Abstract
Purpose of Review
The purpose of this review article is to analyze the current information about diagnosis, prognosis, treatment, and interventional therapies regarding pulmonary embolism (PE) treatment. In addition to review the outcomes obtained by pulmonary embolism response teams.
Recent Findings
Several important contributions in the PE management have been recently published. New scoring systems, such as the PERC rule and YEARS, are used to effectively rule out PE; and stratification scores such as Bova and Hestia were validated. New evidence was favorable to support the use of direct oral anticoagulants in morbidly obese and end-stage renal disease patients; although, not in patients with antiphospholipid antibody syndrome. New studies of catheter-based thrombectomy for acute PE were also published. However, a new statement from the American Heart Association criticizes the lack of randomized trials to support the use of catheter-based interventions in acute PE. Contributions about the cardiopulmonary support in massive PE patients, including ventilation techniques, vasopressors, inhaled pulmonary vasodilators and extracorporeal membrane oxygenation are available. Finally, the advantages and disadvantages of the impact of Pulmonary Embolism Response Teams in the care of acute PE patients.
Summary
Nearly all aspects in the diagnosis, prognosis and care of PE are evolving. In this article, we discuss the epidemiology, diagnosis, risk stratification, and therapeutic approaches to PE. We provide additional focus on advanced therapeutic strategies such as catheter-based interventions, surgical approaches, and cardiopulmonary support. The impact of a multidisciplinary team approach to PE management is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) affects between 300,000 and 600,000 people in the United States per year and is the third leading cause of cardiovascular deaths[1]. A PE occurs when a thrombus originating in the deep veins, usually the lower extremities, breaks off and travels to the pulmonary artery. Patients’ symptoms range from completely asymptomatic to shortness of breath, hypotension, syncope, collapse, and death. The treatment of PE has evolved during the past several decades, with the introduction of new anticoagulant drugs, thrombolytics, minimally invasive procedures to revascularize the pulmonary artery, and advances in cardiopulmonary support. In this article we will review the current approaches to treating acute PE.
Epidemiology Etiology and Hypercoagulable States
The exact prevalence of PE is unknown. Risk factors and the estimated annual incidence associated with PE increase with age; being about 1.4/1,000 in people 40–49 years old to 11.3/1,000 in people 80 years old and above [2]. In autopsy studies, only 30–45% of patients were diagnosed with PE before death [3]. In patients presenting with a deep vein thrombosis (DVT) the incidence of PE is about 40–50% [4]. The prevalence of PE is higher in Caucasian and African Americans over Hispanics, Asians and Native Americans [5]. Also, mortality is higher in Caucasians and African Americans [6].
Risk factors for PE include recent surgery (odds ratio [OR] = 21.7), trauma (OR = 12.7), hospitalization (OR = 8), malignancy (OR = 6.5, 4.1), central venous catheters or pacemakers (OR = 5.6), and neurologic disease with extremity paresis (OR = 3.0) [7].
Hypercoagulable states are inherited or acquired. The most common inherited hypercoagulable states are Factor V Leiden, and prothrombin gene mutation in Caucasians, [8•], while elevated factor VIII, sickle cell trait, and Protein C deficiency are most common in African Americans [9]. Other inherited hypercoagulable states are protein S and antithrombin deficiency. The testing for inherited hypercoagulable states should be performed exclusively in the out-patient as it will not impact in-patient treatment and results could be affected by anticoagulation therapy.
The presence of antiphospholipid antibodies is associated with venous and arterial thrombosis and is considered an acquired hypercoagulable state. The International Society of Thrombosis and Haemostasis (ISTH) defines the presence of lupus anticoagulant, beta 2 glycoprotein, or cardiolipin antibodies as antiphospholipid antibodies. To diagnose a patient with antiphospholipid antibody syndrome, two positive test are required at least 12 weeks apart, in addition with unprovoked venous or arterial thrombosis or recurrent miscarriages [10]. In patients with suspected antiphospholipid antibody syndrome, we recommend using anti Factor-Xa levels to monitor anticoagulation.
Diagnosis
Symptoms of PE can vary and include palpitations, shortness of breath, dizziness, hemoptysis, pleuritic chest pain, and in severe cases hypotension and shock. An incidental PE is found in 1–3% of patients during a radiologic exam performed for different reasons [11•]. The outcomes of PE are closely related with the clinical presentation. Mortality is less than 1% in asymptomatic patients, but it increases to 30% in patients with hypotension, and up to 65% in patients requiring cardiopulmonary resuscitation [12].
Chest X-Rays are usually non-diagnostic of PE but rule out other pathologies. Some findings described in patients with PE are an area of oligemia (Westermark’s sign), atelectasis with elevation of the hemidiaphragm and linear disk-shaped densities, a hilum increased in size (Fleischner’s sign), pleural effusion and a rounded opacity (Hampton’s sign) [13].
The most common electrocardiographic manifestation of PE is sinus tachycardia; however, a new right bundle branch block, the presence of a prominent S wave in lead I, a Q wave and inverted T wave in lead III (S1Q3T3) as a sign of acute cor pulmonale, or a new onset of atrial fibrillation or flutter, could also be present in acute PE [14].
When PE is suspected, it is necessary to determine the probability of PE based on risk factors and symptoms before using further diagnostic methods. This can be accomplished through a number of scoring systems designed to accurately exclude the diagnosis of PE before imaging studies such as the Well’s criteria and the Geneva Score [15, 16]. Other models such as the YEARS and the PE rule-out criteria (PERC) have been recently developed [17, 18]. These probability models are explained in Table 1.
D-Dimer is the final product of the degradation of fibrin and should be obtained in patients with moderate to high clinical suspicion of DVT or PE. D-Dimer has a high sensitivity but a low specificity since other conditions such as trauma, surgery, cancer can also increase its levels. The diagnosis of PE can be effectively ruled out in patients with low pretest probability of PE and D-dimer values less than 500 ng/mL [19••].
