Abstract
Recent Findings
Each year 40,000 people are diagnosed with primary brain tumors. Malignant gliomas account for more than 50% of them and are universally fatal despite aggressive surgery and combined chemoradiotherapy. Identification of novel therapeutic targets has been challenging. New areas of interest are now focusing on exploiting special metabolic adaptations these tumors have developed in response to the stress caused by their rapid growth rate, exceeding their vascular supply.
Purpose of Review
This article is an attempt to review a controversial strategy of modulating tumor biology by understanding their metabolic characteristics, how they differ from normal healthy cells, and implementing therapies that include nutritional interventions.
Summary
Although fasting as part of the management of brain malignancies is controversial and may be contrary to popular opinion, developing supportive evidence in targeting their special metabolic profile is promising and fascinating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly invasive and angiogenic glioblastomas (GBM) and anaplastic astrocytomas comprise the majority of primary brain tumors; despite the best available treatment options such as maximum surgical resection, radiation therapy, and chemotherapy, median survival is only about 12–18 months after diagnosis, with less than 10% survival at 5 years [1]. Unfortunately, complete surgical resection of these tumors is difficult to achieve with their diffusely infiltrative growth pattern and usual proximity to the eloquent cortex; furthermore, the effectiveness of systemic chemotherapy is often blunted due to the selective properties of the blood brain barrier (BBB). Approved by the FDA in 2015, Temozolomide (TMZ) is an alkylating agent used concurrently with radiation therapy as an adjuvant therapy, and currently remains the standard of care for the upfront management of high-grade malignant gliomas. TMZ is not directly active and undergoes non-enzymatic conversion to 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC) at physiological pH [2, 3]. The main cytotoxic effect caused by TMZ is due to the alkylating guanine at the O6 and N7 positions, which precipitates the failure of DNA mismatch repair, which induces a blockage in the cellular cycle at the G2-M boundary, and finally induces apoptosis [4]. Common side effects include nausea, fatigue, headache, constipation, and myelosuppression.
Most chemotherapeutic agents cause significant damage to normal tissues, particularly those with high a proliferative rate, resulting in significant short- and long-term adverse effects, leading the dose-limiting factor. The current approach to treatment is targeting therapeutics based on underlying genetic aberrations and the gene or protein expression of GBMs; however, the evolution of our understanding of tumor biology and possible targeted therapies has achieved limited success. Hence, there is an emerging interest in targeting the differences in metabolism and glucose utilization between GBM cells and normal cells.
A New Concept: Differential Stress Resistance
Multiple researchers have described the dysregulated glucose metabolism seen in most cancer cells, suggesting that with tumor cells’ increased reliance on glucose, treatments aiming to exploit their unique cellular metabolism may be keys to improving the efficacy of current therapies [5•]. Carcinogenic cells, regulated by oncogenes, lose their self-control mechanism for cellular division, and contrary to common perception, their unabated growth rate creates a vulnerability, at least from the metabolic point of view. A normal cell, under starvation state, will react adaptively to a scarcity of resources by switching to a “standby” or protective mode to avoid self-extinction. Cancer cells, on the other hand, are unable to implement an appropriate adaptation to a resource poor environment; they will continue aggressive growth even if it implies increased risk of self-destruction.
Laviano et al. showed [5•] that chemotherapy-induced oxidative stress shrinks the tumor volume but also negatively affects normal cells; however, in conditions of short-term starvation (STS), before or after chemotherapy, normal cells developed a starvation-induced differential stress resistance (DSR). DSR is a metabolic mechanism proposed as a possible “protective shield” available to normal cells during chemotherapy and radiotherapy treatment where normal cells can re-channel the available energy resources, in part by reducing insulin-like growth factor1 (IGF-1) signaling and, hence, activating their protective mechanisms. In vitro studies have shown that this differing response between normal and cancer cells can be induced by reducing the available glucose, the IGF-1 levels, and downregulating the mTOR/S6K system [6, 7].
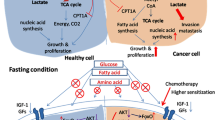
Cancer cells harbor mutations in growth signaling genes such as IGF1R, phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog (PTEN), and RAS that contribute to self-sufficient proliferation signaling and their unresponsiveness to starvation signals. These effects have been further described in the literature [7, 8] (See Fig. 1).
Effect of starvation in cancer cells. Fasting in cancer cells increases insulin-like growth factor 1 (IGF-1). It induces downstream signaling proteins such as phosphoinositide 3-kinase (PI3 K) which in turn induces target of rapamycin (TOR), protein kinase B (AKT), protein kinase A (PKA), and Ras proteins. As result of activation of this downstream regulatory pathway, stress resistance genes are not fully functional, so cancer cells lack protective mechanisms found in normal cells. Also, activation of RAS and certain oncogenes makes cancer cells more vulnerable to chemotherapy via inducing oxidative stress and hence lesser proliferation
In mice, pre-clinical studies have consistently revealed that intermittent fasting cycles, applied during the chemotherapy treatment in immunocompromised mice with neuroblastoma and other cancers, lead to decrease in the tumor size as compared to normally fed mice, suggesting that combining fasting with standard of care chemotherapeutic agents may provide a more effective anti-tumor effect [6, 7].
