Abstract
Purpose of Review
The aim of this review is to summarize and sum up the recent evidence on the topic of abdominal wall reconstruction post oncologic resection, comparing the use of mesh versus autologous reconstruction.
Recent Findings
Recent findings show a more accepting approach towards more complex reconstructions that aim at a dynamic and robust abdominal wall reconstruction. Musculocutaneous free flaps using the anterolateral thigh flap, with a vastus lateralis, or the latissimus dorsi flap are being used more for replacing musculo-fascial and full-thickness defects to restore abdominal domain. Those autologous reconstructions are best combined with a mesh for robust musculo-fascial layer closure. Different mesh options are available for different cases depending on defect and the contamination status of the wound.
Summary
Post-oncologic abdominal wall reconstruction is a complex procedure that should be well planned in multidisciplinary teams. The surgical options should be set up on a case-by-case basis weighing the different benefits and risks of autologous, mesh, or combined reconstruction. The more robust the reconstruction, the less complications encountered, especially with hernia formation rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oncologic excision an abdominal wall tumor often involves an en bloc resection of the neoplasm, along with a margin of normal tissue, creating a full-thickness defect in the abdominal wall [1]. There is an immediate need for coverage of exposed organs and vital structures in the abdominal cavity [2]. Reconstruction of the abdominal wall is of central importance, frequently necessitating intervention from a Plastic surgeon. The reconstructive surgeon needs to address both soft tissue coverage and abdominal wall support to prevent herniation of the abdominal viscera. Proper preoperative planning is essential. Patient factors such as body habitus, previous abdominal surgeries, medical comorbidities, and previous radiation therapy need to be considered. Defect factors should also be well-thought-out as defects can differ significantly in complexity. It is vital to assess the size and depth of the defect, its location, and its composition [3]. A thorough understanding of the abdominal wall anatomy and proper surgical planning are essential for a successful reconstruction.
Numerous options exist for the closure of the iatrogenic defect including primary fascial closure, component separation, the use of a synthetic or biologic mesh or autologous reconstruction with either pedicled or free flaps [4•]. Although the optimal technique for abdominal wall reconstruction continues to be a subject of continuing debate, knowledge of each techniques advantages and disadvantages are imperative to allow the surgeon to make a proper decision about the mode of reconstruction to be used.
In this review, we will address the challenges in the post-oncologic abdominal wall reconstruction with special focus on the benefits and pitfalls of reconstruction with a mesh versus autologous reconstruction.
Goals of Reconstruction
Regardless of the defect specifications, the goals of abdominal wall reconstruction are the same. This includes restoration of the integrity of the abdominal wall and its dynamic function, protection of the abdominal viscera, prevention of hernia formation, and decrease of the overall complications; these complications include seroma formation, infection, enterocutaneous fistula formation, wound breakdown, exenteration of internal organs, and bowel strangulation [5, 6].
The abdominal wall is a dynamic organ and so the repair needs to resist stress and strain. Every attempt should be made to achieve fascial closure either by primary fascial repair, component separation, or the addition of a flap [7]. The repair can be reinforced with a mesh. A mesh-reinforced repair has a hernia occurrence rate of 8% and an overall complication rate of 32% when compared to a bridging mesh only with no fascial closure where the hernia occurrence rate is 56% and the overall complication rate can be as high as 74% [8].
Preoperative Planning
A multidisciplinary approach is needed when addressing abdominal wall tumors. Input from the medical oncologist, surgical oncologist, radiation oncologist, and reconstructive surgeon is required. With a proper understanding of the treatment plan, expectations of the defect size, and the need for radiotherapy, the reconstructive surgeon is better able to formulate a reconstructive plan.
Preoperative evaluation also includes risk stratification. As with all abdominal wall reconstructions, one needs to consider the patients general condition. Factors such as tobacco use, obesity, diabetes mellitus, and nutritional status should be optimized prior to any intervention [9]. Prior abdominal surgeries also increase the risk of complications. Patients with virgin abdomens, and no previous incisions, distortion, or loss of domain, generally have a lower risk of complications. Patients with multiple abdominal scars, prior colostomies, or already placed meshes need to be addressed more thoroughly as the reconstructive plan could be more complex [10•].
Preoperative imaging is of great value in planning abdominal wall reconstruction. It aids in predicting the size and thickness of the defect with visualization of the abdominal wall perforators necessary in free flaps. Usually, imaging is done as part of the work-up for the abdominal wall tumor, as a computed tomography or magnetic resonance imaging. A computed tomography angiography may be necessary for assessment of perforators in patients planned for free flap coverage, especially in history of previous abdominal surgery or irradiation [11].