Pulmonary angiography was once the gold standard test to diagnosis PE. However, computer tomography pulmonary angiography (CTPA) has become the new gold standard being a non-invasive, easy to perform test and is available in most hospitals. The 4 or 16-slice CTPA offers images of equal or better quality than pulmonary angiography and has the ability to detect segmental PE with a sensitivity of 83–100% and specificity of 89–96% and detects sub-segmental PE with a specificity of 92% [20]. On the other hand the sensitivity and specificity of CTPA to detect RV strain is lower when compared to echocardiography [21]. Notably, the use of CTPA is not risk-free and is associated with radiation exposure and contrast-induced nephropathy [20].
The ventilation–perfusion scan (VQ scan) is an alternative method to diagnose PE and involves two components. In the ventilation component, the patient is asked to inhale a radionuclide gas (xenon-133, krypton-81 or technetium-99), and in the perfusion component, the patient receives an intravenous injection of technetium macroaggregated albumin. Both images are compared looking for areas of ventilation/perfusion mismatch and are interpreted as low, medium, or high probability for PE. Although this scan can be used in patients with renal insufficiency, it has a high rate of non-conclusive results (up to 30%) [22••]. The VQ scan is also used in follow-up for thrombus resolution in patients who are at risk of developing chronic thromboembolic pulmonary hypertension [23].
Echocardiography does not directly diagnose PE but is an important tool to measure severity and prognosis of the patient. Echocardiography evaluates biventricular systolic function, pulmonary pressure, and the presence of a patent foramen ovale (PFO). The most common measurements obtained from echocardiography are the right ventricle (RV)/ left ventricle (LV) ratio to evaluate for RV dilatation (normal ≤ 1); the RV fractional area change (normal ≥ 35%); and the tricuspid annular plane systolic excursion or TAPSE (normal < 15 mm) to evaluate RV systolic function. McConnell’s sign (RV akinesia in the mid free wall with hyperkinetic function at the apex) is a distinct echocardiographic finding in patients with moderate to severe PE [24]. The hypokinesis of the RV is independent predictor of early death in normotensive PE patients [25]. The presence of a PFO in PE patients has been associated with an increased risk of stroke [26]. In acute PE, we recommend performing the baseline echocardiogram including the injection of agitated normal saline to rule out a PFO. In patients presenting with a paradoxical embolism it is recommended to perform a pulmonary embolectomy and the closure of the PFO. However, there is no consensus about management of a PFO in acute PE patients without neurologic symptoms.
Pulmonary Embolism Risk Stratification
The prognosis of a patient with a PE is dictated by the clinical characteristics at presentation. The American Heart Association has classified PE into massive (acute PE with sustained hypotension not due to a cause other than PE, pulselessness, or persistent profound bradycardia with signs or symptoms of shock), sub-massive (acute PE with evidence of RV dysfunction, overload, or ischemia), and low-risk PE (normal RV morphology and function) [27].
Several risk stratification models to predict 30-day mortality have been developed to guide management of patients with an acute PE. The Pulmonary Embolism Severity Index or PESI score stratifies risk of death into 5 classes with a risk of death from 0% for class I to 24.5% for class V [28]. The Bova score was validated for hemodynamically stable PE patients, and is divided into low, moderate or severe risk with a risk of death from 3.1%-10% at 30 days [29••]. The Hestia score is designed to select patients who are eligible for out-patient PE treatment. If one element is positive, the patient requires hospitalization [30]. These different predictions models are explained in Table 2. Elevated lactate levels on admission have also been associated to an increased mortality [31].
Therapeutic Approaches
The initial treatment of PE is anticoagulation; however, some situations require the use of thrombolytics, catheter-based therapies, or surgical pulmonary embolectomy/endarterectomy to prevent death.
Bleeding is a feared complication of therapeutic anticoagulation and should be considered before starting therapy. The “Registro Informatizado de Enfermedad Tromboembolica” (RIETE) registry has developed the RIETE bleeding score which evaluates the risk of bleeding in patients on therapeutic anticoagulation This score includes history of major bleeding, an elevated creatinine, the presence of anemia or malignancy, the clinical presentation of the PE and if patient is > 75 years old [32].
Parenteral Anticoagulation
Unfractionated heparin (UFH) works potentiating the action of antithrombin to inhibit factors IIa, IXa and Xa; also UFH has anti-inflammatory and antineoplastic properties [33] Unfractionated heparin is administered continuously with weight-based dosing and requires monitoring of the activated partial thromboplastin time (aPTT) or anti-factor-Xa. The half-life of UFH is 45 min and can be reversed with protamine. Unfractionated heparin is the treatment of choice in patients with renal insufficiency and those with morbid obesity (body mass index > 40) [34]. About 3% of patients who receive UFH will develop heparin-induced thrombocytopenia (HIT) characterized by a drop in platelets with or without venous or arterial thrombosis. Under clinical suspicion of HIT the “4Ts score” (Thrombocytopenia, Timing, Thrombosis and oTher causes) should be calculated. This score stratify patients into low, moderate or high probabilities of having HIT [35]. Patients with low probability of HIT should continue on heparin with no further testing. Patients with intermediate or high probability should be taken off all heparin products immediately and a non-heparin anticoagulant should be started to prevent thrombosis. Immunoassays testing for anti-platelet factor-4 antibodies and the confirmatory serotonin release assay should also be ordered [36•].
Low Molecular weight heparins (LMWH) are synthetized from unfractionated heparin and possess longer half-lives that allow less frequent administration. The most used are Enoxaparin, Dalteparin, Tinzaparin, Nadroparin. They also work by potentiating the action of antithrombin. LMWH are administered on a weight-based dose and do not require frequent testing. Alternatively, drug activity can be monitored using anti-factor-Xa. LMWH can also be reversed with protamine. In a randomized controlled trial, Dalteparin was non-inferior than UFH to treat PE.[37] The American College of Chest Physicians (ACCP) guidelines recommend the use of LMWH for treatment of venous thromboembolism (VTE) after direct oral anticoagulants (DOACs) and warfarin. LMWH are recommended as a first-line treatment for cancer-associated VTE since they reduced the risk of recurrent events [38, 39]. LMWH still carry a 0.2% of developing HIT [40].
