Abstract
Purpose of review
Heart failure is a growing problem across the world. Although many advances have been made in heart failure therapy, patients with cardiogenic shock still have a grim prognosis. The aim of this article is to discuss the current state of mechanical circulatory support and future directions.
Recent findings
Mechanical support can be classified as temporary or durable. Temporary support ranges from the intra-aortic balloon pump to extracorporeal membrane oxygenation. Durable support consists of left ventricular assist devices that are long-term and can be used as a bridge to transplant or destination therapy. Many advances continue be made in terms of size, thrombogenic potential, and infection risk.
Summary
As the supply of heart transplants is limited, mechanical support options for a growing heart failure population are becoming increasingly important. Deciding when to initiate and selecting the right device are of utmost importance and should be a multidisciplinary approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure is a growing pandemic across the world and is projected to affect close to eight million people by the year 2030 [1]. Despite advances in pharmacotherapy for chronic systolic heart failure, few advances have been made in the management of patients with acute systolic heart failure complicated by cardiogenic shock. Though the etiology of cardiogenic shock is diverse and varied, the most common cause is secondary to acute myocardial infarction (AMI) with left ventricular dysfunction complicating 2–8% of all acute ST-elevation myocardial infarctions (STEMI) [2]. The TRACE study demonstrated that 30-day mortality was higher in patients with acute myocardial infarction complicated by cardiogenic shock than in those without shock (62% vs. 9%) as well as 6-year mortality (88% vs. 45%). In fact, an 11-year observational study also demonstrated higher rates of mortality with AMI complicated by shock than those without shock (44.8% vs. 30.6%) [3].
Cardiogenic shock is a complex state that is defined as a failure to maintain cardiac output and is characterized by a severely reduced cardiac index (less than 2.0 L/min) along with signs and symptoms of end-organ hypoperfusion, including hypotension, cold extremities, oliguria, and altered mental status. Myocardial infarction leads to left ventricular systolic and diastolic dysfunction, resulting in hypotension. This in turn leads to decreased coronary perfusion and potentiates poor cardiac output. Cardiogenic shock is accompanied by a systemic inflammatory response, activation of systemic neurohormones, and worsening ischemia which propagate this cycle. In the SHOCK trial, the investigators demonstrated that revascularization of patients in cardiogenic shock resulted in a 6-month mortality benefit over patients who were managed with medical therapy [4]. However, even after reperfusion, areas that are stunned continue to exhibit dysfunction. Consequently, mechanical circulatory support (MCS) may be necessary for further recovery. The aim of this article is to discuss the current state of mechanical support and the future direction ahead.
Mechanical circulatory support
Advanced systolic heart failure and cardiogenic shock are typically characterized by elevated systemic vascular resistance as a compensatory mechanism to maintain systemic perfusion. However, this ultimately becomes maladaptive as it results in increased afterload. Thus, standard medical therapy involves afterload reduction. Patients in cardiogenic shock are limited in the usage of such therapies due to hypotension. Though inotropic therapy can be used as a temporizing measure, they lead to increased myocardial oxygen demand and can increase long-term mortality. Thus, MCS can be an option for such patients. MCS decreases myocardial oxygen demand and can provide augmentation of cardiac output and can also decrease afterload. Mechanical support is classified as use for bridge to recovery, bridge to transplant, or destination therapy. The type of support depends on many factors including single ventricle versus biventricular support, temporary versus durable support, and level of support needed. In this article, we outline the various types of support and advances made in each type.
Temporary devices
Intra-aortic balloon pump
Counterpulsation with the intra-aortic balloon pump (IABP) is one of the most established forms of mechanical circulatory support and therefore one of the most commonly used. Typically placed through femoral artery access, it involves placing a balloon that inflates by helium, distal to the left subclavian artery and proximal to the renal arteries. The IABP inflates during diastole, leading to increased coronary perfusion, as coronary perfusion occurs during this portion of the cardiac cycle. Conversely, it deflates during systole, causing a vacuum and decreasing afterload and increasing forward flow. This allows for a modest increase in cardiac output, approximately 0.5–1.0 L/min. Inflation can be timed via electrocardiography, arterial pressure waveforms, or now newer algorithms to detect when the aortic valve opens and closes. Contraindications to the aortic balloon pump include severe aortic insufficiency, aortic dissection, and severe peripheral arterial disease. Given the femoral access, a complication that needs to be monitored for is distal limb ischemia. Weaning of the IABP involves decreasing the frequency of counterpulsation from 1:1, which refers to inflating every beat, to 1:2 and 1:3 and assessing hemodynamics.
