Abstract
Cardiogenic shock due to acute myocardial infarction, postcardiotomy syndrome following cardiac surgery, or manifestation of heart failure remains a clinical challenge with high mortality rates, despite ongoing advances in surgical techniques, widespread use of primary percutaneous interventions, and medical treatment. Clinicians have, therefore, turned to mechanical means of circulatory support. At present, a broad range of devices are available, which may be extracorporeal, implantable, or percutaneous; temporary or long term. Although counter pulsation provided by intra-aortic balloon pump (IABP) and comprehensive mechanical support for both the systemic and the pulmonary circulation through extracorporeal membrane oxygenation (ECMO) remain a major tool of acute care in patients with cardiogenic shock, both before and after surgical or percutaneous intervention, the development of devices such as the Impella or the Tandemheart allows less invasive forms of temporary support. On the other hand, concerning mid-, or long-term support, left ventricular assist devices have evolved from a last resort life-saving therapy to a well-established viable alternative for thousands of heart failure patients caused by the shortage of donor organs available for transplantation. The optimal selection of the assist device is based on the initial consideration according to hemodynamic situation, comorbidities, intended time of use and therapeutic options. The present article offers an update on currently available mechanical circulatory support systems (MCSS) for short and long-term use as well as an insight into future perspectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and historical overview
Over the last 40 years, a variety of mechanical circulatory support systems (MCSS) have been developed to offer on the one hand short-term circulatory stabilization of patients either developing cardiogenic shock due to myocardial infarction, percutaneous, and surgical procedures, or being at high risk for further diagnostic cardiac interventions and on the other side provide a permanent alternative or bridging to cardiac transplantation. These devices include aortic balloon pumps [1], total artificial hearts [2], extracorporeal membrane oxygenation systems [3], portable pump oxygenators [4] and ventricular assist devices (VADs).
Historically, John Gibbon in 1953 was the first to apply successfully in clinical practice the concept of the mechanical support to the cardiopulmonary system using for an atrial septal defect repair the cardiopulmonary bypass [5]. DeBakey and coworkers were the first, who implanted a VAD in 1963 without success due to the patient’s death on the fourth postoperative day. Nevertheless, the first successful implantation of a pneumatically driven VAD as bridge to recovery (BTR), leading to the patient’s discharge, was reported in 1966 by the same group [6]. Soon thereafter, the team of Denton Cooley performed the first successful implantation of a pneumatically driven artificial heart as bridge to transplantation (BTT) [7], while DeVries and colleagues undertook the first successful implantation in 1984 of the Jarvik-7-100 total artificial heart [8]. A temporarily moratorium in 1991 regarding the use of the total artificial heart, due to the associated high morbidity was terminated in 1994, through the food and drug administration (FDA) approval of an left VAD as a “bridge to transplantation” treatment, arising from the technical evolution of the devices and the concurrently limited applicability of heart transplantation (Fig. 1).
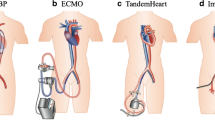
Optional use of circulatory assist devices used in cardiogenic shock. Cardio pulmonary support (CPS) includes a membrane oxygenator. Intravascular systems like the Impella are dependent on left myocardial filling an arterial afterload, and sufficient pulmonary function. If weaning is not successful other options like bridge to transplantation or destination therapy with a left or biventricular permanent assist device (LVAD, BVAD) should be considered in selected cases
Long-term mechanical circulatory support
VADs in cardiac surgery
General considerations
VADs are mechanical pumps constructed to support one or more ventricles of the failing heart. Depending on the supported cardiac chamber the devices are classified as: LVADs to augment the left ventricle, RVADs for right ventricle support and Bi-VADs if both left and right ventricle function is replaced by the system. With regard to the technological characteristics of the blood pumps there are two main types: positive displacement pulsatile and rotary continuous flow pumps.
The advantage of pulsatile pumps is on the one hand maintaining the physiological condition of pulsatility, but on the other these devices had high rates of complications such as infections and mechanical failure due to their large size and their complex pump function. The blood flow in pulsatile pumps is generated either pneumatically or by a pusher plate against a segmented polyurethane blood sac. Inlet and outlet valves ensure correct unidirectional flow at the appropriate time, characterized by inlet jets establishing a large vortex during filling, which washes the whole chamber [9].
The principle of continuous flow VADs arose from the desire of minimizing the pump size and to move away from the external venting. Thus, the advantages of continuous flow pumps are the simpler smaller designs, including fewer moving parts, and lower power consumption. They work either by an axial or a centrifugal mechanism, with the rotor impeller being levitated magnetically in both pump designs [10]. Centrifugal pumps produce higher pressures at lower flow rates, while axial pumps usually generate higher flows with lower pressure rises, and require, therefore, much faster rotational speeds. In addition, centrifugal pumps are bigger and heavier compared to their axial counterparts, which due to their tubular shape are easier to implant. The impact of the non-physiological pulseless blood flow on the circulation and organ function remains debatable [9], although there are series suggesting that the continuous blood flow is not only physiologically entirely well tolerated, but it improves neurocognitive dysfunction caused by severe heart failure, in the same degree as pulsatile devices do [11].