Fondaparinux is a synthetic analog of a unique pentasaccharide which works by blocking factor-Xa but requires antithrombin as a co-factor. The half-life is longer compared to LMWH, at 17–21 h, and once a day dosing is effective. Fondaparinux has been shown to be non-inferior to unfractionated heparin for the treatment of acute PE [41]. Fondaparinux is considered safe to treat patients with HIT since it rarely induces HIT, although few cases have been reported [42]. The ACCP guidelines does not recommend using Fondaparinux in patients with PE in the setting of HIT since there is not enough evidence to support its use [43]. On the other hand, the American Society of Hematology (ASH) recommends the use of Fondaparinux in the setting of HIT only in low-risk PE patients with average risk of bleeding [36].
Direct thrombin inhibitors (DTI) inhibit factor-Xa without the need of antithrombin. Lepirudin and Desirudin are derived from Hirudin, which is a natural anticoagulant present in the blood-sucking leeches. Bivalirudin is a synthetic bivalent analog of Hirudin, while Argatroban is a univalent reversible DTI [44]. The current FDA approved DTIs are Lepirudin, Desirudin, Bivalirudin and Argatroban.
Bivalirudin is a synthetic peptide analog of the hirudin that directly inhibits factor-Xa, has a half-life of 25 min and is cleared by both renally and by enzymatic degradation. Bivalirudin is monitored using aPTT. The ASH and the ACCP guidelines recommends the use of Bivalirudin for treatment of VTE in patients with HIT [36, 43]. Bivalirudin also has been extensively studied in patients with acute coronary syndromes [45].
Argatroban is a synthetic drug derived from the L-arginine amino acid which reversibly binds the active site of thrombin, has a half live of 40–50 min, and is cleared by the liver. It is monitored using aPTT and is also approved included in the ASH and ACCP guidelines to treat venous thromboembolic events in HIT patients [46].
Oral Anticoagulation
Warfarin was approved to be used in humans in 1952 and was synthetized from moldy silage made from sweet clover [47]. Warfarin works inhibiting the Vitamin K epoxide reductase complex 1 (VKORC1), preventing the final carboxylation of the Vitamin K-dependent factors (II, VII, IX and X) and its final activation, producing a depletion of active vitamin K depending factors [48]. Warfarin also inactivates the natural anticoagulants proteins C and S and may initially produce a hypercoagulable state prior to reaching therapeutic levels. This occurs because proteins C and S have shorter half-lives (8 and 30 h respectively) when compared to Factor II (60 h) and factor X (48–72 h). It is important to administer a different parenteral anticoagulant, while a complete depletion of Factor II and X occurs which usually takes between 5–6 days [49]. Warfarin requires frequent testing of prothrombin time (PT) to calculate the international normalized ratio (INR). Warfarin has multiple interactions with food (leafy green vegetables which have high levels of vitamin K) and other medicines. When the INR level is critically elevated (> 5) or there is major bleeding, Warfarin should be reversed using fresh frozen plasma (FFP), prothrombin complex concentrates (PCC), or vitamin K [50].
A rare complication is warfarin-induced skin necrosis. Risk factors associated with warfarin-induced skin necrosis are the use of initial large warfarin doses; patients with protein C resistance; deficit of proteins C and S, or antithrombin; obesity; and antiphospholipid antibodies [51].
Direct oral anticoagulants have revolutionized oral anticoagulation therapy after 50 years of Warfarin being the only available oral anticoagulant. DOACS work by either directly inhibiting factor-Xa or directly inhibiting thrombin. The factor-Xa inhibitors include Rivaroxaban, Apixaban, and Edoxaban and work by inhibiting the prothrombinase complex-bound and clot-associated with factor-Xa, thus decreasing the production of thrombin. Dabigatran, on the other hand, is a competitive inhibitor of thrombin. Rivaroxaban is eliminated mostly by the kidneys and does not inhibit the cytochrome P450 enzymes. Apixaban is eliminated mostly in the gastrointestinal tract but about 30% is eliminated by the kidneys. Edoxaban is cleared mostly in the gastrointestinal tract. Dabigatran is administered as a prodrug, converted to the active form in the liver, is excreted by the kidneys and is not metabolized by the cytochrome P450 enzymes.
All DOACS have shown safety and efficacy in the treatment of VTE [52,53,54,55] and are recommended for treatment of PE in hemodynamically stable patients [36, 56]. There are no studies comparing safety and efficacy between DOACS. They are easy to use and do not require frequent testing.
In cancer-associated thrombosis, two randomized trials demonstrated that Apixaban and Edoxaban were non-inferior to Dalteparin to treat VTE events [57, 58]. Also, Apixaban was effective to prevent VTE in high-risk cancer patients [59]. There are situations where the use of DOACS are controversial. Morbidly obese patients (BMI > 40 kg/m2) were excluded from previous randomized studies. Therefore the ISTH do not recommended the use of DOACS in morbidly obese patients due to the lack or prospective data [60]. However, retrospective data suggest that DOACS are non-inferior than Warfarin in this population [61•]. We consider DOACS a reasonable option in morbid obese patients; however, a close monitoring for recurrent VTE and bleeding is required.
The use of DOACS in patients with end-stage renal disease and hemodialysis is also controversial. Two pharmacokinetic and pharmacodynamic studies for Rivaroxaban and Apixaban showed no increase in maximum concentration of the drug after a single dose administration in dialysis patients [62, 63]. In an analysis of 25,523 patients from Medicare with atrial fibrillation on hemodialysis, Apixaban was associated with a lower risk of bleeding and stroke compared with Warfarin [64••]. Also, the RENAL-AF study showed that Apixaban was superior than Warfarin [65]. However, information is less robust for VTE [66]. We still consider DOACs a reasonable alternative in end-stage renal disease patients with close monitoring for recurrent VTE and bleeding complications.
In patients with antiphospholipid antibodies, the use of DOACS should be avoided. The TRAPS study comparing Rivaroxaban vs. Warfarin in patients with triple positive antiphospholipid antibodies was terminated earlier due to increased number of adverse events in the Rivaroxaban arm [67••].