Prior to the age of revascularization, studies did not demonstrate a benefit with the intra-aortic balloon pump since it could not improve blood flow past a fixed stenosis. The GUSTO trial showed a trend for lower 30-day and 1-year mortality for patients treated with fibrinolysis and IABP versus those with just fibrinolysis. However, the National Registry of Myocardial Infarction (NRM-2) showed no mortality benefit after primary angioplasty [5]. The IABP-SHOCK II trial, a prospective, randomized trial, randomly assigned patients to IABP and no IABP after shock from acute MI. Fifty-two percent of patients in the IABP group died compared with the 51% of patients in the control group over a span of 12 months (relative risk [RR] 1.01, 95% CI 0.86–1.18, p = 0.91). In addition, there was no difference in reinfarction, repeat revascularization, or stroke. Interestingly, there was a 10% crossover rate from the control to the IABP group and a trend toward eventual use of a ventricular assist device in the control group [6]. Although there has been no demonstrated mortality benefit, the IABP is still widely used, mostly due to availability and ease of insertion. Overall, the American College of Cardiology and American Heart Association (ACC/AHA) have collectively given insertion of temporary mechanical support for refractory cardiogenic shock a class IIa recommendation, and the European Society of Cardiology gives it a class IIb [7, 8].
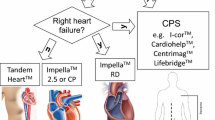
Impella
The Impella is a type of temporary left ventricular assist device (LVAD) that is placed percutaneously and crosses the aortic valve to rest in the left ventricle. It utilizes the Archimedes screw principle and aspirates blood from the left ventricle through a continual axial flow pump to the aorta. The Impella 2.5 and CP devices are able to generate an output between 2.5 and 4 L/min, and are typically inserted peripherally via the femoral artery. The Impella 5 can generate an output of 5.0 L/min, however requires a large 22-F sheath and a surgical cutdown. The RP Impella is a continuous axial flow device that is designed to provide right ventricular support. Though similar in configuration, it is placed through venous access and crosses the pulmonic valve to rest in the pulmonary artery. The Impella directly decreases ventricular stress, wall stretch, and preload, without increasing afterload. Contraindications to the Impella include severe aortic stenosis or insufficiency, LV thrombus, mechanical aortic valve, or severe peripheral arterial disease. The Impella has varying levels of support, using nomenclature of P1 (lowest degree of support) to P9 (highest degree of support). Continuous monitoring of this device by echocardiogram is necessary as it needs frequent positioning. Additionally, hemolysis is a significant complication, requiring vigilant monitoring of coagulation factors.
The initial study that promoted the use of the Impella device was the ISAR-SHOCK trial in 2008 which was a randomized, prospective trial of twenty-six patients with cardiogenic shock, randomized to IABP versus Impella. After 30 min, the Impella group had a larger increase in cardiac output (0.49 ± 0.46 L/min/m2) versus the IABP group (0.11 ± 0.31 L/min/m2). However, there was no difference in 30-day mortality [9]. The PROTECT II was a larger randomized study of four hundred and fifty-two high-risk PCI patients with depressed LV function using the Impella 2.5. This trial was stopped for futility as the 30-day incidence of major adverse events was similar (35.1% for Impella vs. 40.1% for IABP). Again, the Impella group was found to have better hemodynamics [10]. The RP Impella was studied separately, including a prospective multicenter trial where thirty patients with right ventricular failure unresponsive to medical therapy had the Impella RP device implanted. Cardiac index increased from 1.8±0.2 L/min/m. The overall survival at 30 days was 73.9% [11]. Based on these studies, the FDA has approved the Impella for treatment of cardiogenic shock within 48 h of acute myocardial infarction or post-cardiotomy.
An innovative approach for biventricular failure is the BiPella system which employs a simultaneous left-sided and right-sided Impella configuration. In 2017, Kuchibhotla et al. published a retrospective analysis of twenty patients in cardiogenic shock supported by BiPella. All patients either had Impella 2.5, Impella CP (most common), or Impella 5 for left-sided support and Impella RP for right-sided support. All patients experienced decreased cardiac filling pressures and increased cardiac output. However, in-hospital mortality was 50%. Predictors of survival were identified as younger age, implantation of LV and RV support simultaneously, lower pulmonary artery pressures, and lower pulmonary vascular resistance prior to deployment [12•]. In 2019, a multicenter retrospective analysis of fourteen patients demonstrated similar results of increased survival with simultaneous deployment of left- and right-sided support and lower pulmonary artery pressures [13]. These observations corroborate the idea that elevated pulmonary vascular resistance and pulmonary pressures correspond to more severe and long-standing disease, and portent a poorer prognosis irrespective of therapy. Although both were small studies, they have prompted the case for this non-surgical means of acute biventricular support.