With regard to the clinical intent at the time of device implantation, four broad indications are defined: (a) the bridge to transplant intent (BTT) performed on patients eligible for transplantation, while listed for a transplant; (b) destination therapy (DT) for patients ineligible for transplantation having refractory heart failure symptoms; (c) bridge to decision (BTD) including patients requiring MCS with the option of reevaluation of their candidacy for transplantation after improvement of clinical parameters through the MCS; and (d) bridge to myocardial recovery (BTR) applied to patients with non-ischemic heart failure, with the goal to restore myocardial function targeting the explantation of the device [12]. The decisions: firstly to apply a VAD to a patient, and secondly the precise choice of the device is often difficult, thus the criteria for referral vary greatly among institutions. However, heart failure confirmed by typical signs such as pulmonary capillary wedge pressure >20 mmHg, cardiac index <2.0 L/min/m2, or systolic blood pressure <80 mmHg, despite best medical management, should be present [13].
According to their technical characteristics and reflecting their development through the years VADs are divided, in general, into three groups:
First-generation VADs
The first-generation LVADs were the first devices initially introduced into clinical practice consisting of large pulsatile, positive displacement pumps with a lot of moving parts. Thus, those pumps were limited to patients with a body surface area greater than 1.5 m2. The prototypes are the Novacor left ventricular assist system (LVAS, WorldHeart, Salt Lake City, UT, USA), the Thoratec IVAD (implantable ventricular assist device) and the HeartMate XVE (later called HeartMate I; Thoratec Corporation, Pleasanton, CA, USA) [14, 15]. These early devices were powered by two rechargeable batteries that provided 4–6 h of power and were usually worn in a shoulder holster, vest, or belt [16]. Other commonly utilized pulsatile flow MCSS, in which the blood pump lies external to the patient are: the Thoratec PVAD (paracorporeal ventricular assist device), the Berlin Heart Excor (Berlin Heart AG, Berlin, Germany) and the Toyobo LVAS (Toyobo Co Ltd, Osaka, Japan). Their main implantation indications are the temporary use for BTT and BTR (Table 1) [13].
Second-generation VADs
The second-generation VADs consisted of axial pumps, utilizing continuous blood flow without valves. Their smaller size enables the implantation in patients with small body surface areas. Second-generation VADs include the Jarvik 2000 (Jarvik Heart, New York, NY, USA), the MicroMed DeBakey VAD (MicroMed Technologies, Woodlands, TX, USA) and the HeartMate II (Table 1). The HeartMate II represents to date the most frequently used second-generation pump worldwide [17–20].
Third-generation VADs
Third-generation VADs provide like second-generation pumps continuous blood flow, utilized from an axial or a centrifugal rotor. The impeller or rotor consists of a mechanism forced by hydrodynamic or electromagnetic energy, reducing in that way, the moving parts and the areas of contact.
The magnetic-levitation (maglev) system can be distinguished into three types [21]:
-
1.
External motor-driven system, where the magnetic coupling force to the impeller is induced by a motor, while the impeller suspension is controlled by a separate levitation system. The impeller is levitated in the axial or z-direction. Disadvantage of the system is the need of mechanical bearings in the external motor prone to mechanical wear.
-
2.
Direct-drive motor-driven system, where the impeller becomes the motor rotor and rotates through magnetic flux realized through an external stator, while a separate levitation system is incorporated into the system to provide magnetic suspension. This system follows the principle one-axis control combination with hydrodynamic force and
-
3.
Self-bearing or bearingless motor system, where the levitation and drive coils share the same stator core to make it as if the bearing does not exist. In this system levitation is implemented in both, radial and axial, directions [21].
Their smaller size approaching almost that of an AA battery enables the relative non-invasive complete intrapericardial implantation, adjacent to the heart with improved patient outcomes [22]. Third-generation MCSS include the Levacor VAD (WorldHeart), HeartWare HVAD (HeartWare International, Inc, Framingham, MA, USA), VentrAssist (Ventracor Ltd., Sydney, Australia, since 2010 Thoratec Corporation, Pleasanton, CA, USA), DuraHeart (Terumo Heart Inc, Ann Arbor, MI, USA) and the Berlin Heart Incor (Berlin Heart, Berlin, Germany) (Table 1). Historically, the DuraHeart was the first third-generation device entering European clinical trials in 2004 [23]. In summary third-generation devices consist of smaller, potentially more reliable LVADs, which make long-term circulatory assist available to a wider range of the heart failure population, particularly those who are ineligible for transplantation or those with smaller body surface area [13].
