Abstract
High temperature applications of new class of iron-based filler metals provide brazements with high corrosion resistance and mechanical properties. These brazements are cost-effective alternative to those made of the conventional brazing alloys. However, a wiser usage demands a deeper understanding of the wetting as well as gap filling behavior in conjunction with the resulting microstructure, which is mainly influenced by the applied brazing cycles. Therefore, this paper presents results of the investigation of specific brazing fundamentals for the new iron-based brazing alloy Fe-24Cr-20Ni-10Cu-7P-5Mn-5Si. Followed by DTA/DSC measurements, the spreading and gap filling behavior were examined by using stainless steel AISI 304 as base material. In wetting tests and wedge-gap experiments, the influence of the applied brazing temperature and the dwell time were investigated for vacuum brazing processes. The resulting microstructure was evaluated using a scanning electron microscope (SEM), equipped with an energy dispersive X-ray spectroscopy (EDS). Finally, strength tests were conducted in order to determine the influence of the brazing parameters on the mechanical properties of the joint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper- and nickel-based brazing filler metals are the most significant brazing alloys in the field of high temperature brazing [1]. Regarding the nickel-based filler metals, acceptable joint strengths in combination with a high thermal stability can be achieved by considering the engaged brazing gap [2–4]. Compared to these filler metals, copper alloys have an increased ductility but a lower strength and often exhibit a poorer corrosion resistance [5–7]. Furthermore, the raw material price of copper and nickel are significantly higher than the raw material price of iron.
Taking these considerations into account, the development of new iron-based brazing filler metals has been in the focus of many publications [8–13]. In order to minimize the microstructural influence of the base material during the brazing process, one of the key ideas was to reduce the brazing temperature to temperatures below 1100 °C [1]. Additional goals of these approaches were to produce joints with high strengths and a high corrosion resistance.
A promising iron filler metal for this purpose is the commonly available alloy Fe-24Cr-20Ni-10Cu-7P-5Mn-5Si. With regard to the brazing process, this study presents an investigation of the brazing fundamentals for this brazing alloy.
2 Materials
Within this study, all basic investigations were performed on an austenitic stainless steel X5CrNi18-10 (AISI 304) with a thickness of 2 mm as a base material. This steel is commonly used for components which are exposed to corrosion and moderate loadings, e.g., in the food or chemical industry as well as for automotive components. The steel possesses a face-centered cubic structure, has a good machinability, and a high corrosion resistance.
The Young’s Modulus and the ultimate tensile strength at room temperature of the steel are given in Table 1.
The brazing filler metal BrazeLet F300 was used in form of a paste for the brazing procedure. The chemical composition of this alloy is given in Table 2 [13]. According to the manufacturing company, the melting range of this filler metal ranges between 1000 and 1070 °C. The recommended brazing temperature is 1120 °C [15].
3 Experimental procedures
For a thorough characterization of the new brazing filler metal, the melting range of this alloy was measured by means of a differential thermal analysis (Linseis STA PT 1400). For a precise determination, the measurements were conducted in vacuum with a heating rate of 1 K/min. The subsequent brazing experiments were conducted in vacuum of approximately 10−5 to 10−6 mbar. For the wetting and the wedge splitting experiments, three different brazing temperatures of 1100, 1110, and 1120 °C were applied. Three dwell times, 5, 10, and 15 min were used. Taking into consideration that the past binder needs enough time to outgas, the brazing process was stopped for 35 minutes at 450 °C. The general heating rate was above 10 K/min. Prior to the brazing process, the base material was cut into coupons of 30 × 30 mm for the wetting test and into coupons of 30 × 40 mm for the wedge splitting experiments. All specimens were prepared by using a silicon carbide grinding paper with a grit of 1200 and underwent a subsequent cleaning process in an ultrasonic bath for 5 min.