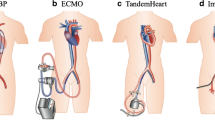
TandemHeart
The TandemHeart system is a percutaneous biventricular support system that was developed in 2005. It utilizes a 21-F catheter which traverses into the right atrium and requires a trans-septal puncture into the left atrium. It then drains oxygenated blood from the left atrium and then pumps it into the femoral artery. The TandemHeart provides continuous flow circulatory support up to 5 L/min. Similar to the Impella, the TandemHeart decreases left ventricular filling pressures and decreases wall stress. Contraindications to placing this device include left atrial or right atrial thrombus and severe peripheral arterial disease. Two clinical trials in 2005 and 2006 randomized patients in cardiogenic shock after myocardial infarction to either TandemHeart or IABP. The trial in 2005 demonstrated that the TandemHeart group had statistically significant improvements in cardiac output (p = 0.007), cardiac power index (p = 0.004), pulmonary capillary wedge pressure (p = 0.003), and serum lactate level (p = 0.03). However, complications including limb ischemia and coagulopathies were higher, and overall, there was no mortality difference at 30 days [14, 15].
Percutaneous LVADs should be considered in patients with cardiogenic shock refractory to pharmacotherapy. The 2013 AHA/ACC guidelines give percutaneous LVADs, including TandemHeart, a class IIb/LOE C indication in refractory shock [7]. However, appropriate patient selection and assessment of the clinical situation are paramount.
ECMO
Extracorporeal membrane oxygenation (ECMO) was first studied in the 1970s in respiratory failure and has since undergone many improvements. Though various forms of ECMO are utilized, the venous-arterial ECMO (VA-ECMO) is utilized in cardiogenic shock. VA-ECMO involves placing a cannula that withdraws deoxygenated blood from the inferior vena cava, oxygenates it, and then returns it to the arterial side via flows of up to 10 L/min. In contrast to other forms of mechanical support, ECMO provides biventricular support. The only true contraindication to ECMO support is a preexisting condition which would preclude recovery such as severe neurological injury or end-stage malignancy.
Several considerations need to be made with ECMO. First, the system requires full anticoagulation as the oxygenator has the potential for thrombosis. Second, the arterial cannula delivers oxygenated blood to the arterial system. Though this increases the systemic blood pressure and improves end-organ perfusion, it also increases left ventricular after load and wall stress. This is actually counterproductive in the setting of cardiogenic shock where the goal is to decrease myocardial oxygen demand. Mechanisms to counteract this include “venting” the left heart which involves draining blood from the left atrium and connecting it to the ECMO circuit, or concurrent use of an Impella or IABP system. Lastly, if the heart retains enough function to pump poorly oxygenated blood from the lungs, “North-South” syndrome can arise, where the upper half of the body receives poorly oxygenated blood and the lower half of the body receives sufficiently oxygenated blood from the ECMO circuit.
In 2016, a meta-analysis looked at two hundred and thirty-five patients who had cardiogenic shock after acute myocardial infarction. ECMO patients had a 33% higher 30-day survival when compared with patients with IABP support (95% CI, 14–52%; p < 0.001; NNT 13). However, this increase did not hold true when compared with those of TandemHeart or Impella (− 3%; 95% CI − 21 to 14%; p = 0.70; NNH 33) [16]. There have been no large-scale, randomized controlled trials studying ECMO to date.
Because of the ability to implant bedside, extracorporeal CPR (e-CPR) has grown in usage during refractory cardiac arrest. The CHEER trial was a prospective, single-center trial published in 2014 that evaluated twenty-six patients with in-hospital and out-of-hospital cardiac arrest and usage of mechanical CPR, therapeutic hypothermia, and bedside percutaneous cannulation of the femoral artery and vein for commencement of VA-ECMO. Return of spontaneous circulation occurred in 96% of patients and full neurological recovery occurred in 54% of patients [17•]. Although not currently standard of care, this study demonstrates the feasibility and efficacy of VA-ECMO in cardiac arrest and advocates for creation of such a protocol.