Third-generation VADs under development
Devices currently under development incorporating either magnetic and/or hydrodynamic levitation are: the Heartmate III® (Thoratec Corporation, Pleasanton, CA, USA, in collaboration with and Levitronix GmbH, Zurich, Switzerland) [24]; the Arrow CorAide® (Arrow International Inc., Reading, PA, USA in collaboration with the Cleveland Clinic Foundation) [25]; the Magnevad II® (Gold Medical Technologies, Inc., Valhalla, NY, USA) [26]; the Ibaraki University Pump, (Hitachi, Japan) [27]; the MiTi Heart® (MiTiHeart Corporation, Gaithersburg, MD, USA) [28]; and the TMDU/TIT LVAS (co-developed by the Tokyo Medical and Dental University and the Tokyo Institute of Technology). Figure 2 depicts the evolution of VADs from the first-generation devices up to systems under development or evaluation in clinical trials.
Outcomes of LVADs
In the LVADs outcomes analysis, it should always be mentioned the randomized evaluation of mechanical assistance in treatment of chronic heart failure (REMATCH) trial [29] as the landmark of the clinical studies evaluating the efficacy of VAD support in the treatment of HF. The series included 129 patients with HF of New York Heart Association (NYHA) class IV ineligible for heart transplantation, who were randomized to receive either a HeartMate XVE assist device, or maximum optimized medical treatment. The survival rates at 1 and 2 years were in the pump group with 52 and 28 %, respectively, significantly higher compared to those of the medical treatment group, which showed survival rates of 25 and 8 % after the same observation time periods. LVAD placement led to a 48 % relative reduction in the risk of death during a 30-month follow-up, and a 27 % absolute reduction in 1-year mortality. Despite the trial-proven marked survival advantage of MCSDs over chronic medical therapy, it should not be overseen that survival in the device group was still low and furthermore the neurological event rate with LVAD therapy was 4.35-fold higher than that observed in patients receiving only medical therapy [29]. However, the ongoing improvement in the field of surgical techniques, postoperative care and devices design resulted to a decrease of the mortality of patients with mechanical support to a level of approximately 9 % in some centers [30].
The efficacy of a third-generation assist device (HeartWare® HVAD centrifugal pump) was evaluated among others in the ADVANCE trial [31]. It consists of a study conducted in the USA, which compared the HVADR as a BTT device with commercially available devices (mostly the HeartMate® II). At 1 year, 86 % of the 140 enrolled patients were still alive, and the device was shown to be non-inferior to established LVADs [31]. Device-related morbidity like bleeding, infections, and perioperative right heart failure were the most common adverse events, which are typical in patients with all types of LVAD [17, 20, 32].
In the same manner, two pivotal trials [17, 32] comparing the second-generation HeartMate® II axial pump to its pulsatile counterpart (HeartMate® XVE), demonstrated significantly improved probability of survival and freedom from stroke and device failure at 2 years (actuarial survival 58 and 24 % with the HeartMate® II and the HeartMate® VXE, respectively). Consequently, the FDA approved the HeartMate® II in 2008 for BTT, and for DT in 2010. The benefits of the HeartMate® II extended up to at least 18 months (72 % actuarial survival), and patients had marked improvements in their NYHA functional class and quality of life [20].
Fundamental information regarding the changes in the landscape of MCSDs provides the annual reports of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), a collaborative database, which collects data since its launch in 2006 on MCSD implants in the USA. Since the aforementioned FDA approval of the HeartMate II continuous flow pump for BTT in 2008, there was not only a radical shift towards an extended use of continuous flow pumps, reflected by the fact that since 2010, among patients stratified to a DT designation, essentially 100 % received this type of device, but also a gradual change is observed with regard to the treatment strategies, reflected by the increased use, approaching a rate of 40 %, of implants designated as DT. The recently published fifth annual report of INTERMACS, analyzing data of 6,561 mechanically supported adult patients, demonstrated actuarial survival rates at 1 and 2 years among continuous flow pumps of 80 and 70 %, respectively. With regard to the treatment intent, DT carries a slightly higher risk than BTT therapy due to the availability of transplantation for some BTT patients in the event of device-related complications. When adjusted for risk factor prevalence in each group, the difference in predicted 1-year survival is approximately 5 % [33].
Total artificial heart (TAH)
Although the majority of the cases suffering from end-stage HF are well supported with univentricular assist devices, there is a subset of patients requiring biventricular support, depending on their clinical condition. These patients are more critically ill and tend to have worse outcomes compared to those with only LVAD support. Currently, there are limited assist devices available for biventricular support; however, the selection of what type of device to implant depends on institutional preferences. TAH is one available option when long-term support of both ventricles is required, and two devices are to date predominantly in clinical use: the CardioWest pneumatic TAH (TAH-t; SynCardia, Inc., Tucson, AZ, USA) and the hydraulic AbioCor implantable replacement heart (IRH) (ABIOMED, Danvers, MA, USA).
