Abstract
In this study, solid-state diffusion bonding of Ti-6Al-4V alloy using a pure copper interlayer was conducted, and the effect of bonding pressure (0.5 to 4 MPa) on microstructure and mechanical properties was investigated. The joint zones were analyzed by optical microscope (OM) and scanning electron microscope (SEM), and microhardness and shear strength tests were also employed to evaluate mechanical behaviors of the joints. The results showed that diffusion of Cu into the Ti alloy led to the formation and the stability of β-Ti as a strengthening phase in the joint zone. Moreover, the increase in bonding pressure made the joint width be decreased to the minimum value. The maximum shear strength of 571 MPa was achieved for the bond made with 4 MPa bonding pressure, whereas with a decrease in pressure, the shear strength fell to 358 MPa that could be attributed to the increase in the joint width and the formation of brittle intermetallic compounds at the interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ti alloys possess many good characteristics such as excellent corrosion resistance, high specific strength, and favorable fatigue properties [1]. Considering these advantages, the Ti alloys have been widely applied in aerospace, biomedical, and chemical industries [2, 3]. With the increased use of titanium, many welding methods like friction stir welding [4, 5], diffusion bonding [6, 7], GTAW [8, 9], electron beam welding [10, 11], and laser welding [12] have been investigated to obtain a sound joint. Of these joining methods mentioned, diffusion bonding is an attractive techniques where sound metallurgical bond and mechanical properties in the joint zone are important. In this process, two bond surfaces are held together at the temperature range between 0.5 melting point of material and room temperature under a pressure without leading macroscopic plastic deformation in the base material [13]. Presence of a pressure on the bonding surface along with lack of liquid formation in the joint avoid the creation of defects such as voids and hot cracking led by the fusion welding techniques. Furthermore, according to the works done by some researchers [14, 15], using an appropriate interlayer can lead to the formation of strengthening phases and alleviate residual stress concentration in the joint zone. Based on the Cu-Ti binary phase diagram [16], high solubility of Ti in Cu at the bonding temperature indicates that pure copper can be a suitable interlayer to join the Ti alloys. Moreover, high interdiffusion of Cu and Ti in the joint zone can lead to the formation of various strengthening metallic phases at the interface. Hence, the resultant joint is expected to exhibit the properties much better than that of fusion welded joints.
One of the most challenging aspects of diffusion bonding is to optimize the process parameters. Temperature, pressure, and time of the bonding process, as the most important parameters, play a vital role to fabricate joint assembly. To the best of our knowledge, the bonding pressure is one of the disregarded parameters in diffusion bonding of Ti alloys. Hence, the current study has proposed the mechanism of solid-state diffusion bonding and the effect of bonding pressure on microstructure evolution and mechanical properties of Ti-6Al-4V joints.
2 Experimental procedure
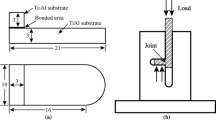
In this work, a pure copper (Cu) foil was employed to join Ti-6Al-4V alloy (6.1 Al-4.05V-bal. Ti). Schematic of the joint is shown in Fig. 1. At first, specimens were cut into dimensions of 10 × 10 × 5 mm, ground, and then polished up to 1000 grit. In order to remove contamination from the bond surface, the ultrasonic cleaning in acetone was done. After preliminary preparation, the pure Cu interlayer with 100-μm thickness was kept between the parent metal and a fixture was then applied for uniaxial pressures of 0.5, 1, 2, and 4 MPa onto the samples. Diffusion bonding was carried out in a furnace under vacuum of 10−2 mBar at 900 °C for a bonding time of 60 min. Generally, the suitable bonding temperature for Ti-6Al-4V is suggested to be about 60 °C lower than the β transus temperature. Hence, cautions were taken for designing of joining parameters such as applied pressure, furnace temperature, bonding time, and thickness of the interlayer. In this study, the bonding parameters were empirically obtained according to a number of previous works related to thermal processes of Ti-6Al-4V and the binary Ti-Cu system [6, 16, 17].
After the bonding process, all the samples were cross-sectioned transversely and then polished and etched by Keller’s reagent (90 ml distilled water, 5 ml HNO3, 2 ml HF, and 3 ml HCl). In order to characterize the joint interface, optical microscopy (OM) and scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS) were applied. Microhardness measurement across the joint zone was determined by a Vickers diamond indenter with the load of 50 g. The shear strength test was also carried out by MTS30/MH tensile testing machine at a cross-head speed of 1 mm/min. Before the shear test, the edges of the bonded samples were removed by machining to 10 × 10 × 10-mm dimension. A special fixture was then used to apply pure shear stress at the bond line during the experiment. Schematic of the fixture is illustrated in Fig. 2.
3 Results and discussion
3.1 Microstructure characterization
Bonding pressure is one of the key parameters in the solid-state diffusion bonding highly affecting the microstructure and the mechanical properties of the joints. It is believed that the distribution of elements would be different for each selected bonding pressure insofar as different microstructures can form at the interface.