Defect Consideration
When deciding on the mode of reconstruction, it is vital to think of the defect and its composition. The abdominal wall in multilayered composed of skin, fat, fascia, and muscle; therefore, defects can be quite diverse. There are many different systems for classification of the defect and that can be confusing. The general concept is the same; one needs to consider both layer composition and location. For central defects, every effort should be made to attempt primary closure of the musculo-fascial layer. Component separation could be done to help achieve domain [12]. In case of a larger defect where component separation is not enough for primary fascial closure, then a pedicled or free flap is warranted. A reinforcing mesh or a bridging mesh could be used if necessary. Special consideration should be given for central defects in the epigastric area. Even with component separation, primary closure is rarely achieved and frequently a free flap is needed [3]. For more lateral defects, primary reapproximation of the musculo-fascial layer is often difficult as the fascia in that area is not very giving. The goal is to achieve a long-lasting durable repair often with an interposition mesh or a flap [13].
After oncologic resection of the tumor, a soft tissue defect is often created. It is extremely important to achieve a tension-free closure in these cases, especially if patients are scheduled to receive radiotherapy. Proper wound healing is essential to allow the patient to receive the post-surgical oncologic treatment with no delay. If sufficient local tissue is available, with minimal undermining, primary closure could be achieved. The less the undermining, the fewer wound healing complications. If there is not enough local tissue for closure, then a flap, local, pedicled, or free, is needed [14]. Large defect size, especially when more than 15 cm, has been associated with an increased risk of hernia recurrence regardless of the method of reconstruction used [8].
Mesh Reconstruction
Mesh Material
The ideal mesh should have the following properties: non-carcinogenic, chemically inert, resists mechanical strain, has minimal foreign body reaction, and unlikely to be allergenic [15]. Selection of the proper mesh material could be a bit challenging. Mesh material could be broken down into three categories: Synthetic, biologic, and composite. The synthetic meshes can be either non-absorbable or absorbable, including the biosynthetics [16]. Non-absorbable meshes include polypropylene, polyester, and polytetrafluorethylene. Glycolic acid (Vicryl) and polyglycolic acid (Dexon) are examples of absorbable meshes. Biosynthetic meshes are similar to the synthetic absorbable meshes except that they allow for tissue ingrowth before completely dissolving [17]. Both porcine and bovine bioprosthetic meshes are available and have been found to have similar outcomes in regard to postoperative complications; however, porcine acellular dermal matrix may be prone to intraoperative device failure [18]. An example of biosynthetic mesh is the Bio-A mesh composed of polyglycolic acid and trimethyl carbonate. These biosynthetic meshes can be supplemented by an extra layer to form the composite meshes. A composite mesh usually has different properties on each of the visceral and parietal side. In general, a coat of a temporary degradable strand is added to create a barrier between the mesh and the abdominal viscera. This will decrease the risk of adhesion and complication associated with the polypropylene, polyester, and polytetrafluorethylene meshes [19].
Biologic meshes are those derived from human, porcine, or bovine tissue. Examples of biologics include the human acellular dermal matrices such as AlloDerm (Allergan, Branchburg, N.J.). These are decellularized non-crosslinked collagen scaffolds that allow tissue ingrowth. The main advantage of biologic meshes is that they have the ability to resist infections and can be used in contaminated fields [20]. Biologic meshes have a 10.9% risk of an infectious wound complication as compared to 36.5% in the synthetic meshes [21]. Biologic meshes have a comparable hernia recurrence rate with the synthetics, contrary to common belief. The risk of hernia formation is more related to the placement of the mesh, defect size and composition, body mass index (BMI), and patient comorbidities rather than the type of mesh used [22].
Mesh Placement
In abdominal wall reconstruction, the mesh can be placed to reinforce the fascial repair or as a bridging interposition mesh in cases where primary repair could not be done. In general, there are four different locations for mesh placement: Onlay, sublay, underlay, and inlay. Each technique has its advantages and disadvantages with respect to hernia recurrence and complications [23]. Onlay placement of the mesh is the simplest technique where the mesh is placed in the subcutaneous space anterior to the rectus fascia. This requires undermining of the skin and subcutaneous fat to allow for proper mesh placement, which conceptually places the repair at a higher risk of seroma formation and subsequent surgical site infection. Even though this has been postulated, many studies have failed to prove statistically significant difference in seroma rates [24].