The CardioWest TAH is the descendent of the famous Jarvik-7 heart, and is the only commercially available TAH approved as a temporary device for BTT. It is implanted orthotopically after partial cardiectomy replacing the ventricles, all four valves and the proximal portion of each great vessel. The pneumatic-driven device consists of two separate polyurethane chambers representing each ventricle, housing a four-layered seamless diaphragmatic membrane. The half of this membrane is attached to the interior of the chambers, while the other half is mobile. The unidirectional blood flow is achieved through an inflow and outflow valve in each chamber (inflow valve: 27 mm Medtronics Hall tilting disc valve, outflow valve: 25 mm Medtronics Hall tilting disc valve). An external console through a reinforced pneumatic driveline supplies the device with compressed air, thus the patient is always tethered and dependent to the external console, and therefore, restricted to a hospital setting. More than 900 CardioWest TAH-ts have been implanted worldwide, and in several series the device performed safe, comparable to currently used biventricular assist devices and LVADs [34, 35].
Further development on the CardioWest TAH focuses on decreasing the size of the external module to facilitate patient convenience and mobility. Currently, a smaller portable external console (Freedom driver) is under investigation.
The AbioCor (Abiomed, Danvers, MA, USA) Implantable Replacement Heart (IRH) was designed as a totally implantable TAH system and unlike the CardioWest TAH is an electrically driven, volume-displacement pump using a transcutaneous energy transmission (TET) coil to charge the implantable battery. The device is intended exclusively as an alternative to transplantation for DT. It consists of internal implantable and external components.
The implantable units include the thoracic pump, the internal battery, a controller, and an internal TET coil. The pump consists of two pumping chambers (ventricles) sandwiching the electrohydraulic unit. The casing material is titanium, and all blood-contacting surfaces including the four valves within the device are made up of polyether urethane. The electrohydraulic unit is a centrifugal pump rotating at 5,000–9,000 rpm. The internal battery is the power source for the artificial heart and takes the size of earlier versions of implantable defibrillators. It can provide power autonomous unattached to the external source for a short period of time ranging from 15 to 30 min. Thus in everyday use, it is constantly being recharged from the external source. The controller is of the same size as the internal battery and is implanted along with it in the preperitoneum. It operates as the communicator between the thoracic unit and the external console, and monitors the hydraulic chamber pressures and pump speed and has the ability to adjust pump parameters like rate, motor speed and balance between left- and right-atrial pressures [36]. The internal TET coil is responsible for energy transmission through inductive coupling when an external TET coil is placed over it and is placed in the infraclavicular region.
The external components include an external TET coil, a console, a radiofrequency communicator box and an external battery pack. The external TET coil is placed and firmly held in position by adhesive dressing. The external console receives system-related information from the internal controller via radiofrequency telemetry. It constantly traces the hydraulic pressures, provides stroke volume, pump rate and heart rate data with the ability to adjust the parameters through the internal controller.
To date, there is limited clinical experience with this device. The initial clinical experience suggests that the AbioCor TAH might be effective as DT in patients with biventricular end-stage congestive HF [37–39]. Compared to the CardioWest TAH, the AbioCor is larger in size precluding its use in smaller patients [40], thus the manufacturer is proceeding with the development of a second-generation device that will be smaller and designed to perform well over 5 years.
The evolution of TAH devices as well an overview of the multiple types of long-term MCSDs and their technical characteristics are given in Fig. 3 and Table 1, respectively.
Short-term mechanical circulatory support in cardiogenic shock and cardiac interventions
Today, we define cardiogenic shock as systolic blood pressure below 90 mmHg for at least 15 min, heart rate above 60/min and cardiac index below 2.2 L/min/m2 in patients with myocardial dysfunction. The clinical symptoms of infarct-related cardiogenic shock including paleness, dyspnea, cyanosis, pulmonary rales, impaired renal and neurologic functions, weak pulse, and tachycardia were described by von Herrick more than 100 years ago [41]. Cardiogenic shock may occur as a result of acute myocardial damage caused by myocardial infarction, acute myocarditis, structural heart disease, rhythm disorders, and acute pulmonary hypertension such as pulmonary embolism. The prognosis of cardiogenic shock mainly depends on the treatment during the acute phase including early revascularization of occluded vessels and sufficient hemodynamic stabilization. Even today, mortality rate during the first 30 days still reaches 40 % under optimal treatment [42].
MCSS can provide stabile hemodynamic conditions and therefore, prevent multi-organ dysfunction syndrome (MODS). Sufficient perfusion pressure is essential for prevention of MODS at a level of 65 mmHg [43]. Early initiation and sufficient support at flow rates above 3.0 L/min should be achieved in the early state of cardiogenic shock after circulatory arrest. External compression devices have been introduced into clinical routine recently for early stabilization in acute circulatory arrest until initiation of invasive circulatory support.