When patients receiving DOACS present with life-threatening bleeding, or require urgent surgery, anticoagulation should be reversed immediately. Prothrombin complex concentrate (PCC) and activated prothrombin complex concentrate (APCC) are used to control bleeding. Two agents to reverse the anticoagulant effect of DOACS, Idarucizumab and Andexanet, are available. Idarucizumab is a monoclonal antibody fragment that binds and inactivates 100% of the Dabigatran in 4 h after administration, reversing the bleeding risk. However, it is associated with 6.8% of thrombotic complications at 90 days [68••]. Andexanet is a recombinant modified human factor-Xa which binds and sequesters factor-Xa inhibitors keeping them in the intravascular space, restoring the activity of endogenous factor-Xa. In patients treated with Andexanet the anti-factor-Xa activity was reduced by 94% within 2–5 min from administration and the thrombin activity was regenerated by 100%.[69] Andexanet was also associated with 10% of thrombotic events within 30 days [70••]. Reversing DOACS are not risk-free and should be administered considered risk and benefits.
The mechanism of action of different anticoagulants and thrombolytics is explained in Fig. 1
Coagulation cascade, anticoagulant and fibrinolytic system, and mechanism of action of anticoagulation and thrombolytic therapy. HMWK = High molecular weight Kallikrein; F = Factor; PK = Plasma Kallikrein; a = activated; Ca + + = Calcium; TF = Tissue Factor; ACP = Activated Protein C; TM = Thrombomodulin; FDP = Fibrin degradation products; PAI-1 = Plasminogen Activator Inhibitor 1; tPA = Tissue Plasminogen Activator; uPA = Urokinase Plasminogen Activator; TAFI = Thrombin Activatable Fibrinolysis Inhibitor; LMWH = Low Molecular Weight Heparin. Numbers in a circle corresponds with number of the anticoagulant or fibrinolytic drug listed on the box at the left side of the figure. Numbers are placed
Advanced Therapies to treat Pulmonary Embolism
Systemic Thrombolytics.
In hemodynamically unstable PE patients, an urgent revascularization of the pulmonary artery is required. Systemic thrombolytics activate plasminogen forming plasmin which breaks down fibrin causing clot dissolution. The FDA approved thrombolytics to treat PE in the United States are Streptokinase, Urokinase and Alteplase. Reteplase and Tenecteplase are approved for acute coronary syndromes. Alteplase is the most commonly used and a dose of 100 mg is administered over a period of 2 h. However, a reduced dose of 50 mg provides similar results [71••]. Before administering thrombolytics an assessment of the bleeding risk should be performed. Absolute contraindications for systemic thrombolytics are a history of intracranial hemorrhage, structural intracranial disease, history of an ischemic stroke within 3 months, active bleeding, recent brain or spinal surgery, recent head trauma and the presence of a bleeding disorder. Relative contraindications include uncontrolled hypertension, recent surgery or invasive procedure, traumatic cardiopulmonary resuscitation, pericarditis and pericardial effusion, pregnancy, and diabetic retinopathy [56]. The European Society of Cardiology (ESC) and the ACCP guidelines recommend the use of thrombolytics in hemodynamically unstable PE patients [56, 72••]. However their use in hemodynamically stable patients is controversial. In the PEITHO trial, the use of systemic thrombolytics in intermediate-risk PE patients improved outcomes but was associated with an increased risk of bleeding [73]. Similar results were found thereafter in a systematic review and meta-analysis [74].
Catheter-Based Interventions
The use of catheter-based interventions (CBI) is an alternative to treat massive and sub-massive PE. CBI includes catheter-directed thrombolysis (CDT) and catheter-based thrombectomy (CBT). The simplest form of CDT is using a catheter to deliver small amounts of thrombolytics directly into the pulmonary artery over a prolonged period of time, reducing the risk of bleeding associated with systemic thrombolytics. A different technique includes the use of acoustic waveforms to enhance the action of thrombolytics directly to the thrombus using the EkoSonic™ Endovascular System (Boston Scientific, Marlborough, MA, USA). Only one randomized study using the EkoSonic catheter demonstrated improvements in RV function over a period of 24 h [75]. Most of the information regarding CDT for PE come from single-arm studies or registries [76, 77]. When CDT was compared with systemic thrombolytics, CDT demonstrated a decrease in mortality, with no significant difference in intracranial bleeding; although, CDT had a higher all-cause bleeding events rate [78•]. CDT has been also associated with a higher cost of hospitalization with no difference in length of stay [79]. On the other hand, shorter treatment and smaller doses of thrombolytics appear as effective as standard protocols [80•], reducing the risk of bleeding.
Catheter-based thrombectomy produces a fragmentation and aspiration of the thrombus. There are multiple catheters available such as the Penumbra Indigo ® catheter (Penumbra Inc, Alameda, CA, USA) and the FlowTriever® catheter (Inari Medical, Irvine, CA, USA). CBT do not require thrombolytics or a prolonged ICU stay like CDT. Both techniques showed similar outcomes in death and bleeding in a retrospective review [81•]. Only the EkoSonic and FlowTriever catheters are FDA approved to treat acute PE. A recent statement from the American Heart Association emphasizes the lack of randomized trials and high-quality evidence for these interventions; however they consider reasonable its use in high-risk patients, but not in intermediate or low risk [82••]. Table 3 summarizes all the CBI and CBT studies.
The ESC and the ACCP guidelines recommend using CBI in patients with high-risk PE in which systemic thrombolytics are contraindicated or have failed [56, 72]. Data from our hospital suggest that patients treated with CBI have less recurrent PE events than patients treated medically. However, the in-hospital mortality was higher when compared with those treated with surgical pulmonary embolectomy.
Surgical Pulmonary Embolectomy and Endarterectomy
Surgical pulmonary embolectomy (SPE) is the surgical extraction of the thrombus from the pulmonary artery and can be performed with or without cardiopulmonary bypass. The surgery is performed through a median sternotomy incision. The common indications for SPE are acute PE with RV dysfunction, PE with a clot in-transit, and a PE with a PFO and paradoxical embolism. Several cardiac surgery groups have showed excellent results with a survival rate from > 90% in patients with massive PE at discharge [83, 84]. Patients with acute on chronic PE would require pulmonary thromboendarterectomy (PTE) which is a more extensive and complex procedure. PTE is performed under cardiopulmonary bypass and often uses periods of circulatory arrest to perform a complete endarterectomy with dissection into the segmental branches of the pulmonary artery. The data for PTE in patients with acute on chronic PE are unclear; however, in patients with chronic thromboembolic pulmonary hypertension, PTE has a reported 84–92.5% survival at 5 years [85]. Optimal timing for surgery is also unclear, an early approach could prevent further insult to the RV; however performing a more complex surgery in an unstable patient could increase the risk of complications. On the other hand, if patients are hemodynamically stable, they could be managed with anticoagulation with or without CBI, and PTE could be deferred after recovering from the acute PE.
