joint. When the brazing temperature is 1040 °C and 1050 °C, the brazing seam achieves a good quality, see Fig. 10b and c. A few brittle phases accumulated in brazing seam; thus, the reaction layer is not clear at lower 1040 °C. But it is obvious that the microstructure contains a homogenous mixture of hard FeCrNiP phases (black) and ductile FeCrNi phases (gray) at 1050 °C. However, more and more hard brittle intermetallic compounds such as FeCrNiP and CrB phases accumulate in the interface between brazing seam and substrates after the brazing temperature was prolonged, see Fig. 10d. The thickness of the reaction layer has increased with the propagation of black brittle phase into substrate at 1060 °C. The reaction layer keeps enlarging so that the thickness of brazing seam increases gradually. Notably, the decrease of shear strength at 1070 °C is ascribed to the cracks propagated along joints, see Fig. 10e. There are a couple of possibilities regarding the cause of this cracking; chief among them is the residual stress created by high temperature.
Figure 11 explains the observation results of T-joint specimens brazed at typical brazing temperatures. From Fig. 11a, it is clear that the filler metal is not sufficient to fill the gap at 1030 °C. The main reason is because the brazing temperature did not reach the melting temperature of the filler metal. When the brazing temperature is 1050 °C, see Fig. 11b, the fillet is not favorable with a partly unfilled brazing gap. This is not erosion but instead, a small mass of filler metal could not fulfill the requirement to form fillet at the edges of the brazed area [15]. When the brazing temperature is 1070 °C, see Fig. 11c, the uniform brazed joint was formed by a smooth fillet meniscus without obvious erosion of substrate.
Figure 12 shows the average shear strength variation with holding time. SEM images of the microstructures of the brazed joints are shown in Fig. 13. Figure 14 shows the XRD patterns of a cross-section of brazed joints at 1050 °C for different holding times. It is apparent that the brazed joint resulted in medium strength values within the range of 74 to 77 MPa. The maximum shear strength was obtained at a holding time of 20 min. When a short holding time (10 min), see Fig. 13a, is employed, the filler metal was not fully melted; thus, obvious brazing defects like unfilled gap were observed. When the holding time is prolonged, more and more FeCrNi ductile solid solution and dispersed Mo2C compounds in brazing seam near substrate were generated by intense interfacial reactions, which form a strong metallurgical bond and corresponding high mechanical properties, as shown in Figs. 13b and 14b. The reaction layer keeps enlarging so that the thickness of brazing seam increases gradually at the holding time of 40 min, see Figs. 13c and 14c. However, further prolonged holding time causes the nonmetallic elements P diffuse into the base metal and generate hard and brittle FeCrNiP phases, as shown in Figs. 13d and 14d. As a result, the shear strength of brazed joint is gradually decreased.
As shown in Fig. 15, holding time has a significant effect on the metallographic microstructure of T-shape joint. The filler is completely melted and a super smooth fillet is formed at a short time of 20 min. However, a long time at 1050 °C will allow the diffusion and hence erosion is intensified and worse the mechanical property of brazed joint.
To conclude in fact, both the brazing temperature and the holding time were critical factors for controlling the microstructure and hence the mechanical properties of the brazed joints. The optimum brazing parameters were achieved at 1050 °C-20 min.
3.4 Comparison of the properties between new-type iron-based filler metal and other commercial filler metals
After a series of tests, based on the results, the optimal composition of the iron-based filler metal named BJUT-Fe (Fe50.75Ni20Cr12Cu3Si4P7B0.25Mo3) was determined, as well as the optimal brazing parameters. Furthermore, a performance comparison test was conducted between the new-type BJUT-Fe filler metal and F300 provided by the Höganäs Company. Commercial nickel-based filler metals of BNi2, BNi5, and HBNi613 were also compared [14]. The chemical compositions and brazing temperatures of BJUT-Fe filler metal are shown in Table 4, in comparison with other commercial filler metals such as F300, BNi2, BNi5, and HBNi613 [13, 14].