In addition, MCSS can provide hemodynamic stable conditions in elective high risk cases during a short period of time. In principle, such high risk percutaneous coronary interventions (PCI) are defined as treatment of unstable patients with an ejection fraction less than 25 % or a target vessel supplying more than half the myocardium [44]. Intravascular and extracorporeal assist devices can be used for high risk PCI and other complex cardiac interventions such as trans-catheter aortic valve implantation (TAVI). Optimal selection of the support device is essential for successful treatment without risk of hemodynamic compromise during such treatment (Table 2).
External compression devices
External compression can be achieved by mechanical support devices at compression rates between 80 and 100 per minute. External compression devices allow safe transfer in witnessed cardiac arrest for further diagnostic and therapeutic treatment. The Autopulse™-System (Zoll Med. Corp., Chelmsford, MA, USA) consists of a CPR board with an integrated computer system and accumulator performing rhythmic thorax compression by a surrounding compression band. It is possible to perform coronary angiography and simple coronary interventions in patients being stabilized with such a system. The LUCAS™-system (Physio-Control, Redmond, WA, USA) provides active compression and also decompression of the thorax at a rate of 100 per minute [45]. However, angiographic projections are limited by the bulky driving unit. Coronary angiography and echocardiography as well as coronary interventions are feasible with these external temporary external compression devices (Table 3). They may give time for further therapeutic considerations or as a bridge to invasive circulatory support.
Intra-aortic balloon counter pulsation (IABP)
The IABP has been well established in cardiovascular emergency medicine for more than 40 years. The abrupt inflation of 40 mL balloon with helium gas in the descending aorta during diastole results in an increase in mean arterial and diastolic blood pressure therefore, optimizing organ perfusion pressure. The abrupt decompression of the balloon during early systole decreases myocardial afterload and myocardial oxygen consumption [46]. In severe myocardial dysfunction, an increase of left ventricular ejection fraction by up to 10 % can be achieved by IABP. However, recent studies did not prove any benefit in myocardial infarction complicated by cardiogenic shock after successful coronary intervention. The IABP can be inserted by percutaneous approach. The circulatory support is only 0.5–0.8 L/m [42].
Axial flow pumps
Axial flow devices provide continues circulatory support pumping 2.5–4.0 L/min from the left ventricle into the ascending aorta. A combination with IABP is feasible in selected patients (i).
The Impella™ systems (ABIOMED Inc., Danvers, MA) are available at different sizes (12–14F sheaths for percutaneous insertion). They are used for treatment of cardiogenic shock and during high risk cardiac interventions [47]. Figure 4a, b illustrates such a patient being treated with the Impella™ LP2.5 during high risk PCI of last remaining vessel. In contrast to the IABP, the Impella™ works independently of left ventricular function and cardiac rhythm.
Hemodynamic stabilization with an axial flow pump in cardiogenic shock. a PCI in acute dissection of proximal left anterior descending coronary artery (asterisk). High risk of hemodynamic compromise due to chronically occluded right coronary artery and circumflex artery. The Impella was implanted before coronary stent (hash) implantation via the right femoral artery through a 14F sheath. (RAO 30° projection). b It continuously drains blood from the left ventricle (LV) through the inlet housing (white arrows) into the ascending aorta (Aao) at flow rates up to 4 L/min. The outlet (dotted arrows) is below the micro motor (M) delivering blood flow to the coronary arteries (RCA, LCA) and systemic circulation
Extracorporeal circulatory support
Extracorporeal cardiopulmonary support (CPS) systems consist of a membrane oxygenator (MO) combined with a centrifugal pump. The blood is withdrawn through a venous cannula, placed in the vena cava and right atrium. After extracorporeal gas exchange in the MO, the blood is pumped in the abdominal aorta through an arterial cannula with 15–21F diameters. The Lifebridge™ (Zoll Med. Corp., Chelmsford, MA, USA) system and the Cardiohelp™ (MAQUET, Rastatt, Germany) system are CE certified and premounted semi-automatic emergency system well established in interventional cardiology and emergency medicine [48]. The i-cor system (Xenios AG, Heilbronn, Germany) consists of a membrane oxygenator and a diagonal pump allowing pulsatile support. CE mark is expected in 2014 for this portable system. All CPS devices can be connected to patient’s circulation even under continuous chest compression and cardiac arrest situations. These are also used for high risk PCI allowing longer inflation times in last remaining coronary vessels or left main. These devices can be used in the setting of extremely low left ventricular ejection fraction in so-called low-gradient aortic stenosis patients for trans-catheter aortic valve implantation (TAVI). In addition, they may be helpful in case of hemodynamic instability during TAVI. Since the large cannulas may occlude the external iliac artery, an antegrade cannulation of the femoral artery is recommended when these systems are necessary for a longer time period (>6 h). Another side effect is the increase in afterload and significant drop in red blood cell account due to hemolysis and hemodilution. A modification of external circulation system is the Tandemheart™ (CardiacAssist, Inc., Pittsburgh, PA, USA) consisting of a centrifugal pump only without a MO [49]. It directly pumps arterial blood from the left atrium through a 21F trans-septal cannula (62 or 72 cm long) into the iliac artery (Fig. 5).