In a sublay repair, the mesh is placed in a retro rectus position, anterior to the posterior rectus sheath. This is in opposition to the underlay placement of the mesh, where it is placed posterior to the posterior rectus sheath in the extra or intra peritoneal space. In a review by Sosin et al. in 2018 on 6227 ventral hernia repairs, the location of mesh placement did affect the hernia recurrence rate in a statistically significant manner. The onlay placement had the highest recurrence rate at 12.9%, followed by the underlay at 10.9% and 5.8% for the sublay placement. There was no statistically significant difference in the overall complication rates between the different techniques [25].
The inlay repair refers to placement of the mesh in the defect between the edges of the rectus fascia. This is otherwise known as a bridging or interposition mesh placement. This form of repair has the highest rate of hernia recurrence because of the lack of primary fascial closure. In this technique, the mesh is the single closure layer, it is avascular, and is expected to bear all the load of the reconstruction [26]. Bridged repairs have hernia recurrence rates at 33% in comparison to 6.2% in reinforced repairs and a greater overall complication rate of 59% as compared to 30% in reinforced repairs [27].
Autologous Reconstruction
Autologous reconstructive options of the abdominal wall vary depending on several factors: mainly the type and size of defect, the degree of contamination, and the type of tumor resected. The type of defect can be further divided into the missing layers of the abdominal wall that need to be reconstructed: skin and subcutaneous tissue, muscle, or full-thickness defects [2]. Based on the aforementioned criteria, the autologous reconstruction of the abdominal wall defects needs to be tailored to each specific patient.
Skin and Subcutaneous Tissue
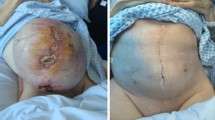
Shallow defects involving only skin and subcutaneous tissue are relatively easy to reconstruct. Negative pressure dressing can be used as a bridging therapy when in doubt about the wound contamination status or when awaiting final pathology results of the resection margins (Fig. 1). Skin grating is also a simple and easy reconstructive option and allows clearer monitoring of the area for tumor recurrence [10•] (Fig. 2).
Local flaps, whether advancement or propeller flaps, are frequently utilized as they provide a similar color match and a superior cosmetic result. Well-planned incisions are critical to preserve blood supply and limit complications. In patients with sufficient abdominal wall laxity, random pattern flaps such as advancement flaps, rotational flaps, VY flaps, or bipedicled flaps can be used. Transverse defects, especially infra-umbilical, can be closed using a standard abdominoplasty technique [28]. Minimizing skin tension through progressive tension sutures and layered incisional closure has been shown to decrease complications [29]. Because of the created dead space, the use of closed suction drains is recommended [28]. Tissue expansion is usually not an immediate option in post-oncological reconstructions as tumor resection cannot be delayed. Reconstruction with tissue expanders can be bridged with skin grafting until sufficient local skin is recruited to cover the defect [30].
With a better understanding of the abdominal wall blood supply and with advancement in microsurgical techniques, perforator flaps have become acceptable reconstructive options. Fasciocutaneous flaps based on perforators of the superior or inferior epigastric, internal mammary or superficial circumflex iliac perforator vessels are able to provide coverage for any area of the abdominal wall [31•].
When local options are insufficient, especially in the setting of larger defects, prior abdominal wall surgery or prior radiation neighboring regional flaps can be utilized [14]. The workhorse regional flaps for defects restricted to skin and subcutaneous tissue mainly constitute the perforator anterolateral thigh (ALT) flap (Fig. 3). The ALT flap can be raised a perforator flap based on its septocutaneous or musculocutaneous perforators. The flap kept attached at its pedicle, the descending branch of the lateral circumflex femoral vessels, can be passed through a tunnel under the rectus femoris and sartorius to reach the lower to mid-abdominal wall and up to supraumbilical cases in some reports [32].
Moving up the reconstructive ladder, and with proper microsurgical training availability of microsurgical equipment, the use of free flaps can be employed. Free tissue transfer allows the recruitment of distant skin to cover almost any defect of the abdominal wall. As a general concept any free flap can be used. The most commonly used free fasciocutaneous flaps are the ALT and the thoracodorsal artery perforator (TdAP) flap (Fig. 4). The TdAP flap spares the Latissimus dorsi muscle. Multiple recipient vessels are available, including the internal mammary vessels and the deep inferior epigastric vessels. If unavailable, the superficial femoral artery could be used. A vein graft is frequently needed or an arteriovenous loop with the saphenous vein could be utilized [14].