Durable mechanical support
HeartMate 3
With the rate of advanced heart failure increasing globally with and a limited amount of transplants available, durable mechanical support with surgically implanted LVADs is becoming increasingly utilized. Common complications of LVADs include pump thrombosis and stroke. In addition, older LVAD models were large and bulky, making implantation difficult. However, newer models, such as the HeartMate 3, have addressed such complications. Smaller than its predecessors, the HeartMate 3 is a centrifugal pump that does not require a pump pocket and has a slimmer apical sewing cuff. This allows for less surgical procedures and decreased recovery time. The HeartMate 3 is different from its predecessors in that it is a centrifugal pump, whereas the HeartMate XVE was pulsatile and the HeartMate 2 was an axial pump. This design, along with the fact that it is a fully magnetically levitated motor, allows for decreased friction and less shearing effects, and the textured surfaces with sintered titanium microspheres simulate a tissue interface with blood. In addition, the HeartMate 3 creates an artificial pulse which decreases the amount of stasis and potential for thrombosis and increases aortic valve opening to decrease valve fusion.
The Momentum III study published in 2018 was a randomized study that assigned a total of three hundred and sixty-six patients to either a centrifugal pump or to the standard axial based pump (HeartMate 2). The trial population were patients with end-stage heart failure refractory to medical therapy and both either bridge to transplant or bridge to destination therapy. The primary endpoint was survival at 2 years free of disabling stroke or survival free of reoperation to replace or remove a malfunctioning device. Secondary endpoints included adverse events such as stroke, bleeding, infection, and quality of life. After 2 years, the primary endpoint was reached more in the centrifugal group (79.5%) versus the axial pump group (60.2%) (p < 0.001). Three patients in the centrifugal pump group underwent reoperation versus thirty patients in the axial pump group, most often for pump thrombosis or severe hemolysis in the latter group. In contrast, the centrifugal group had zero patients with thrombosis that lead to reoperation [18••]. The significantly low rate of thrombosis has prompted clinicians to lower anticoagulation goals, which decreases bleeding potential. In 2018, the FDA approved the HeartMate 3 for destination therapy for patients who are not transplant candidates.
Miniature ventricular assist device
As technology advances, left ventricular assist devices have decreased in size in order to reduce potential complications. The miniature ventricular assist device (MVAD) weighs about 78 g and can provide flows from 1 to 7 L/min. The unique aspect of the MVAD system is that the motor lies in the inflow cannula. It utilizes a hybrid system of passive magnetic and hydrodynamic forces to suspend its motor which has wide helical channels, which in turn decrease sheer stress. Because of its significantly smaller size, it can be implanted via thoracotomy as opposed to sternotomy and can be used in smaller patients, extending its use to the pediatric population. In 2014, the MVAD system was tested in sheep with promising results. Out of the nine animals, seven animals survived to the end of the study and none of the survivors had evidence of end-organ damage by laboratory analysis or pathological analysis (two died from non-device-related complications). In addition, the device demonstrated hemocompatibility as none of the devices had thrombus and blood cell morphology and coagulation parameters were within normal limits [19]. This study demonstrated the safety and efficacy of miniature devices but human testing is still pending.
Heart Assist 5
As gastrointestinal bleeding is a common complication of durable devices, anticoagulation is often problematic and devices with decreased thrombogenic potential are being sought after. The Heart Assist 5 is another one of the smallest and lightest axial flow pumps at this time, weighing just less than 100 g and around the size of a quarter. It is also implanted in the pericardium as opposed to the abdomen, and because of its size, it extends into usage for the pediatric population. It is unique in that it houses a small probe that monitors flow through the outflow graft into the aorta. The hemodynamic information is displayed on the controller and can also be remotely monitored to guide therapy (i.e., volume management, medication changes). A study in 2016 compared the Heart Assist 5 to the Heart Mate 2 and found it to have lower thrombogenic potential due to less platelet activation, lower speed requirements, and less sheer stress as seen in artificial flow models [20]. As thrombogenic potential becomes increasingly optimized, the need for anticoagulation decreases and may eventually be eliminated.