SEM micrograph of a bond at the Cu/Ti interface is presented in Fig. 3 (the region shown by SEM is marked as λ in Fig. 4b). The EDS analysis of selected regions is presented in Table 1. As observed, certain interdiffusion of Cu and Ti occurred between the interlayer and the parent metal. The figure shows that the joint zone is divided into two distinguished areas. Area 1 is located adjacent to the substrate and includes more than 12 wt% Cu. It is considered that the diffusion of Cu, as a β-stabilizer element, into the Ti alloy lowers α-β eutectoid transformation temperature and leads to the stability of β phase at the Cu/Ti interface [17]. Consequently, α-β needle-like structure is formed in area 1. Two distinct regions are specified in the center of the joint (area 2). The chemical analysis of these regions indicates that the significant amount of Ti diffused along the interface. This can be due to the higher diffusivity of Ti into pure Cu than that of vice versa [18]. Depending on EDS analysis of regions B and C (See Table 1) and Cu-Ti binary phase diagram, formation of the intermetallic compounds such as TiCu and Ti2Cu in the joint center are expected.
Figure 4 illustrates microstructure of the joints made with bonding pressures from 0.5 to 4 MPa. Bonding process with the 0.5 MPa pressure resulted in a joint width of 500 μm. In Fig. 4a, it is clear that the intermetallic phases formed at the interface were larger in size compared to intermetallics formed when a higher bonding pressure was applied. Upon increasing the bonding pressure to 1, 2, and 4 MPa, the joint width was measured at 250, 150, and 100 μm, respectively. Generally, the increase of the bonding pressure causes two phenomena in the joint zone: (i) a decrease in the joint width because of the greater squeezing action on the intermetallic layer and (ii) an increase in the joint width due to the increase of surface to surface contact and the enhancement of Cu diffusion into the substrate [19]. In this study, the results reveal that increasing the bonding pressure led to the reduction in the joint width and the formation of finer intermetallic phases at the interface. Hence, it can be concluded that the squeezing action far outweighs to the diffusion rate on the width of joint zone. Another point should be noted is the trend of reduction rate in the joint width (See Fig. 5). As observed, with the increase in bonding pressure from 0.5 to 4 MPa, the reduction rate is remarkably decreased. It is believed that the effect of squeeze action in high bonding pressure (2–4 Mpa) pales into the insignificance compared to the interdiffusion of elements across the joint zone. It means that the migration of Cu and Ti atoms in the joint zone can be easier in higher bonding pressure and lessen the effect of squeeze action on the joint width. It is also worth-mentioning that the width of eutectoid area in all the specimens is in the same value. Regarding Cu-Ti phase diagram, β-Ti dissolves about 12 wt% Cu at 900 °C. Therefore with the diffusion of Cu into the Ti alloy, the eutectoid structure, saturated by Cu, impedes more diffusion of interlayer into the parent metal.
3.2 Mechanical properties
Microhardness profiles of the joint zone for the bonds made by pressures of 0.5, 1, 2, and 4 are shown in Fig. 6. Considering the overall disposition of microhardness distribution, the profiles are divided into three main areas. The hardness value of 350 VHN at the two side of the joint zone indicates the presence of Ti-6Al-4V. Adjacent to the Ti alloy, the hardness values dramatically dropped to about 310 VHN which is similar for all the samples. It is believed that diffusion of Cu into the Ti alloy and subsequently stabilization of β phase lead to a slump in the hardness value of this area. Approaching the center of joint zone, an upward trend appeared which can be attributed to the diffusion of Ti along the interface and the formation of the intermetallic compounds such as TiCu and Ti2Cu in the joint zone. In this area, the hardness value reached to more than 370 VHN and maintained the same level along the joint zone. The measurements also indicate that the hardness values in the joint center are approximately alike for all the samples. It reveals that the size and the shape of intermetallic compounds, influenced by bonding pressure, do not change the hardness values in the joint center. According to the microhardness test, the bonding pressure and the joint width have an inverse relation so that with the increase in the pressure and the squeezing action, the joint width decreases to the minimum value. It should be noted that there is a good match between the hardness test and the optical micrographs.
Figure 7 represents shear strength of the bonds as a function of the bonding pressure. It has been found that the differences in the bonding pressure led to significant differences in the bonding strength values. Soaring in the shear strength can be contributed to the increase in squeezing action, subsequently a decrease in the joint width as well as the formation of finer intermetallics at the interface. Moreover, formation of β-Ti as a strengthening phase led to the enhancement in mechanical properties of the joint. The fractured surface of the bonded sample also approves the effect of joint width on the shear strength (See Fig. 8 and Table 1). In fact, the fracture occurred through the intermetallics formed in the joint center. So, it stands to reason that with the increase of joint width (decrease in bonding pressure), the conditions for propagation of cracks can be provided easier. In this study, the maximum strength was measured for the joint made with 4 MPa bonding pressure (571 MPa) that is considerably higher than the values attained during diffusion bonding of Ti alloys done by some researchers [2, 20].