Muscle/Fascia Defects
In defects involving the muscular layer of the abdominal wall, more complex reconstruction methods need to be employed. The musculo-fascial defect needs to be replaced by a strong layer that will protect the abdominal contents and prevent hernia formation.
Primary musculo-fascial closure should always be attempted. Component separation is a simple reconstructive technique and comes in handy in critical patients where operative time is of essential value. Component separation allows this musculo-fascial closure to occur using advancement of the lateral abdominal wall muscles [33] (Fig. 5). Depending on the size of defect, different components of the abdominal wall might need to be released [34]. Posterior sheath release is required for smaller and medium-sized defects, while a release of the external oblique muscle fascia is required for larger defects [34]. The component separation technique is mainly used for midline and paramedian defects. The bilateral advancement of the abdominal wall components allows coverage of up to 10 cm in the epigastric area, 20 cm at the waist, and 6 cm in the suprapubic region [35]. It may also be used to decrease the bridging gap between the abdominal wall muscles when primary repair is not possible. The main advantage is that it spares donor site morbidity. The use of Botulinum toxin A 2 weeks preoperatively may aid in primary fascial closure by relaxing the abdominal wall muscles [36].
If component separation is not sufficient to allow primary fascial closure, regional flaps can be used. The ALT flap is again one of the most utilized flaps in abdominal wall reconstruction. In musculo-fascial defect, the ALT flap can be harvested as a musculocutaneous flap, including the vastus lateralis muscle, enabling replacement of like with like. The tensor fascia lata (TFL) flap provides a robust layer of fascia for abdominal wall reinforcement [37]. Reserved for larger defects, the subtotal thigh flap, based on the lateral circumflex branch, can be used for large soft tissue musculo-fascial coverage [38]. The subtotal thigh flap incorporates a larger skin paddle, along with the possibility of including multiple muscles, the TFL, vastus lateralis, and rectus femoris (RF).
Free flaps are often used whenever regional flaps are insufficient or cannot reach the resulting defect. In addition to the aforementioned flaps (ALT and TFL) that can also be used as free flaps, the latissimus dorsi (LD) musculocutaneous flap is a strong option [39]. The LD is a large muscle and thus able to cover large defects. There is minimal donor site morbidity. The downside to using the flap is frequent intraoperative repositioning which will prolong operative time.
An understudied field is the reconstruction of the abdominal wall using neurotized flaps. Reports of using neurotized ALT flaps incorporating the vastus lateralis have been published with encouraging results for a dynamic and stronger reconstruction and decreased postoperative hernia rate [7, 40].
Full-Thickness Defects
Similar to musculo-fascial defects, full-thickness defects of the abdominal wall need a strong reconstructive layer to decrease the incidence of hernias later on. An ALT flap harvested with the tensor fascia lata (TFL) provides both a robust layer to support the abdominal contents and a large skin paddle for skin closure. For larger defects, the subtotal thigh flap or combining multiple free flaps can be used; bilateral ALT free flaps can be harvested and used to close sizeable defects (Fig. 6). Full-thickness defects can be treated similar to musculo-fascial defects in general. A gain in full-thickness defects is the access to intra-abdominal vessels such as the gastroepiploic vessels, without impinging an additional morbidity of opening the peritoneal layer that is already violated [14].
Conclusion
Abdominal wall reconstruction is a challenging procedure that should not be underestimated. The surgical plan should be tailored to each patient. Different patients will have different defect location, different layers involved, and different anatomies, surgical history, and medical history, permitting or prohibiting certain procedures. Hence, the surgeon should take a detailed history and perform a proper physical exam preoperatively. A multidisciplinary team approach cannot be stressed enough. The tumor characteristics need to be discussed and the need of radiotherapy addressed as all these factors will help guide the form of reconstruction used. The decision of whether to use mesh, autologous, or combined reconstruction is dictated by the multiple defect factors and the patient’s general condition. In larger defects, a combined reconstruction may be necessary to establish a robust abdominal wall. The mesh can reinforce the autologous repair decreasing the rate of hernia formation [41]. The aim remains to be reestablishment of the abdominal domain and support the intra-abdominal contents while simultaneously sealing the outer soft tissue defect.