Cardiopulmonary Support
Maintaining adequate coronary and brain oxygenation while awaiting revascularization of the pulmonary artery is crucial to assure survival and acceptable quality of life after PE.
In hemodynamically unstable patients, it is important to optimize the fluid status of the patient, as volume contraction could contribute to hypotension and fluid overload and could worsen RV dysfunction. The ESC guidelines recommend a fluid challenge with 500 ml of normal saline over 15–20 min. Monitoring pulmonary pressure with a pulmonary artery catheter (Swan-Ganz catheter) is not recommended in PE patients. Alternatively, central venous pressure monitoring could provide a good indirect measure of the patient’s fluid status [72••].
Pulmonary vasodilators are not routinely used in the treatment of PE. Pulmonary vasodilators improve the hemodynamics of the pulmonary artery and RV but worsen the pulmonary ventilation–perfusion mismatch and oxygenation. Due to the risk of hypotension, inhaled pulmonary vasodilators are preferred over intravenous. Inhaled nitric oxide has demonstrated reduction of the pulmonary artery pressure and improves RV function in patients with acute PE, but has no impact on clinical outcomes [86•]. Also, nitric oxide carries a potential risk of methemoglobinemia. Inhaled epoprostenol has been used effectively in patients with acute respiratory distress syndrome [87]; however, there is no evidence to support its use patients with acute PE. Intravenous epoprostenol showed no improvement in RV function or pulmonary hemodynamics in patients with acute PE [88]. Inhaled pulmonary vasodilators are not currently recommended to treat PE.
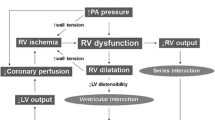
In patients with massive PE, the use of inotropes and vasopressors are necessary to maintain adequate coronary perfusion and cardiac output. Low-dose epinephrine improve RV dysfunction due to inotropic properties [89]. Dobutamine improve arterial pressure, increases RV contractility, normalizes RV–pulmonary artery coupling and improves cardiac output [90]. Animal studies suggest benefits using milrinone and levosimendan in acute PE by improving RV contractility, decreasing pulmonary hypertension with lower risk of tachyarrhythmias [91, 92].
Patients with PE often present with shortness of breath and hypoxia. Contrary to the management of respiratory failure in patients with parenchymal lung disease, in patients with acute PE intubation should be avoided as it can precipitate hemodynamic collapse. During induction, patients can suffer systemic hypotension, decreasing preload of the RV and cardiac output. Positive pressure ventilation can worsen the pulmonary artery pressure and RV dysfunction. Sedative agents also have a negative effect in systemic pressure. Instead, high flow nasal cannula or mask oxygenation would be preferred in hypoxic acute PE patients. It is important to monitor the oxygenation and CO2 levels as hypoxia and hypercapnia increase the pulmonary artery pressure. If intubation is inevitable, tidal volumes of 6–8 mL/Kg of ideal body weight are recommended [93•].
The early use of venous-arterial extracorporeal membrane oxygenation (VA-ECMO) during a massive PE, facilitates recovery of the RV and prevents end-organ dysfunction [94]. The use of VA-ECMO has also been associated with autolysis of the thrombus due to the development of thrombocytopenia, some degree of disseminated intravascular coagulation, and an acquired Von Willebrand syndrome [95, 96]. The survival in patients with acute PE treated with VA-ECMO in varies from 48 to 95%, [97, 98] probably due to differences in patient selection protocols. In our hospital, the use of VA-ECMO is reserved for patients with hemodynamic instability at high risk of death and/or worsening systemic malperfusion (i.e., massive PE). The Pulmonary Embolism Response Team consortium recommends using this VA-ECMO in patients with cardiac arrest or refractory shock, [99] and aligns with the ESC guidelines recommendations [72••].
Multidisciplinary Approach to Pulmonary Embolism
Multiple novel therapies have been developed during the last several years with similar effectiveness and safety profiles, but the lack of randomized studies among these therapies make the decision process complicated. In order to maximize resources, hospitals have created multidisciplinary teams to approach patients with acute PE and select the best therapy for each case. Pulmonary embolism response teams (PERT) are usually integrated by some of the following specialties: interventional radiology, interventional cardiology, vascular surgery, cardiac surgery, emergency medicine, pulmonary and critical care, hematology, vascular medicine, and internal medicine. Multiple models exist depending each hospital, but a treatment plan is always discussed across all the members. After the creation of multidisciplinary teams, there has been a reported improvement in quality of care for PE patients [100•]. Interestingly, however, a 10-year analysis after PERT creation suggested there was no difference in clinical outcomes (bleeding or mortality) with an increased number of procedures specially among sub-massive PE patients [101••]. The current approach in our hospital is explained in Fig. 2.
Multidisciplinary approach to patients with Pulmonary Embolism. PE = Pulmonary Embolism; VA-ECMO = Venous Arterial extracorporeal Membrane Oxygenation; CBT = Catheter-Based Intervention; CV support = Cardiovascular support not including VA-ECMO; ICU = Intensive Care Unit; 48 h = 48 h; CDT = Catheter-directed thrombolysis; CBT = Catheter-based thrombectomy; SPE = Surgical pulmonary embolectomy; PTE = Pulmonary Thromboendarterectomy. Anticoagulation is started in all patients unless contraindicated. Test required for stratification are an echocardiogram (with agitated saline to rule out patent foramen ovale), venous duplex ultrasound of the lower extremities, troponin, pro-BNP and lactate. Pulmonary Embolism are stratified following the American Heart Association clinical classification. [27] Sub-Massive Pulmonary embolism are sub-classified using Bova Score.[29••] Clinical Improvement: includes improvement in tachycardia, shortness of breath at rest and walking, oxygen saturation > 90%, in addition with improvement of pulmonary pressure, right ventricular function in the echocardiogram
Conclusion
The management of patients with PE requires a timely diagnosis and accurate risk stratification plan to guide therapy. The prognosis of patients with massive pulmonary embolism has improved with the increasing availability of therapeutic options such as systemic thrombolytics, and advanced technology such as CBI and SPE. In addition, advances in cardiopulmonary support and ventilation strategies ensure an acceptable post-PE quality of life. On the other hand, in hemodynamically stable PE patients, although advanced therapies are commonly used, the definite benefits are not completely understood. The creation of multidisciplinary teams has improved the quality of care of patients with PE and are unique to each hospital based on individual resources. The use of DOACS and pulmonary embolism clinics have improved the adherence to treatment and post-PE care. In summary, early recognition of patient’s risk and the use of the appropriate therapeutic approach will impact outcomes of acute PE patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous Thromboembolism. A Public Health Concern. American Journal of Preventive Medicine. 2010;38(4):S495-501.