4 Conclusion
-
1.
The solid-state diffusion bonding of Ti-6Al-4V using Cu interlayer under different bonding pressures was performed successfully.
-
2.
Microstructure characterization showed that diffusion of Cu into the parent metal lowers α-β eutectoid transformation temperature and leads to stability of β phase in the joint zone. Furthermore, increase in bonding pressure led to the reduction in the joint width and the formation of finer intermetallic compounds at the interface.
-
3.
The bonding pressure has a straight relation with the shear strength so that the maximum value (571 MPa) was recorded for the bond made with 4 MPa bonding pressure.
References
(1990) ASM Handbook, properties and selection: nonferrous alloys and special purpose materials, ASM International Handbook Committee, tenth ed., vol. 2
Kim Y, Kim E, Song Y, Lee S, Kwon Y (2014) Microstructure and mechanical properties of hot isostatically pressed Ti–6Al–4V alloy. J Alloys Compd 603:207–212
Hamilton B, Oppenheimer S, Dunand D, Lewis D (2013) Diffusion bonding of Ti-6Al-4V sheet with Ti-6Al-4V foam for biomedical implant applications. Metall Mater Trans B 44B:1554–1559
Wang J, Su J, Mishra R, Xu R, Baumann J (2014) Tool wear mechanisms in friction stir welding of Ti–6Al–4V alloy. Wear 321:25–32
Russell MJ, Blignault C, Horrex NL, Wiesner CS (2008) Recent developments in the friction stir welding of titanium alloys. Weld World 52:12–15
Lee H, Yoon J, Park C, GunKo Y, Shin D, Lee C (2007) A study on diffusion bonding of superplastic Ti–6Al–4V ELI grade. J Mater Process Technol 187:526–529
Zakipour S, Samavatian M, Halvaee A, Amadeh A, Khodabandeh A (2015) The effect of interlayer thickness on liquid state diffusion bonding behavior of dissimilar stainless steel 316/Ti-6Al-4V system. Mater Lett 142:168–171
Yang Z, Qi B, Cong B, Liu F, Yang M (2015) Microstructure, tensile properties of Ti-6Al-4V by ultra-high pulse frequency GTAW with low duty cycle. J Mater Process Technol 216:37–47
Prilutsky VP, Akhonin SV (2014) TIG welding of titanium alloys using fluxes. Weld World 58:245–251
Tsai C, Wang L (2014) Improved mechanical properties of Ti–6Al–4V alloy by electron beam welding process plus annealing treatments and its microstructural evolution. Mater Des 60:587–598
Wang SQ, Li WY, Zhou Y, Li X, Chen DL (2016) Tensile and fatigue behavior of electron beam welded dissimilar joints of Ti–6Al–4V and IMI834 titanium alloys. Mater Sci Eng A 649:146–152
Cao X, Jahazi M (2009) Effect of welding speed on butt joint quality of Ti–6Al–4V alloy welded using a high-power Nd:YAG laser. Opt Lasers Eng 47:1231–1241
Lee H, Yoona J, Yi Y (2008) Fabrication of titanium parts by massive diffusion bonding. J Mater Process Technol 201:280–284
Rahman A, Cavalli MN (2012) Diffusion bonding of commercially pure Ni using Cu interlayer. Mater Charact 69:9 0–9 6
Samavatian M, Halvaee A, Amadeh A, Khodabandeh A (2015) Transient liquid phase bonding of Al 2024 to Ti−6Al−4V alloy using Cu−Zn interlayer. Trans Nonferrous Met Soc China 25:770–775
Baker H (2004) Alloy phase diagrams, ASM international, vol 3. Handbook, ASM
Zakipour S, Halvaee A, Amadeh A, Samavatian M, Khodabandeh A (2015) An investigation on microstructure evolution and mechanical properties during transient liquid phase bonding of stainless steel 316L to Ti–6Al–4V. J Alloys Compd 626:269–276
Castoldi L, Visalli G, Morin S, Ferrari P, Alberici S, Ottaviani G, Corni F, Tonini R, Nobili C, Bersani M (2004) Copper–titanium thin film interaction. Microelectron Eng 76:153–159
Atieh A, Khan T (2014) TLP bonding of Ti-6Al-4V and Mg-AZ31 alloys using pure nielectro-deposited coats. J Mater Process Technol 214:3158–3168
Cepeda-Jimenez CM, Carreno F, Ruano OA, Sarkeeva AA, Kruglov A, Lutfullin R (2013) Influence of interfacial defects on the impact toughness of solid state diffusion bonded Ti–6 Al–4V alloy based multilayer composites. Mater Sci Eng A 563:28–35
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission IX - Behavior of Metals Subjected to Welding
Rights and permissions
About this article
Cite this article
Samavatian, M., Zakipour, S. & Paidar, M. Effect of bonding pressure on microstructure and mechanical properties of Ti-6Al-4V diffusion-bonded joint. Weld World 61, 69–74 (2017). https://doi.org/10.1007/s40194-016-0390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-016-0390-0