3.1 Wetting experiments
In order to characterize the spreading behavior of the filler metal with regard to the brazing temperature and the dwell time, three specimens were used for each parameter change. In terms of reproducibility, the brazing paste was applied on each specimen in the same way. The paste was placed at the center of all specimens in a 9-mm diameter braze device. To ensure a defined height of the braze device, 0.2 g of the paste were applied on each specimen. Subsequently, the spreading behavior and the wettability were examined in the resulting spreading areas. These areas were recorded by taking light microscope pictures, which were afterwards analyzed by using the image processing software Fiji. Reflecting the work of [13], the wetting of the specimen was determined as the spreading ratio S of the melted area Am to the area of the initial brazing filler metal Ai (63.6 mm2).
3.2 Wedge splitting tests
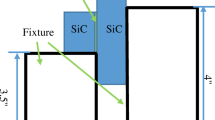
For the wedge splitting experiments, the specimens were positioned as shown in Fig. 1 prior to the brazing process. The base plate was placed flat on the used fixture and the cover plate was placed at one end of the base plate. The other end of the cover plate was set on a ceramic prop. In order to enable a spreading of the brazing filler metal into the widening brazing gap, four small equal grooves were machined into the cover plate with a distance of 6 mm. The depth of the grooves was approximately 2.5 mm.
With the objective to observe the phase formation as a function of the applied brazing gap, the needed height of the ceramic prop was calculated prior to the experiments. As a geometrical result of the angular relationship (Fig. 2) of the plates to each other, the simplified equation 2 could be used to calculate the needed height.
Knowing the defined gap length (b) as well as the maximum gap length (w) and with the aim to investigate the brazing width up to 400 μm, the needed height of the prop was calculated as 460 μm. Concerning the gap length b and the maximum gap length a, a distance of 3 mm was defined in order to prevent a damage of the prop by the brazing filler metal during the tests. An assumed increase of 0.005 leads to a necessary brazing length of 20 mm for a gap of 400 μm.
Regarding the application of the brazing filler metal and especially of the amount of the brazing material, the volume of the brazing gap was determined first. In accordance with the law of mass conservation, but without any detailed information about the spreading behavior on the austenitic steel, the amount of paste was set to 100 mm3. Concerning the form of application, it was important to guarantee that the filler metal would only spread through the grooves in the cover plate and not along the sides of the plates. Therefore, a paste volume of 13 × 3.8 × 2 mm3 was applied directly on the center of the sample, next to the overlying cover plate. Thus, an unwanted spreading could be prevented.
3.3 Tensile strength experiments
Round test specimens were manufactured for tensile strength testing according to dimensions shown in Fig. 3.
For a better application of the brazing paste, a chamfer was implemented next to the brazing area. The diameter of the specimens was approximately 11 mm at the brazing area. The temperature recommended by the manufacturer was selected as the brazing temperature. Hence, the brazing tests were conducted at a temperature of 1120 °C whereby the dwell time was varied between 5, 10, and 15 min. For the configuration of the brazing gap, which also was varied between 50, 100, and 150 μm, two rectangular bars were spot-welded after adjusting the gap by using a feeler gauge. One result of the experiment is shown in Fig. 4.
3.4 Metallographic examinations
The microstructural analyses of the wetting and the wedge splitting gap were carried out using a light microscope and by means of a scanning electron microscope (SEM) which is equipped with an energy dispersive X-ray spectroscopy (EDS). The formation of phases was analyzed by SEM, EDS, and additional hardness measurements on cross-sections of the specimens. The fracture pattern was analyzed for the fractographical examination by means of SEM in addition to 3D profilometer measurements.
4 Results and discussions
The detailed investigation of the melting range of the used brazing alloy reveals a good compliance with the specification of the manufacturer. With a melting range between 1015 and 1070 °C, a difference of 15 K was measured in the solidus temperature. According to Höganäs, the solid temperature should be 1000 °C. The difference in the solidus temperature could have been produced by a minor contamination of the thermocouple in the DTA or could be considered a measurement uncertainty. Nevertheless, the brazing parameters were determined from the results of the measured liquidus temperature.
4.1 Wetting and spreading behavior
The spreading behavior of the brazing alloy F300 on stainless steel (AISI 304) is in general very good. A visual observation after the brazing process shows that the brazing alloy is completely molten on all test specimens. The spreading looks mostly circular and uniform. In Fig. 5, the results of all spreading experiments are visualized, dependent on the applied temperature and dwell time. The spreads for the lowest brazing temperature of 1100 °C and a dwell time of 5 min are compared to the highest brazing temperature of 1120 °C and a dwell time of 15 min was essentially smaller. For the dwell times of 5 and 10 min, almost all spreads seemed to be completely circular. The surfaces are silvery with small dark areas. The shapes look very dense and homogenous. With a temperature of 1120 °C, small dark areas occurred, especially at the edge of the spreads.