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: ∙ Of importance
Yezhelyev MV, Deigni O, Losken A. Management of full-thickness abdominal wall defects following tumor resection. Ann Plast Surg. 2012;69(2):186–91.
Tang R, Gu Y, Gong D-Q, Qian Y-L. Immediate repair of major abdominal wall defect after extensive tumor excision in patients with abdominal wall neoplasm: a prospective review of 27 cases. Ann Surg Oncol. 2009;16(10):2895–907.
Rohrich RJ, Lowe JB, Hackney FL, Bowman JL, Hobar P. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg. 2000;105(1):202–17.
• Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg. 2000;232(4):586. This reference represents the basis of abdominal wall reconstruction with the different options available.
Patel NG, Ratanshi I, Buchel EW. The best of abdominal wall reconstruction. Plast Reconstr Surg. 2018;141(1):113e–e136.
Khansa I, Janis JE. The 4 principles of complex abdominal wall reconstruction. Plast Reconstr Surg Global Open. 2019;7(12):e2549.
Vranckx J, Stoel A, Segers K, Nanhekhan L. Dynamic reconstruction of complex abdominal wall defects with the pedicled innervated vastus lateralis and anterolateral thigh PIVA flap. J Plast Reconstr Aesthet Surg. 2015;68(6):837–45.
Booth JH, Garvey PB, Baumann DP, Selber JC, Nguyen AT, Clemens MW, et al. Primary fascial closure with mesh reinforcement is superior to bridged mesh repair for abdominal wall reconstruction. J Am Coll Surg. 2013;217(6):999–1009.
Liang M, Holihan J, Itani K. Ventral hernia management: expert consensus guided by systematic review [published online March 15, 2016]. Ann Surg.
• Khansa I, Janis JE. Modern reconstructive techniques for abdominal wall defects after oncologic resection. J Surg Oncol. 2015;111(5):587–98. This reference represents the basis of abdominal wall reconstruction with the different options available.
Sacks JM, Broyles JM, Baumann DP, editors. Considerations in abdominal wall reconstruction. Seminars in plastic surgery; 2012: Thieme Medical Publishers.
Ko JH, Wang EC, Salvay DM, Paul BC, Dumanian GA. Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg. 2009;144(11):1047–55.
Baumann DP, Butler CE, editors. Lateral abdominal wall reconstruction. Seminars in plastic surgery; 2012: Thieme Medical Publishers.
Mericli AF, Baumann DP, Butler CE. Reconstruction of the abdominal wall after oncologic resection: defect classification and management strategies. Plast Reconstr Surg. 2018;142(3S):187S–S196196.
Shankaran V, Weber DJ, Lawrence R, Reed I, Luchette FA. A review of available prosthetics for ventral hernia repair. Ann Surg. 2011;253(1):16–26.
Cobb WS. A current review of synthetic meshes in abdominal wall reconstruction. Plast Reconstr Surg. 2018;142(3S):64S–71S.
Lak KL, Goldblatt MI. Mesh selection in abdominal wall reconstruction. Plast Reconstr Surg. 2018;142(3S):99S–106S.
Clemens MW, Selber JC, Liu J, Adelman DM, Baumann DP, Garvey PB, et al. Bovine versus porcine acellular dermal matrix for complex abdominal wall reconstruction. Plast Reconstr Surg. 2013;131(1):71–9.
Jacob B, Hogle N, Durak E, Kim T, Fowler D. Tissue ingrowth and bowel adhesion formation in an animal comparative study: polypropylene versus proceed versus parietex composite. Surg Endosc. 2007;21(4):629–33.
Köckerling F, Alam N, Antoniou S, Daniels IR, Famiglietti F, Fortelny R, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia. 2018;22(2):249–69.
Darehzereshki A, Goldfarb M, Zehetner J, Moazzez A, Lipham JC, Mason RJ, et al. Biologic versus nonbiologic mesh in ventral hernia repair: a systematic review and meta-analysis. World J Surg. 2014;38(1):40–50.
Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE. Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg. 2017;224(3):341–50.
Holihan JL, Nguyen DH, Nguyen MT, Mo J, Kao LS, Liang MK. Mesh location in open ventral hernia repair: a systematic review and network meta-analysis. World J Surg. 2016;40(1):89–99.
Haskins IN, Voeller GR, Stoikes NF, Webb DL, Chandler RG, Phillips S, et al. Onlay with adhesive use compared with sublay mesh placement in ventral hernia repair: was chevrel right? An Americas hernia society quality collaborative analysis. J Am Coll Surg. 2017;224(5):962–70.