Figure 16 shows the spreading ratio comparison results of selected filler metals. BJUT-Fe exhibits a sufficient wetting and an excellent flowing behavior on the SUS304 stainless steel at 1050 °C, which is similar to that of F300. Actually, BJUT-Fe has a lower amount of Cr (12 wt.%) than that of F300 (20 wt.%), thus the fluidity diminishes a little bit. But the addition of B element (0.25 wt.%) also improves the wettability. Besides, it is apparent that both BJUT-Fe and F300 iron-based filler metals have a higher fluidity and better wettability than that of BNi2 and BNi5. Whereas, due to the high phosphorus content (compared with BNi2 and BNi5), HBNi613 show excellent wetting and flowing behavior with the maximum spreading ratio of 11.6%.
Figure 17 describes the mechanical properties comparison results of selected filler metals. For BJUT-Fe, the value of shear strength of the brazed joints is even a little higher than that of F300 and BNi5, but is lower than that of BNi2 and HBNi613. According to the previous explanation, the microstructure of the brazed joint has become finer due to Mo addition in BJUT-Fe filler metals, the mixture of FeCrNi solid solution ductile phases, several FeCrNiP brittle phases, and precipitated Mo2C phase was the main cause for achieving relative good strength. Whereas, the shear strength of brazed BNi2 joints exhibits the maximum value of 108 MPa.
Table 5 presents the corrosion resistance comparison results of selected filler metals. The weight loss ratio of BJUT-Fe is close to that of F300. It means that BJUT-Fe iron-based filler metal has better corrosion resistance than F300 in 10% H2SO4 solution after 48 h (2 days) immersion time. According to the literature [15], F300 has corrosion resistance similar to BNi5 and HBNi613. BNi2 has a worse corrosion resistance compared with BNi5 and HBNi613 due to its low Cr content. Therefore, it can be deduced that BJUT-Fe filler metals have corrosion resistance better than BNi2, and also comparable to BNi5 and HBNi613, which contains a higher content of Cr and does not contain Mo. Mo made a positive effect on the corrosion for iron-based filler metal.
3.5 Standard tensile test for the butt-joint at room temperature and 600 °C
The results of strength of the brazed butt-joints measured by tensile test at room temperature and at high temperature (600 °C) are shown in Table 6. As can be seen from the table, the joint brazed by BJUT-Fe filler metals in the form of amorphous brazing foil (ABF) has good mechanical properties at room temperature; the average tensile strength reaches 233 MPa, which even exceeds that of F300 filler metals. It reaches 75% of tensile strength of commercial BNi2 filler metal. In contrast, the average tensile strength of the joints brazed by BJUT-Fe filler metal in the form of paste is only 129 MPa, thus the joints fracture during the mechanical machining procedure for preparing high-temperature tensile test specimen. As a consequence, it is not possible to evaluate the high-temperature tensile strength of joints brazed by BJUT-Fe (paste). Moreover, this new iron-based filler in the form of ABF exhibits desirable mechanical properties at high temperature. The average tensile strength at 600 °C reaches 95 MPa, approximately half of BNi2 (180 MPa) and one third of high-temperature tensile strength of SUS304 stainless steel substrate (359 MPa) [16].
In addition, holding time has a significant impact on the mechanical properties of brazed joints at high temperature. Figure 18 shows the stress and strain curves of the joints under different holding times 20, 30, and 40 min. The deformation behavior is completely elastic-plastic. The joints brazed at 20 min are found to have higher strength than those brazed at 30 and 40 min. With the extension of holding time, Si, P, and other elements in the filler metal will diffuse into the substrate, and the brittle phases are formed at high temperature, which deteriorates the high-temperature mechanical properties of joints.
4 Conclusions
With respect to developing a new iron-based filler metal as a good alternative to Ni-based filler metals for the purpose of reducing raw material cost, this study aimed at decreasing brazing temperature but improving wettability and mechanical properties as well as corrosion resistance of brazed SUS304 stainless steel joints by chemical composition design and brazing parameters optimization. Primary conclusions are summarized as follows:
-
(1).
Iron-based filler metal containing 20 wt.% Ni, 12 wt.% Cr, 3 wt.% Cu, 4 wt.% Si, 7 wt.% P, and 3 wt.% Mo and Fe as balance element exhibits a lower liquidus temperature of 952 °C, which enables brazing to be performed at remarkable lower temperature of 1050 °C.
-
(2).