For a dwell time of 15 min, the spreads are much larger and not completely circular. Dark areas inside the silver shape emerge more often and are larger in size. In general, the spreads look homogenous but not as tight as the spreads for shorter dwell times.
A detailed view on the spreading size shows that the braze alloy has extended itself to a spreading factor of up to 7 compared to the applied brazing paste area. Therefore, it is quite obvious that for all investigated parameters the wetting angles on the specimens have to be very small. The influence of the temperature and the dwell time on the spreading ratio in Fig. 6 show an obvious correlation. When applying a low brazing temperature in combination with a short dwell time, the resulting spreading ratio is minimal with a value of 4.82. In general, it can be observed that the differences in the spreading are quite small for short dwell times. Increasing this parameter up to 15 min, the differences in the spreading ratio expand up to a value of about ΔS = 1.1. The best spreading factor of S = 6.76 was achieved for a brazing temperature of 1120 °C with a dwell time of 15 min.
4.2 Metallographic examination
The microstructure and the composition of the wedge splitting specimens were analyzed by an optical microscope, a scanning electron microscope (SEM), and an integrated energy dispersive X-ray spectroscope (EDS). For these investigations, the samples were prepared by means of metallographical techniques. A SEM-analysis of the cross-section of a joint (Fig. 7) in combination with the appropriate EDS-analysis (Table 3), and hardness measurements allows a closer view on the microstructural formation. According to the EDS and the hardness measurement results, five different phases can be distinguished.
First, a phase rich in iron with a moderate content of silicon (position 1 and 2) can be recognized. This phase is very soft and often appears as a large area, especially for brazing gaps smaller than 100 μm (Fig. 8).
In contrast to the soft phase, a particularly hard phase is located at positions 3 and 7. Concerning the composition of this phase, the high amount of phosphor in connection with iron and chromium is responsible for the high hardness level and their most likely brittle behavior. Visually, this phase can be recognized as a dark area with a needle-shaped structure. With regard to the mentioned comparison with the brazing alloy BNi-2, the value of the hardness confirms the assumption that the strength is reduced for brazing gaps larger than 150 μm. In case of brazing AISI 304 with the most common nickel-based brazing alloy BNi-2, this phenomenon of reduced strength properties is known for brazing gaps larger than approximately 50 μm. Above this level, the content of boron and silicon in the braze tends to form brittle phases in the seam, which reduce the tensile strength significantly [16]. If these brittle phases occur as uninterrupted precipitates, as indicated in Fig. 8 for brazing gaps larger than 150 μm, the obtainable strengths can decrease significantly down to 50 % of the nominal strength [17]. Therefore, first the tensile strength testing of joints with a 150-μm gap was performed.
The third recognized phase is characterized by its high amount of copper. This phase has a moderate hardness and can be clearly identified as the white regions in the cross-sections (position 5 and 6). Concerning the frequency distribution of this phase, no clear structure can be observed. Most likely, this phase appears punctiform at the border area between the base material and the brazing seam, especially for brazing gaps larger than 100 μm (Fig. 8).
The most commonly developing phase has an intertwining mesh structure and can be found at position 4. The high amount of iron, chromium, and nickel in conjunction with low phosphor content is typical for this phase. In contrast to the mentioned hard phase at position 3 or 7, a low content of copper seems to reduce the hardness of the phase. In general, the appearance of this phase with its intertwining structure can be recognized for all investigated brazing gaps.
For a brazing gap of 50 and 100 μm, one special phase occurs in a form of a “fishbone” pattern across the brazing seam (Fig. 8 position 8). This kind of structure appears at the border area between the base material and the brazing seam for a brazing gap of 100 μm. It is characteristic for this phase that it is as soft as the base material.