Future development, perspectives
On the one hand mechanical circulatory long-term support has evolved from a last resort life-saving therapy to an established viable alternative for thousands of HF patients, while on the other hand percutaneous implanted support devices achieve temporary hemodynamic stabilization of high risk patients, or those suffering of severe cardiogenic shock.
In general, in the last decade continuous technological developments led to the introduction in clinical use of smaller and more efficient implantable pumps. However, we are still far away from the ideal MCSD consisting of a totally percutaneously, implantable supporting device and towards this direction exists enough space for further development.
Ongoing research aims the development of devices that can be implanted endovascularly minimizing surgical trauma and subsequently reducing associated complications like infection and bleeding. Examples are devices like the Cardiobridge (Hechingen, Germany), a foldable pump for temporary support enabling via low entrance profile in the groin sufficient blood flow rates (>3 L/min) [50], or the new version of the Synergy pump, a completely endovascularly by interventional cardiologists implantable pump, under development by Circulite Inc. The first version Synergy Pocket Micro-Pump (CircuLite, Inc, Saddle Brook, NJ, USA) represents the first miniaturized pump utilizing partial circulatory support with a blood flow up to 4.25 L/min, and is placed superficially in a “pacemakerlike” pocket through a small right thoracotomy [51]. Additionally, the manufacturer works on the development of a modified device for right heart support.
Other research topics in the field of long-term support are: the further improvement of the already existing, but up to now only in two devices (LionHeart 2000 LVAD; Arrow International, Reading, PA, USA and AbioCor TAH; Abiomed, Danvers, MA, USA) used transcutaneous energy transmission system (TETS), and the development and incorporation of sophisticated algorithms in the pump function which will allow on the one side pulsatility- and automatic impeller speed-control, and on the other side will prevent retrograde flow during the weaning phase from the device. The native heart load control system (NHLCS) developed for the EVAHEART LVAS by researchers at Sun Medical Inc., Japan, addresses, for example, these requirements [52, 53].
Regarding future perspectives in TAHs, there is a growing interest encouraged by the experience and durability of continuous flow LVADs in non-pulsatile TAHs using continuous flow pumps. Under investigation, following this principle are the new rotary TAH, BiVACOR BV Assist (BiVACOR Pty Ltd., Brisbane, Australia) [54, 55] and an undulation pump using, total artificial heart UPTAH4 at the University of Tokyo [56, 57] among others. In addition, further research moves towards enhanced biocompatibility of the devices like the CARMAT-TAH (CARMAT SA, Vélizy-Villacoublay Cedex, France) an implantable, electro-hydraulically driven, pulsatile flow pump with four bioprosthetic valves and blood-pumping surfaces consisting of processed bioprosthetic pericardial tissue and expanded polytetrafluorethylene (ePTFE), potentially allowing for the reduction of anti-coagulation [58]. Actually status and development perspectives are shown in Table 4.
Apart from the technological evolution, future development of MCSD is also being geared to the constantly changing clinical profiles of the patients, who need or will need VAD support. The refinement of the clinical classification of patients with HF as proposed by the INTERMACS study [59] and the use of validated risk stratification models preceded further development of sophisticated therapeutic strategies, suggesting in some cases temporary circulatory support, known as “bridge to bridge therapy” aiming to “prepare” patients for permanent LVAD support. Thus, a gradual change took place in the role of LVAD support as DT away from “a last option”—treatment to in some cases an “elective” therapy. Forthcoming results of two currently running studies will clarify the efficacy of such an “elective” support management.
The first is the risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients trial (ROADMAP, (http://clinicaltrials.gov-ID#NCT01452802), a prospective, multicenter, non-randomized, controlled, observational study evaluating the effectiveness of the Thoratec HeartMate II left ventricular assist system (LVAS) compared to optimal medical management (OMM). The trial enrolls ambulatory advanced HF patients not yet dependent on intravenous inotropic support, who are typically classified as INTERMACS profiles 4–6, within the existing FDA approved indication for DT. Estimated completion date is December 2015.
The second is the randomized evaluation of VAD Intervention before Inotropic Therapy trial (REVIVE-IT), a randomized trial of the heartware ventricular assist system (VAS) versus best medical treatment in patients with advanced HF and whose illness is not severe enough to qualify them for cardiac transplantation or permanent LVAD therapy according to current guidelines. Preliminary results are not awaited before the beginning of 2016 [22].
Summary
From the early days of mechanical support for cardiopulmonary bypass to the modern-era of assist devices, the technology has been evolving rapidly, with both frequent advancements to the particular device types and more recently, a dramatic shift toward the use of newer generation continuous flow miniaturized machines. The current use of percutaneously inserted pumps is leading to a paradigm shift in the treatment of severe refractory cardiogenic shock. In the acute setting, these devices may provide temporary circulatory support until the benefits of reperfusion are achieved. If weaning is impossible, these devices may serve as a bridge to decision or transplant. In patients who are ineligible for transplant, implantable VADs or TAH provide an option of viable permanent destination therapy. As a consequence the availability of new blood-pump technology and well-trained teams, in combination with appropriate patient selection is anticipated to improve long-term survival.