Goldhaber SZ. Venous thromboembolism: Epidemiology and magnitude of the problem. Best Practice and Research: Clinical Haematology. 2012;25(3):235–42.
Pineda LA, Hathwar VS, Grant BJB. Clinical suspicion of fatal pulmonary embolism. Chest. 2001;120(3):791–5.
Meignan M, Rosso J, Gauthier H, Brunengo F, Claudel S, Sagnard L, et al. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Archives of Internal Medicine. 2000;160(2):159–64.
Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: Racial contrasts in incidence and in-hospital case fatality. J Natl Med Assoc. 2006;98(12):1967.
Tang Y, Sampson B, Pack S, Shah K, Yon Um S, Wang D, et al. Ethnic differences in out-of-hospital fatal pulmonary embolism. Circulation. 2011;123(20):2219–25.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism. Arch Intern Med. 2000;160(6):809–15.
• Carroll BJ, Piazza G. (2018) Hypercoagulable states in arterial and venous thrombosis: When, how, and who to test? Vasc Med (United Kingdom). 23(4):388–99. This article explains how and when to perform a hypercoagulable work up in VTE patients.
Buckner TW, Key NS. (2012) Venous thrombosis in blacks. Circulation. 125(6):837–9
Giannakopoulos B, Passam F, Ioannou Y, Krilis SA. How we diagnose the antiphospholipid syndrome. Blood. 2009;113(5):985–94.
• Klok FA, Huisman M V. (2017) Management of incidental pulmonary embolism. The European respiratory journal. 49(6). This article discusses the management of asymptomatic incidentally found PE.
Wood KE. Major pulmonary embolism: Review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121(3):877–905.
Worsley DF, Alavi A, Aronchick JM, Chen JTT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: Observations from the PIOPED study. Radiology. 1993;189(1):133–6.
Ullman E, Brady WJ, Perron AD, Chan T, Mattu A. Electrocardiographic manifestations of pulmonary embolism. Am J Emerg Med. 2001;19(6):514–9.
Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98–107.
Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, Perrier A. Prediction of pulmonary embolism in the emergency department: The revised geneva score. Ann Intern Med. 2006;144(3):165–71.
van der Hulle T, Cheung WY, Kooij S, Beenen LF, van Bemmel T, van Es J, et al. (2017) Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 390(10091):289–97. This article introduces a new scoring system to diagnose pulmonary embolism
Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772–80.
•• Kearon C, de Wit K, Parpia S, Schulman S, Afilalo M, Hirsch A, et al. (2019) Diagnosis of Pulmonary Embolism with d -Dimer Adjusted to Clinical Probability. N Engl J Med. 381(22):2125–34. This article introduces a new scoring system to diagnose pulmonary embolism
Doğan H, de Roos A, Geleijins J, Huisman M, Kroft L. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagnostic and Interventional Radiology. 2015;21(4):307.
Dudzinski DM, Hariharan P, Parry BA, Chang Y, Kabrhel C. (2017) Assessment of Right Ventricular Strain by Computed Tomography Versus Echocardiography in Acute Pulmonary Embolism. Acad Emerg Med. 24(3):337–43. This article explains how to appropriately evaluate the right ventricle by echocardiogram and CT.
•• van der Hulle T, Cheung WY, Kooij S, Beenen LF, van Bemmel T, van Es J, et al. (2017) Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 390(10091):289–97. This article introduces a new scoring system to diagnose pulmonary embolism
Waxman AD, Bajc M, Brown M, Fahey FH, Freeman LM, Haramati LB, et al. (2017) Appropriate use criteria for ventilation-perfusion imaging in pulmonary embolism: Summary and excerpts. Journal of Nuclear Medicine. 58(5):13N-5N. This article explains how to use VQ scan to evaluate a patient for pulmonary embolism.
Dilorenzo MP, Bhatt SM, Mercer-Rosa L. How best to assess right ventricular function by echocardiography. Cardiol Young. 2015;25(8):1473.
Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165(15):1777–81.
Goliszek S, Wisniewska M, Kurnicka K, Lichodziejewska B, Ciurzyński M, Kostrubiec M, et al. Patent foramen ovale increases the risk of acute ischemic stroke in patients with acute pulmonary embolism leading to right ventricular dysfunction. Thromb Res. 2014;134(5):1052–6.
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the american heart association. Circulation. 2011;123(16):1788–830.
Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041–6.
•• Bova C, Vanni S, Prandoni P, Morello F, Dentali F, Bernardi E, et al. A prospective validation of the Bova score in normotensive patients with acute pulmonary embolism. Thromb Res. 2018. May 1;165:107–11. This article validates a new scoring system for pulmonary embolism risk stratification
Zondag W, Mos ICM, Creemers-Schild D, Hoogerbrugge ADM, Dekkers OM, Dolsma J, et al. Outpatient treatment in patients with acute pulmonary embolism: The Hestia Study. J Thromb Haemost. 2011;9(8):1500–7.
Vanni S, Jimenez D, Nazerian P, Gigli C, Parisi M, Morello F, et al. Prognostic value of plasma lactate in acute pulmonary embolism: the multicentre Thrombo-Embolism Lactate Outcome study. Eur Heart J. 2013. https://doi.org/10.1016/j.annemergmed.2012.10.022.
Ruíz-Giménez N, Suárez C, González R, Nieto JA, Todolí JA, Samperiz ÁL, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100(07):26–31.