Sosin M, Nahabedian MY, Bhanot P. The perfect plane: a systematic review of mesh location and outcomes, update 2018. Plast Reconstr Surg. 2018;142(3S):107S–S116116.
Boukovalas S, Sisk G, Selber JC, editors. Abdominal Wall Reconstruction: An Integrated Approach. Seminars in plastic surgery; 2018: Thieme Medical Publishers.
Giordano S, Garvey PB, Baumann DP, Liu J, Butler CE. Primary fascial closure with biologic mesh reinforcement results in lesser complication and recurrence rates than bridged biologic mesh repair for abdominal wall reconstruction: a propensity score analysis. Surgery. 2017;161(2):499–508.
Khansa I, Janis J. Management of skin and subcutaneous tissue in complex open abdominal wall reconstruction. Hernia. 2018;22(2):293–301.
Janis JE, Khansa I. Evidence-based abdominal wall reconstruction: the maxi-mini approach. Plast Reconstr Surg. 2015;136(6):1312–23.
Wooten KE, Ozturk CN, Ozturk C, Laub P, Aronoff N, Gurunluoglu R. Role of tissue expansion in abdominal wall reconstruction: a systematic evidence-based review. J Plast Reconstructive & Aesthetic Surgery. 2017;70(6):741–51.
• Roubaud MS, Baumann DP, editors. Flap reconstruction of the abdominal wall. Seminars in plastic surgery; 2018: Thieme Medical Publishers. This reference represents the basis of abdominal wall reconstruction with the different options available.
Fernandez-Alvarez JA, Barrera-Pulido F, Lagares-Borrego A, Narros-Gimenez R, Gacto-Sanchez P, Gomez-Cia T. Coverage of supraumbilical abdominal wall defects: the tunnelled-pedicled ALT technique. Microsurgery. 2017;37(2):119–27.
Maloney SR, Schlosser KA, Prasad T, Kasten KR, Gersin KS, Colavita PD, et al. Twelve years of component separation technique in abdominal wall reconstruction. Surgery. 2019;166(4):435–44.
Ramirez OM. Inception and evolution of the components separation technique: personal recollections. Clin Plast Surg. 2006;33(2):241–6.
Heller L, McNichols CH, Ramirez OM, editors. Component separations. Seminars in plastic surgery; 2012: Thieme Medical Publishers.
Soltanizadeh S, Helgstrand F, Jorgensen LN. Botulinum toxin A as an adjunct to abdominal wall reconstruction for incisional hernia. Plast Reconstr Surg Global Open. 2017;5(6):1358.
Song Z, Yang D, Yang J, Nie X, Wu J, Song H, et al. Abdominal wall reconstruction following resection of large abdominal aggressive neoplasms using tensor fascia lata flap with or without mesh reinforcement. Hernia. 2018;22(2):333–41.
Lin SJ, Butler CE. Subtotal thigh flap and bioprosthetic mesh reconstruction for large, composite abdominal wall defects. Plast Reconstr Surg. 2010;125(4):1146–56.
Kim SW, Han SC, Hwang KT, Ahn BK, Kim JT, Kim YH. Reconstruction of infected abdominal wall defects using latissimus dorsi free flap. ANZ J Surg. 2013;83(12):948–53.
Iida T, Mihara M, Narushima M, Todokoro T, Hara H, Yoshimatu H, et al. Dynamic reconstruction of full-thickness abdominal wall defects using free innervated vastus lateralis muscle flap combined with free anterolateral thigh flap. Ann Plast Surg. 2013;70(3):331–4.
Henry CR, Bradburn E, Moyer KE. Complex abdominal wall reconstruction: an outcomes review. Ann Plast Surg. 2013;71(3):266–8.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RK and FG worked on literature review and manuscript writing. AI worked on manuscript writing. All authors contributed significantly to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Reem Karami, Fadi Ghieh, and Amir Ibrahim declare no conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Ethics Approval
Not applicable.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Plastic Surgery.
Rights and permissions
About this article
Cite this article
Karami, R., Ghieh, F. & Ibrahim, A. Post-Oncologic Abdominal Wall Reconstruction: Mesh Versus Autologous Tissue. Curr Surg Rep 8, 26 (2020). https://doi.org/10.1007/s40137-020-00272-4
Published:
DOI: https://doi.org/10.1007/s40137-020-00272-4