References
Trost JC, Hillis LD (2006) Intra-aortic balloon counterpulsation. Am J Cardiol 97:1391–1398
DiGiorgi PL, Rao V, Naka Y, Mehmet CO (2003) Which patient, which pump? J Heart Lung Transplant 22:221–235
Mielck F, Quintel M (2005) Extracorporeal membrane oxygenation. Curr Opin Crit Care 11:87–93
Zhang T, Cheng G, Koert A et al (2009) Functional and biocompatibility performance of an integrated maglev pump-oxygenator. Artif Organs 33:36–45
Gibbon JH (1954) Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med 37:171–185
DeBakey ME (2005) Development of mechanical heart devices. Ann Thorac Surg 79:S2228–S2231
Gemmato CJ, Forrester MD, Myers TJ, Frazier OH, Cooley DA (2005) Thirty-five years of mechanical circulatory support at the Texas Heart Institute—an updated overview. Tex Heart Inst J 32:168–177
DeVries WC, Anderson JL, Joyce LD et al (1984) Clinical use of the total artificial heart. N Engl J Med 310:273–278
Fraser KH, Taskin ME, Griffith BP, Wu ZJ (2011) The use of computational fluid dynamics in the development of ventricular assist devices. Med Eng Phys 33:263–280
Caccamo M, Eckman P, John R (2011) Current state of ventricular assist devices. Curr Heart Fail Rep 8:91–98
Strüber M, Meyer AL, Malehsa D, Kugler C, Simon AR, Haverich A (2009) The current status of heart transplantation and the development of artificial heart systems. Dtsch Arztebl Int 106:471–477
Kirklin JK, Naftel DC, Kormos RL et al (2012) The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Trans 31:117–126
Spiliopoulos K, Giamouzis G, Karayannis G et al (2012) Current status of mechanical circulatory support: a systematic review. Cardiol Res Pract 2012:574198
Frazier OH, Rose EA, McCarthy P et al (1995) Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg 222:327–338
McCarthy PM, James KB, Savage RM et al (1994) Implantable left ventricular assist device: approaching an alternative for end-stage heart failure. Circulation 90:II83–II86
Goldstein DJ, Oz MC, Rose EA (1998) Implantable left ventricular assist devices. N Engl J Med 339:1522–1533
Slaughter MS, Rogers JG, Milano CA et al (2009) Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361:2241–2251
Rogers JG, Aaronson KD, Boyle AJ et al (2010) Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 55:1826–1834
Kirklin JK, Naftel DC, Kormos RL et al (2010) Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 29:1–10
Pagani FD, Miller LW, Russell SD et al (2009) Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 54:312–321
Hoshi H, Shinshi T, Takatani S (2006) Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs 30:324–338
Wieselthaler GM, O’Driscoll G, Jansz P, Khaghani A, Strueber M (2010) Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transpl 29:1218–1225
Nishinaka T, Schima H, Roethy W et al (2006) The DuraHeart VAD, a magnetically levitated centrifugal pump: The University of Vienna bridge-to-transplant experience. Circulation 70:1421–1425
Farrar DJ, Bourque K, Dague CP et al (2007) Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J 53:310–315
Gazzoli F, Alloni A, Pagani F et al (2007) Arrow CorAide left ventricular assist system: initial experience of the cardio-thoracic surgery center in Pavia. Ann Thorac Surg 83:279–282
Goldowsky M (2005) Lafaro, Reed G. Magnevad status of design improvements human blood results and preliminary sheep trial. Artif Organs 29:855–857
Masuzawa T, Ohta A, Tanaka N et al (2009) Estimation of changes in dynamic hydraulic force in a magnetically suspended centrifugal blood pump with transient computational fluid dynamics analysis. J Artif Organs 12:150–159
Ren Z, Jahanmir S, Heshmat H et al (2009) Design analysis and performance assessment of hybrid magnetic bearings for a rotary centrifugal blood pump. ASAIO J 55:340–347
Rose EA, Gelijns AC, Moskowitz AJ et al (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345:1435–1443
Long JW, Healy AH, Rasmusson BY et al (2008) Improving outcomes with long-term”destination” therapy using left ventricular assist devices. J Thorac Cardiovasc Surg 135:1353–1360
Aaronson KD, Slaughter MS, Miller LW et al (2012) Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 26(125):3191–3200
Miller LW, Pagani FD, Russell SD et al (2007) Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 357:885–896
Kirklin JK, Naftel DC, Kormos RL et al (2013) Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 32:141–156
Copeland JG, Smith RG, Arabia FA et al (2001) Comparison of the CardioWest total artificial heart, the Novacor left ventricular assist system, and the Thoratec ventricular assist system in bridge to transplantation. Ann Thorac Surg 71:S92–S97