In general, the difference in the microstructural formation for a brazing temperature of 1100 °C and a dwell time of 5 min is shown in Fig. 8, depending on the brazing gap. It is obvious that the microstructure consists of a mixture of relatively ductile phases which partly are even softer than the base material and very hard phases with a high phosphor content. The density of phase precipitation increases with brazing gap widening. With a potential critical brazing gap of 200 μm very hard phases occur in a needle shape structure as a continuous band which may decrease the strength properties of the joint.
4.3 Tensile strength experiments
The tensile strength results shown in Fig. 9 reveal that all tested specimens produced under all applied brazing parameters possess strong tensile strength above 200 MPa. The highest strength of 236 MPa could be achieved with a brazing temperature of 1120 °C, a brazing gap of 100 μm, and a dwell time of 5 min. The lowest tensile strength, 202 MPa was observed on specimens having 50 μm gap after brazing carried out at 1120 °C for a 10-min dwell time. Basically, the differences in the average tensile strengths were smaller than 20 MPa for all investigated brazing parameters. Compared to the commonly used AISI304-BNi-2 brazement, the usage of the brazing filler metal Fe-24Cr-20Ni-10Cu-7P-5Mn-5Si enables a stable process with smaller manufacturing tolerances for the brazing gap. At the same time, the differences in the tensile strength properties lie within the range of 30 to 60 MPa.
Tensile strengths, AISI 304-F300-joint, brazing temperature 1120 °C; AISI 304-Ni620-joint, brazing temperature 1050 °C, dwell time 10 min, brazing gap 60 μm [18]
4.4 Fractographic examination
A two-stage fracture behavior could be recognized for the fractured joints. The upper image of Fig. 10 illustrates the occurrence of spallings at the fringe-area of the broken sample. The SEM-analysis of this area is given in the bottom picture. A kind of a river pattern is visible, which mainly results from an embrittlement. Most likely, the high axial force caused a transcrystalline cleavage fracture that was initiated at the notches of the specimens, caused by the freely solidified brazing seam.
The inner parts of the fractured surfaces, however, reveal another fracture behavior. The inner areas of the fracture mirror show a relatively flat surface without visible peaks or spallings (upper image of Fig. 11). With regard to the reference plane, the cracked surfaces show almost no differences in height at the center of the surfaces. The SEM-analysis of this fracture reveals the appearance of circle and sharp-edge honeycombs which are partly interrupted by intercrystalline separations. It can be stated on the basis of these observations, a residual force fracture as a mixture of a transcrystalline fracture with partially intercrystalline breakups with a honeycombed structure occurs [19].
5 Conclusion
In this study, the brazing characteristics of the iron-based filler metal Fe-24Cr-20Ni-10Cu-7P-5Mn-5Si were investigated. The wetting experiments reveal an excellent spreading behavior on the base material AISI 304. The best spreading ratio of S = 7 could be obtained with a brazing temperature of 1120 °C and a dwell time of 15 min using a vacuum brazing process. Furthermore, it could be observed that the spreading ratio depends more on the dwell time than on the brazing temperature.
On the basis of wedge splitting experiments, the influence of the brazing gap on the microstructure could be evaluated as well. Moreover, the development of different phases was revealed by EDS-measurements in combination with hardness measurements. It could be observed that brittle phases in form of continuous bands occurred with a brazing gap larger than 150 μm.
Additionally, tensile strength tests were conducted with a constant brazing temperature of 1120 °C. The differences in the average strength properties were smaller than 20 MPa, whereby the best result of 236 MPa was achieved for a brazing gap of 100 μm and a dwell time of 5 min. The high joint strengths obtained are in good agreement with the resulting fracture mirror that indicates a two-stage fracture behavior in form of a transcrystalline cleavage and a residual force fracture.