Ludwig R. Therapeutic Use of Heparin beyond Anticoagulation. Curr Drug Discov Technol. 2009;6(4):281–9.
Patel JP, Roberts LN, Arya R. Anticoagulating obese patients in the modern era. British Journal of Haematology. 2011;155(2):137–49.
Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood. 2012;120(20):4160–7.
• Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Advances. 120(20):4160–7. This article provides recommendations for management of heparin induced thrombocytopenia.
Simonneau G, Sors H, Charbonnier B, Page Y, Laaban J-P, Azarian R, et al. A Comparison of Low-Molecular-Weight Heparin with Unfractionated Heparin for Acute Pulmonary Embolism. N Engl J Med. 1997;337(10):663–9.
Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2003;349(2):146–53.
Lee AYY, Bauersachs R, Janas MS, Jarner MF, Kamphuisen PW, Meyer G, et al. CATCH: A randomised clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients. BMC Cancer. 2013;13(1):1–3.
Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: A meta-analysis. Blood. 2005;106(8):2710–5.
Investigators Matisse. Subcutaneous Fondaparinux versus Intravenous Unfractionated Heparin in the Initial Treatment of Pulmonary Embolism. N Engl J Med. 2003;349(18):1695–702.
Bhatt VR, Aryal MR, Shrestha R, Armitage JO. Fondaparinux-associated heparin-induced thrombocytopenia. Eur J Haematol. 2013;91(5):437–41.
Linkins L-A, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and Prevention of Heparin-Induced Thrombocytopenia. Chest. 2012;141(2):e495S-530S.
Lee CJ, Ansell JE. Direct thrombin inhibitors. British Journal of Clinical Pharmacology. 2011;72(4):581–92.
Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, et al. Bivalirudin for Patients with Acute Coronary Syndromes. N Engl J Med. 2006;355(21):2203–16.
Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6):340S-80S.
Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. British Journal of Haematology. 2008;141(6):757–63.
Whitlon DS, Sadowski JA, Suttie JW, Sadowski JA. Mechanism of Coumarin Action: Significance of Vitamin K Epoxide Reductase Inhibition. Biochemistry. 1978;17(8):1371–7.
Tideman PA, Tirimacco R, St. John A, Roberts GW. (2015) How to manage warfarin therapy. Aust Prescr. 2015;38(2):44.
Zareh M, Davis A, Henderson S. Reversal of warfarin-induced hemorrhage in the emergency department. Western Journal of Emergency Medicine. 2011;12(4):386.
Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin-induced skin necrosis Ann Dermatol. 2014;26(1):96.
Investigators E. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med [Internet]. 2013;369(9):799–808.
Bueller HR. Edoxaban versus warfarin for venous thromboembolism. N Engl J Med. 2014;370(1):80–1.
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med [Internet]. 2009;361(24):2342–52.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman M V., Connors JM, et al. (2020) Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 382(17):1599–607. This article provides evidence of efficacy of the use of apixaban in cancer patients.
Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. (2017) Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 378(7):615–24. This article provides evidence of efficacy of the use of edoxaban in cancer patients.
Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. (2019) Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 380(8):711–9. This article provides evidence of efficacy of the use of apixaban to prevent VTE in cancer patients.
Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308.
• Coons JC, Albert L, Bejjani A, Iasella CJ. (2020) Effectiveness and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients with Acute Venous Thromboembolism. Pharmacotherapy. 40(3):204–10. This article provides evidence of efficacy of the use of DOACS in morbid obese patients.
Dias C, Moore KT, Murphy J, Ariyawansa J, Smith W, Mills RM, et al. Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am J Nephrol. 2016;43(4):229–36.
Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56(5):628–36.
•• Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. (2018) Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 138(15):1519–29. This article provides evidence of efficacy of the use of apixaban in patients receiving hemodialysis.
Nigwekar SU, Thadhani R. (2018) Long-Term Anticoagulation for Patients Receiving Dialysis. Circulation. 1530–1533. This article provides evidence of efficacy of the use of DOACS in patients receiving hemodialysis.
Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. (2018) Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2(2):291–8. This article provides evidence of efficacy of the use of apixaban in patients receiving hemodialysis.
•• Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, et al. (2018) Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 132(13). This article provides evidence of safety of the use of rivaroxaban in patients diagnosed with antiphospholipid antibody syndrome.
•• Pollack C V., Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. (2017) Idarucizumab for Dabigatran Reversal — Full Cohort Analysis. N Engl J Med. 377(5):431–41. This article provides evidence of effectiveness and safety of the use of Idarucizumab to reverse dabigatran
Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413–24.
•• Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. (2019) Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 380(14):1326–35. This article provides evidence of effectiveness and safety of the use of Andexanet to reverse anti Xa.
•• Kiser TH, Burnham EL, Clark B, Ho PM, Allen RR, Moss M, et al. (2018) Half-dose versus full dose alteplase for treatment of pulmonary embolism. Crit Care Med. 46(10):1617. This article provides evidence of the effectiveness of reduced doses of thrombolytics to treat pulmonary embolism.
•• Konstantinides S V, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. (2019) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 41(4):543–603. This article is the updated European guidelines to treat pulmonary embolism.
Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for Patients with Intermediate- Risk Pulmonary Embolism for the PEITHO Investigators*. N Engl J Med. 2014;370:1402–11.
Marti C, John G, Konstantinides S, Combescur C, Sanchez O, Lankeit M, et al. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. European Heart Journal. 2015;36(10):605–14.
Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–86.
Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8(10):1382–92.
• Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J, et al. (2018) A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv. 11(14):1401–10. This article presents the evidence of safety and effectiveness of multiple protocols to use catheter directed thrombolytics.
• Geller BJ, Adusumalli S, Pugliese SC, Khatana SAM, Nathan A, Weinberg I, et al. (2020) Outcomes of catheter-directed versus systemic thrombolysis for the treatment of pulmonary embolism: A real-world analysis of national administrative claims. Vasc Med (United Kingdom). 25(4):334–40. This article presents the evidence of safety and effectiveness of catheter directed thrombolytics vs systemic thrombolytics.
Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, et al. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheterization and Cardiovascular Interventions. 2015;86(7):1219–27.