El-Banayosy A, Arusoglu L, Morshuis M et al (2005) CardioWest total artificial heart: bad Oeynhausen experience. Ann Thorac Surg 80:548–552
Kung RT, Yu LS, Ochs B et al (1993) An atrial hydraulic shunt in a total artificial heart. A balance mechanism for the bronchial shunt. ASAIO J 39:M213–M217
Samuels L (2003) The AbioCor totally implantable replacement heart. Am Heart Hosp J 1:91–96
Dowling RD, Gray LA Jr, Etoch SW et al (2004) Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg 127:131–141
Frazier OH, Dowling RD, Gray LA, Shah NA, Pool T, Gregoric I (2004) The total artificial heart: where do we stand. Cardiology 101:117–121
Lederman D, Kung RT, McNair DS (2002) Therapeutic potential of implantable replacement hearts. Am J Cardiovasc Drug 2:297–301
Von Herrick JB (1912) Clinical features of sudden obstruction of the coronary arteries. JAMA 250:1757–1765
Thiele H, Zeymer U, Neumann FJ et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367:1287–1296
Werdan K, Ruß M, Buerke M et al (2012) Cardiogenic shock due to myocardial infarction: diagnosis, monitoring and treatment: a German-Austrian S3 Guideline. Dtsch Arztebl Int 109:343–351
Vogel RA, Shawl F, Tommaso C et al (1990) Initial report of the national registry of elective cardiopulmonary bypass supported coronary angioplasty. J Am Coll Cardiol 15:23–29
Putzer G, Braun P, Zimmermann A et al (2013) LUCAS compared to manual cardiopulmonary resuscitation is more effective during helicopter rescue-a prospective, randomized, cross-over manikin study. Am J Emerg Med 31:384–389
Marrin CA, Rose EA, Spotnitz HM, Bregman D (1982) Mechanical circulatory support via the left ventricular vent: the concept of left ventricular copulsation. J Thorac Cardiovasc Surg 84:426–429
Jung C, Ferrari M, Rödiger C, Fritzenwanger M, Figulla HR (2008) Combined Impella and intra-aortic balloon pump support to improve macro- and microcirculation: a clinical case. Clin Res Cardiol 97:849–850
Ferrari M, Poerner TC, Brehm BR et al (2008) First use of a novel plug-and-play percutaneous circulatory assist device for high-risk coronary angioplasty. Acute Card Care 10:111–115
Vranckx P, Foley DP, de Feijter PJ, Vos J, Smits P, Serruys PW (2003) Clinical introduction of the Tandemheart, a percutaneous left ventricular assist device, for circulatory support during high-risk percutaneous coronary intervention. Int J Cardiovasc Interv 5:35–39
Smith EJ, Reitan O, Keeble T, Dixon K, Rothman MT (2009) A first-in-man study of the Reitan catheter pump for circulatory support in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv 73:859–865
Klotz S, Meyns B, Simon A et al (2010) Partial mechanical long-term support with the circulite synergy® pump as bridge-to-transplant in congestive heart failure. Thorac Cardiov Surg 58:173–178
Umeki A, Nishimura T, Ando M et al (2012) Alteration of LV end-diastolic volume by controlling the power of the continuous-flow LVAD, so it is synchronized with cardiac beat: development of a native heart load control system (NHLCS). J Artif Organs 15:128–133
Ando M, Takewa Y, Nishimura T et al (2011) A novel counterpulsation mode of rotary left ventricular assist devices can enhance myocardial perfusion. J Artif Organs 14:185–191
Timms D, Fraser J, Hayne M, Dunning J, McNeil K, Pearcy M (2008) The BiVACOR rotary biventricular assist device: concept and in vitro investigation. Artif Organs 32:816–827
Greatrex NA, Timms DL, Kurita N, Palmer EW, Masuzawa T (2011) Axial magnetic bearing development for the BiVACOR rotary BiVAD/TAH. Ann Biomed Eng 39:2313–2328
Abe Y, Isoyama T, Saito I et al (2007) Development of mechanical circulatory support devices at the University of Tokyo. J Artif Organs. 10:60–70
Abe Y, Isoyama T, Saito I et al (2011) Results of animal experiments with the fourth model of the undulation pump total artificial heart. Artif Organs 35:781–790
Jansen P, van Oeveren W, Capel A, Carpentier A (2012) In vitro haemocompatibility of a novel bioprosthetic total artificial heart. Eur J Cardiothorac Surg 41:e166–e172
Boyle AJ, Ascheim DD, Russo MJ et al (2011) Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 30:402–407
Acknowledgments
This study was elaborated within the grant of European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrari, M., Kruzliak, P. & Spiliopoulos, K. An insight into short- and long-term mechanical circulatory support systems. Clin Res Cardiol 104, 95–111 (2015). https://doi.org/10.1007/s00392-014-0771-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-014-0771-6