Total artificial heart
Total artificial hearts (TAH) have been an important development for patients requiring biventricular support and who are not candidates for LVADs. Patients with right ventricular failure have worse outcomes with LVADs as a weak right heart cannot accommodate the increased flow generated from the LVAD. The device consists of two polyurethane ventricles and two mechanical single-leaflet tilting valves. The surgical procedure is more complicated as it involves cardiopulmonary bypass, then excising the right and left ventricles as well as atrioventricular valves and then attaching the artificial ventricles. The Jarvik 7 device was the first TAH and utilized pneumatic compression to generate a cardiac output of 6 to 8 L/min. It was eventually renamed CardioWest, and in 2004, Copeland et al. published a 10-year clinical study that demonstrated 79% success rate of bridge to transplant. Total artificial hearts became relevant with the original SynCardia TAH which established a feasible bridge to transplantation [21]. In 2013, the FDA approved a humanitarian use for a smaller version of the SynCardia TAH for destination therapy.
Research continues in this field, namely the CARMAT TAH. This device utilizes an electrohydraulic mechanism to generate pulsatile flow that adapts with exercise. In addition, it has bovine pericardium valves that enhance its hemocompatibility. A case report of two patients published in 2015 by Carpentier et al. demonstrated a patient living up to 9 months post implant [22]. The ReinHeart TAH is a novel system that utilizes a pusher plate between each ventricle chamber alternating between squeezing one chamber while relaxing the other. The preliminary animal data has shown favorable results with 99.4% of blood washing out within three pump cycles leading to decreased pooling of blood and decreasing rates of thrombosis [23]. Limitations include durability of the device as there are numerous moving parts as well as need for systemic anticoagulation. However, other advances have been proposed for increased hemocompatibility including titanium surfaces and grating that promotes adhesion of endothelial cells. These advancements will potentially decrease the rates of pump thrombosis and cerebral vascular accidents in the future.
Transcutaneous energy transmission
A common factor for current forms of durable mechanical support are drivelines which are externalized power cables and are a major source of infection. Although decreasing the size of the driveline has decreased rates of infection, the infection rate remains approximately 19% in the 12 months following implantation [24]. A novel mechanism has evolved called transcutaneous energy transmission (TET) which has allowed complete internalization of the device. The concept involves an external battery which provides direct current which is converted to alternating current and then applied to a coil which sits on the skin. This coil produces an oscillating magnetic field which resonates an internal coil which generates an alternating current and powers an internal battery. Two devices have utilized this mechanism, the AbioCor implantable replacement heart and Arrow International LionHeart. These devices had obvious drawbacks including the need for close TET location and short battery lives which led to inferior outcomes in studies. However, these devices were the first in its kind and have paved the way for further research. The FREE-D system improved on this technology by utilizing more efficient resonant coupling to induce electricity over 2 to 3 m and extinguished the need for skin contact and reduced interference. While both of these technologies are far from perfection, they both symbolize innovative means for reducing the need for externalized drivelines and decreasing rates of infection.
Specific uses
As demonstrated, many different forms of mechanical support are available and several decisions need to be made when deciding on mechanical circulatory support. The first consideration is when to consider MCS. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a North American registry of patients who have an FDA-approved mechanical circulatory support device and provides a classification which is a useful tool to guide this decision. Patients who are INTERMACS profile 2 have evidence of worsening perfusion and end-organ function and are defined as patients who are “sliding” on inotropes. These patients are candidates for initiation of mechanical support. Generally, if the patient likely has irreparable pathology or severe neurological insult, mechanical support should be a multidisciplinary decision. Timing has been looked at in several studies, and the expert consensus is that early recognition and early initiation are important, particularly in the setting of acute myocardial infarction. These studies have contributed to the push for “door to unloading” time [25]. Early recognition of cardiogenic shock and early initiation of mechanical support lead to decreased left ventricular stress and oxygen demand, less inflammatory response, less ischemia, and overall better outcomes.
When to transition from temporary support to durable support is another important point. In patients who have not made meaningful recovery, durable mechanical support should be considered. Recovery can be assessed by echocardiography, by laboratory analysis, and by hemodynamic parameters. In patients with an acute insult such as acute myocardial infarction or acute myocarditis, it is recommended to trial temporary mechanical support for about 1 week before making the decision for bridge to durable device or bridge to transplant [26, 27]. For patients with long-standing cardiomyopathy, the decision for bridge to durable device or bridge to transplant should be made earlier as their chances for recovery are smaller. Temporary mechanical support in these situations should be utilized to permit recovery of cardiac output and to permit time for candidate selection and elective transition to durable device or transplant.