• Stępniewski J, Kopeć G, Musiałek P, Magoń W, Jonas K, Waligóra M, et al. (2021) Hemodynamic Effects of Ultrasound-Assisted, Catheter-Directed, Very Low-Dose, Short-Time Duration Thrombolysis in Acute Intermediate–High Risk Pulmonary Embolism (from the EKOS-PL Study). Am J Cardiol. 141:133–9. This article presents the evidence of safety and effectiveness of a low-dose protocol to use catheter directed thrombolytics.
• Avgerinos ED, Abou Ali A, Toma C, Wu B, Saadeddin Z, McDaniel B, et al. (2019) Catheter-directed thrombolysis versus suction thrombectomy in the management of acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 7(5):623–8. This article compares two different catheter-based techniques to treat pulmonary embolism.
•• Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, et al. (2019) Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association. Circulation. 140(20):e774–801. This article are the American Heart Association recommendations about the interventional therapies to treat pulmonary embolism.
Pasrija C, Kronfli A, Rouse M, Raithel M, Bittle GJ, Pousatis S, et al. (2018) Outcomes after surgical pulmonary embolectomy for acute submassive and massive pulmonary embolism: A single-center experience. J Thorac Cardiovasc Surg. 155(3):1095–1106.e2. This article presents the single center outcomes of the use of surgical pulmonary embolectomy to treat massive pulmonary embolism.
Neely RC, Byrne JG, Gosev I, Cohn LH, Javed Q, Rawn JD, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Annals of Thoracic Surgery. 2015;100(4):1245–52.
Jenkins D. Pulmonary endarterectomy: The potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. European Respiratory Review. 2015;24(136):263–71.
• Kline JA, Puskarich MA, Jones AE, Mastouri RA, Hall CL, Perkins A, et al. (2019) Inhaled nitric oxide to treat intermediate risk pulmonary embolism: A multicenter randomized controlled trial. Nitric Oxide - Biol Chem. 84:60–8. This article presents the efficacy and safety of the use of inhaled nitric oxide to treat acute pulmonary embolism.
Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR. The use of inhaled prostaglandins in patients with ARDS: A systematic review and meta-analysis. Chest. 2015;147(6):1510–22.
Kooter AJ, IJzerman RG, Kamp O, Boonstra AB, Smulders YM. No effect of epoprostenol on right ventricular diameter in patients with acute pulmonary embolism: A randomized controlled trial. BMC Pulm Med. 2010;10(1):1–9.
Boulain T, Lanotte R, Legras A, Perrotin D. Efficacy of epinephrine therapy in shock complicating pulmonary embolism. Chest. 1993;104(1):300–2.
Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, et al. Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2004;32(4):1035–40.
Tanaka H, Tajimi K, Matsumoto A, Kobayashi K. Vasodilatory effects of milrinone on pulmonary vasculature in dogs with pulmonary hypertension due to pulmonary embolism: A comparison with those of dopamine and dobutamine. Clin Exp Pharmacol Physiol. 1990;17(10):681–90.
Kerbaul F, Gariboldi V, Giorgi R, Mekkaoui C, Guieu R, Fesler P, et al. Effects of levosimendan on acute pulmonary embolism-induced right ventricular failure. Crit Care Med. 2007;35(8):1948–54.
• Zhao S, Friedman O. (2020) Management of Right Ventricular Failure in Pulmonary Embolism. Critical Care Clinics. 36(3):505–15. This article provides important guidelines to manage the acute heart failure in pulmonary embolism.
Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. Journal of Thoracic Disease. 2015;7(7):E166.
Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma - Inj Infect Crit Care. 2007;62(3):570–6.
Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfusion Medicine Reviews. 2015;29(2):90–101.
Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, et al. (2018) Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: A multicentre series of 52 cases. Eur Heart J. 39(47):4196–204. This article presents the European outcomes of the use of ECMO to treat massive pulmonary embolism.
Pasrija C, Kronfli A, George P, Raithel M, Boulos F, Herr DL, et al. (2018) Utilization of Veno-Arterial Extracorporeal Membrane Oxygenation for Massive Pulmonary Embolism. Ann Thorac Surg. 105(2):498–504. This article presents the single center outcomes of the use of ECMO to treat massive pulmonary embolism.
Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, et al. (2019) Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clinical and Applied Thrombosis/Hemostasis. 25:1076029619853037. This article presents the recommendations to treat acute pulmonary embolism by the PERT Consortium.
• Jen WY, Kristanto W, Teo L, Phua J, Yip HS, MacLaren G, et al. (2020) Assessing the Impact of a Pulmonary Embolism Response Team and Treatment Protocol on Patients Presenting With Acute Pulmonary Embolism. Hear Lung Circ. 29(3):345–53. This article analyzes the impact of the creation of PERT in the outcomes of pulmonary embolism patients.
•• Rosovsky R, Chang Y, Rosenfield K, Channick R, Jaff MR, Weinberg I, et al. (2019) Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. 47(1):31–40. This article analyzes the impact of the creation of PERT in the outcomes of pulmonary embolism patients.
Kuo WT, Banerjee A, Kim PS, DeMarco FJ, Levy JR, Facchini FR, Unver K, Bertini MJ, Sista AK, Hall MJ, Rosenberg JK, De Gregorio MA. Pulmonaryembolism response to fragmentation embolectomy and catheter thrombolysis (PERFECT). Chest. 2015;148(3):667–73. https://doi.org/10.1378/chest.15-0119.
Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, et al. (2019) A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc Interv. 12(9):859–69. This article presents the effectiveness and safety of catheter based thrombectomy to treat acute pulmonary embolism.
• Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, et al. (2021) Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc Interv. 14(3):319–29. This article presents the effectiveness and safety of catheter based thrombectomy to treat acute pulmonary embolism.
Funding
N/A
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ronson Madathil MD declares he has no conflict of interest. John Anagnostakos MD declares he has no conflict of interest. Gabriel Pereira MD declares he has no conflict of interest. Michael Hall MD declares he has no conflict of interest. Rafael S. Cires-Drouet MD declares he has no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Vasular systems.
Rights and permissions
About this article
Cite this article
Madathil, R., Anagnostakos, J., Pereira, G. et al. Current Management of Acute Pulmonary Embolism. Curr Surg Rep 9, 16 (2021). https://doi.org/10.1007/s40137-021-00293-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s40137-021-00293-7