References
Wielage B, Martinez L, Hoyer I (2004) “New developments in high temperature brazing”, Conference Proceedings “LÖT 2004”, 15.-17. 06 Aachen, p. 365–369
Aida M, Takahashi K, Kanda K, Watanabe K, Ariga T (2013) “Manufacturing of exhaust gas recirculation (EGR) heat exchanger (cooler) for automotive use using an Oxynon® furnance”, Conference Proceedings “LÖT 2013”, 18.-20. 06, Aachen, p. 87–91
Hartmann T, Nützel D (2010) “Nickel-chromium-based amorphous brazing foils for continuous furnace brazing of stainless steel”, Conference Proceedings “LÖT 2010”, 15.–17. 06, Aachen, p. 42–47
Wielage B, Hoyer I (2007) High temperature brazing with modified Ni-base brazing filler metals, Conference Proceedings “LÖT 2007”, 19.-21. 06, Aachen, p. 209–214
Larsson H, Rassmus J (2007) “Properties of pressure fatigue loaded brazed heat exchangers in stainless steel brazed with Cu-, Ni- or Fe-based fillers”, Conference Proceedings “LÖT 2007”, 19.–21. 06, Aachen, p. 135–139
Sjödin P (2004) “Improved performance of brazed plate heat exchangers made of stainless steel type EN 1.4401 (UNS S31600) when using a Iron-based braze filler”, Conference Proceedings “LÖT 2004”, 15.–17. 06, Aachen, p. 94–95
Wolfe C, Eklund T, Rersson O (2004) “Investigation of the corrosion performance of different braze fillers fused onto stainless steel type 1.4401 (UNS S31600)”, Conference Proceedings “LÖT 2004”, 15.-17. 06, Aachen, p. 278–280
Bobzin K, Kopp N, Puidokas S, Tillmann W, Wojarski L, Manka M (2013) “Reliable design procedure of iron-based braze joints”, Conference Proceedings “LÖT 2013”, 18.–20. 06, Aachen, p. 261–266
Hartmann T, Nuetzel D (2009) “New amorphous brazing foils for exhaust gas applications”, Proceedings of the 4th International brazing and soldering conference, April 26–29, Orlando, Florida, USA, p. 110–118
Rangaswamy S, Fortuna D (2007) “Novel high chromium containing braze filler metals for heat exchanger applications”, Conference Proceedings “LÖT 2007”, 19.–21. 06, Aachen, p. 12–16
Hartmann T, Nuetzel D (2007) “Iron containing brazing foils for joining of stainless steels”, Conference Proceedings “LÖT 2007”, 19.-21. 06, Aachen, p. 140–145
Matsu K, Sawada T, Fukumoto S, Miyazawa Y, Ariga T (2010) “Mechanical properties of Fe-Cr based brazing filler metals”, Conference Proceedings “LÖT 2010”, 15.–17. 06, Aachen, p. 48–51
Persson U (2010) “Iron-based brazing filler metals for high temperature brazing of stainless steel”, Conference Proceedings “LÖT 2010”, 15.–17. 06, Aachen, p. 38–41
EN 10088.3 (2005) Stainless steels—part 3: technical delivery conditions for semi-finished products, bars, rods, wire, sections and bright products of corrosion resisting steels for general purposes, German Version, Beuth Verlag
Höganäs AB (2013) BrazeLet F300DW-9201 URL: www.hoganasbrazing.com., date of download: 11.12.2014
Schittny T (1990) “Untersuchung zum Hochtemperaturlöten mit nichtkapillarem Lötspalt,”. Fortschrittsberichte VDI, Band 206, Düsseldorf, Germany
Hoyer I (2005) “Beitrag zur Entwicklung von Hochtem-peraturloten auf Eisenbasis,” Chemnitz, Germany
Bobzin K, Schläfer T, Kopp N, Puidokas S, Tillmann W, Wojarski L, Liu C (2010) “Design systematics and application-oriented testing of join compounds brazed with nickel-based brazing filler metals”, Conference Proceedings “LÖT 2010”, 15.-17. 06, Aachen, p. 17–22
Lange G (1997) Systematische Beurteilung technischer Schadensfälle“, Deutsche Gesellschaft für Materialkunde e.V.,Oberursel
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission XVII - Brazing, Soldering, and Diffusion Bonding
Rights and permissions
About this article
Cite this article
Tillmann, W., Wojarski, L., Manka, M. et al. Investigation of the brazing characteristics of a new iron-based brazing filler metal. Weld World 60, 869–875 (2016). https://doi.org/10.1007/s40194-016-0346-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-016-0346-4