Another decision factor is level of support (i.e., level of flow needed for end-organ perfusion, single ventricle versus biventricular support) and whether support is expected for longer periods of time. In the latter case, clinicians may opt for axillary approach where the patient can remain ambulatory. Anticoagulation is another factor to consider. The IABP system does not require full anticoagulation, particularly if it is placed in 1:1 setting. The next decision to be considered is whether oxygenation will be required. In this setting, the IABP or Impella system would not be ideal and more advanced forms of support should be considered. In the chronic heart failure setting, durable devices are to be considered. Similar decision points of single versus biventricular support need to be considered. Patients with right ventricular failure have worse outcomes with pure LVAD support and BiVAD or TAH should be considered at that point.
Conclusions
Heart failure continues to be a growing epidemic, and as the supply of transplants is limited, mechanical support remains a promising option for many patients. Mechanical support is certainly a growing field; however, the lack of randomized trials remains an obstacle. As the “door to balloon” time continues to evolve, pursuing further research in mechanical circulatory support will be of paramount importance. Whether it is acute cardiogenic shock or chronic heart failure, this new array of devices will usher in a new era of cardiac care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–10.
Steg PG, Goldberg RJ, Gore JM, et al. Baseline characteristics, management practices and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol. 2002;90:358–66.
Singh M, White J, Hasdai D, Hodgson PK, Berger PB, Topol EJ, et al. Long-term outcome and its predictors among patients with ST-segment elevation myocardial infarction complicated by shock: insights from the GUSTO-1 trial. J Am Coll Cardiol. 2007;50:1752–8.
Hochman JS, Sleeper LA, Web JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–34.
Barron HV, Every NR, Parsons LS, Angeja B, Goldberg RJ, Gore JM, et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001;141:933–9.
Thiele H, Schuler G, Neumann FJ, Hausleiter J, Olbrich HG, Schwarz B, et al. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock design and rationale of the intraaortic balloon pump in cardiogenic shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163(6):938–45.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129.
Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–8.
O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–27.
Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34(12):1549–60.
• Kuchibhotla S, Esposito ML, Breton C, et al. Acute biventricular mechanical circulatory support for cardiogenic shock. J Am Heart Assoc. 2017;6(10) Established the efficacy of percutaneous biventricular support in the acute cardiogenic shock setting.
Kapur N, Breton C, O'Kelly R, et al. Simultaneous, not staged, deployment of biventricular micro-axial flow Impella catheters (BiPella) is associated with improved survival for cardiogenic shock involving biventricular failure. J Am Coll Cardiol. 2016;68(18).
Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26.
Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intra-aortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.
Ouweneel DM, Schotborgh JV, Limpens J. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922–34.
• Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94 Established the efficacy of ECMO during cardiac arrest and suggests it should be more widely used in this setting.
•• Mehra M, Goldstein D, Uriel N, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–95 Demonstrated that the HeartMate 3 has better outcomes and lower rates of pump thrombosis compared with the previous HeartMate 2.
McGee E, Chorpenning K, Brown M, et al. In vivo evaluation of the HeartWare MVAD Pump. J Heart Lung Transplant. 2014;33(4):366–71.
Chiu W, Girdhar G, Xenos M, et al. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation-optimized HeartAssist 5 VAD. J Biomech Eng. 2014;136(2).
Copeland J, Smith R, Arabia F, Nolan PE, Sethi GK, Tsau PH, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859–67.
Carpentier A, Latremouille C, Cholley B, et al. First clinical use of a bioprosthetic total artificial heart: a report of two cases. Lancet. 2015;386.
Pelletier B, Spiliopoulos S, Finocchiaro T, et al. System overview of the fully implantable destination therapy-ReinHeart-total artificial heart. Eur J Cardiothorac Surg. 2015;47(1):80–6.
Goldsetin DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31(11):1151–17.
Kapur N, Alkhouli M, DeMartini T, Faraz H, George ZH, Goodwin MJ, et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment–elevation myocardial infarction. Circulation. 2019;139:337–46.
den Uil CA, Akin S, Jewbali L, et al. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017;52:14–25.
Shah P, Pagani F, Desai S, et al. Outcomes of patients receiving temporary circulatory support before durable ventricular assist device. Ann Thorac Surg. 2017;103:106–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
John, A.K., Pirlamarla, P. Mechanical Circulatory Support: a Look Back and a Look Ahead. Curr Emerg Hosp Med Rep 7, 189–195 (2019). https://doi.org/10.1007/s40138-019-00203-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00203-3