B and Mo elements effectively influence the microstructure evolution, mechanical properties, and corrosion resistance of brazed joints. The uniformity of joints microstructure can be improved by adding Mo element, as well as joint strength and corrosion resistance. The maximum shear strength 71.2 MPa with good corrosion resistance in 10% H2SO4 solution was obtained with 0.25 wt.% B and 3 wt.% Mo.
-
(3).
The maximum shear strength of lap-joints reaches 77.0 MPa under the optimum process parameters, i.e., brazing temperature of 1050 °C and holding time of 20 min. Brazing temperature and holding time have a strong influence on the microstructure evolution and shear strength of joints. With the increase of brazing temperature and holding time, quantities of dispersed Mo2C compounds in brazing seam near substrate are generated by intense interfacial reactions, which enlarge the reaction layer and hence form a strong metallurgical bond and corresponding high mechanical properties.
-
(4).
BJUT-Fe exhibits a sufficient wettability, higher shear strength, and better corrosion resistance compared with Höganäs F300 filler metal. The butt-joint brazed by BJUT-Fe filler metals in the form of amorphous brazing foil (ABF) has high tensile strength at room and at 600 °C, which is promising for the future EGR cooler applications.
References
Katsunori O, Shozo N. Iron-base heat-and corrosion-resistant brazing filler metals: Japan, EP2272619A1 [P], 2011-01-12
Bobzin K, Puidokas S, Tillmann W (2013) Reliable design procedure of iron-based braze joints, Proceedings of 10th International Brazing and Filler metaling Conference, Aachen, pp 261–266
Hartmann T, Nuetzel D (2009) New amorphous brazing foils for exhaust gas applications, Proc. 4th International Brazing and Filler metaling Conference, Orlando, AWS, pp 110–118
Pohlman MJ, Kindlimann LE Iron-based brazing alloy composition and brazed assemblies with iron based brazing alloys: USA, 4410604. 1983-10-18
Pouranvari M, Ekrami A, Kokabi H (2014) Diffusion brazing metallurgy of IN718/Ni-Cr-Si-B-Fe/IN718. Weld J 93:60s–68s
Hartmann T, Nuetzel D Iron-based brazing foil and method for brazing: USA, US2008/ 0318082A1. 2008-12-25
Hartmann T, Nuetzel D (2010) New amorphous brazing foils for exhaust gas applications. Proceedings of 9th International Brazing and Filler metaling Conference, pp 42–47
Rangaswamy SDJ Iron-based braze filler metal for high-temperature applications: USA, US2008/0006676A1. 2008-01-10
Jahanzeb N Erosion during brazing in stainless steel grade 304. Sweden, Hoganas, 2012–8
Weinstein M (2015) Properties of selected nickel and iron based brazing filler metals. Technical paper
Xiong HP et al A Fe-Ni-Cr system filler metal for brazing of stainless steel, China, CN201310541371.6. 2013-11-05
Owe M et al (2012) Erosion control of stainless steel brazing alloys, Proc. 5th International Brazing and Filler metaling Conference, Las Vegas, AWS.
Owe M et al Iron-chromium brazed filler metal: Sweden, EP2271460 B1, 2011-11-16
Iron-based brazing filler metal-BrazeLet ® F300 product datasheet, Höganäs Company, 2014
Tillmann W, Wojarski L, Manka M et al (2016) Investigation of the brazing characteristics of a new iron-based brazing filler metal. Welding in the World 60(5):869–875
High-temperature characteristics of stainless steels, A Designers' Handbook Series No. 9004, American Iron and Steel Institute, 2011
Shi KD Study on Fe-Cr-based brazing filler metals as substitutes for Ni-based brazing filler metals. Japan, Gunma University, 2016–8
Acknowledgements
We thank Ms. Yunyue Li for assistance with DSC curve measurement and Prof. Jacek Senkara of Warsaw University of Technology for comments that greatly improved the manuscript.
Funding
This research was supported International Ability Improvement of Young Supervisor Project (2017-2019) and International Seed Fund for Science and Technology Cooperation Project (2018-2019) funded by BJUT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission XVII - Brazing, Soldering and Diffusion Bonding
Rights and permissions
About this article
Cite this article
Li, H., Zhang, X., Mårs, O. et al. The effect of iron-based filler metal element on the properties of brazed stainless steel joints for EGR cooler applications. Weld World 63, 263–275 (2019). https://doi.org/10.1007/s40194-018-00687-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-018-00687-9