Actually, This New Concept is Not That New
The concept of abnormal or altered tumor cell metabolism was described by Dr. Otto Warburg in 1924, a discovery that later won him two Nobel prizes. Normal cells can sense the availability of substrates like glucose and oxygen, and under appropriate resources they depend on mitochondrial oxidative phosphorylation to generate ATP. Once the substrates are not available, normal cells shift their metabolism to an anaerobic pathway of glycolysis. Warburg described how tumor cells, to meet the demands of rapid proliferation, must shift their metabolism into aerobic glycolysis to provide energy regardless of the availability of oxygen [9]—meaning that cancer cells survive by generating ATP via glycolysis rather than oxidative phosphorylation, even when oxygenated.
Glioblastomas have a special glucose addiction, explained by the Warburg effect, not seen in other tumors that can oxidate other substances to produce ATP. Studies suggest that glioma cells present different degrees of glycolysis according to their cellular differentiation, which suggests different mitochondrial reserve capacities and a pronounced sensitivity to chemotherapies that target inhibition of glycolysis, and a resultant cellular death sentence via glucose deprivation [10••]. Studies have shown that malignant cells, unlike normal cells, respond to fasting by promoting oncogenic signaling and protein synthesis, suggesting that targeting metabolic changes can benefit classic therapies, possibly sensitizing tumor cells to anti-cancer agents and broadening the therapeutic window.
Presently, we understand that cancer cell metabolism is even more complex than previously understood, and due to other various genetic and mitochondrial defects, tumor cells cannot utilize ketones effectively and must rely on glucose as their primary energy source [11]. Some of the various cellular defects seen in cancer cells include the following:
-
(1)
Needing a constant source of energy to proliferate, cancer cells employ various underlying mechanisms to maintain this homeostasis. Mitochondrial reactive oxygen species (ROS), upon activation via genotoxic stress, inflammation, hypoxia, and nutrient deprivation, cause autophagy and apoptosis. ROS have been implicated in angiogenesis induction and tumor growth through the regulation of vascular endothelial growth factor (VEGF) and Hypoxia inducible factor-1 HIF-1 [12].
-
(2)
Tumor suppressor 53 (TP53) gene’s alteration is crucial in tumorigenesis; given that metabolic pathways have been implicated as being among the various mechanisms regulating its function, recent studies have focused on the effects of glycolysis and oxidative phosphorylation on TP53 regulation. It has been shown that TP53 functions by slowing down the glycolysis pathway, thereby inhibiting tumor growth [13].
-
(3)
Phosphoinositide 3-kinase/protein kinase A (PI3 K/AKT) is an important factor linking metabolism and cellular growth in cancer; it is activated in cancer cells and promotes glycolysis. Rapid cancer cell death has been observed in a nutrient deprived, specifically glucose deprived environment, and it is known that there are metabolically driven checkpoints which can be upregulated [13].
-
(4)
HIF-1 plays a key role in tumor development via various mechanisms such as VEGF and IGF-1/2. HIF-1 upregulates certain genetic mechanisms under stress conditions [13], and studies which evaluate targeting it in solid malignancies such as colon and breast cancer are underway.
-
(5)
Myc oncogene is considered a key regulator of metabolism and growth of cancer cells. It is not yet clear whether its overexpression is primarily linked to cellular metabolism alterations or is a result of metabolic changes within cancer cells themselves [13].
The above mentioned are but a few examples that highlight the importance of understanding the interplay of genetic and metabolic pathways in tumors. Even though highly complex and not fully understood, known genetic alterations can be targeted to further deepen the effect of starvation on cancer cells.
Options on the Menu
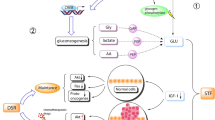
As mentioned, DSR is the protective effect where fasting or STS can selectively protect normal cells from chemo-toxicity without reducing the deleterious effect on cancer cells, possibly due to redistribution of energy and resources from reproduction and growth to cellular maintenance in normal, but not cancer cells. Dr. Rous was the first to suggest that restricted food intake reduced tumor growth [14], but again there are variations on the menu: The ketogenic diet (high fat, low carbohydrate and protein), caloric restriction, and fasting all cause a metabolic change, specifically, a reduction in blood glucose and an increase in blood ketones (See Fig. 2).
Ketogenic Diet (KD)
Simplifying a complex biochemical process, ketogenesis involves the oxidation of fatty acids and acetyl-CoA production to generate β-hydroxybutyrate and acetoacetate, which can be utilized as an effective energy source in the normal brain [15], but not by tumor cells [6]. This selective metabolic response induced by Unrestricted KD has been shown to improve survival in animal models of malignant gliomas, and potentiate the anti-tumor effect of chemotherapies and radiation treatment [11], likely related to decreasing expression of angiogenesis and peritumoral edema by inhibition of stimulating factors like IGF-1, platelet-derived growth factor (PDGF), and epidermal growth factor receptor (EGFR) [12]. Another possible explanation of the protective effect of nutritional ketosis is the reduction of ROS production [16]. Similar effects have been seen in UKD in combination with TMZ [12].
Caloric Restriction (CR)
Caloric Restriction (CR) involves a reduction of 20–40% in daily caloric intake which has been shown to protect a wide variety of organisms against oxidative stress and aging, and to reduce tumor growth and expression of angiogenic and pro-inflammatory biomarkers [cyclooxygenase 2 (COX-2), nuclear factor κB (NF-κB), and macrophage inflammatory protein 2 (MIP-2)] [17] in astrocytoma models [18]. Combining the metabolic benefits of CR and KD (RKD) can reduce inflammation, peritumoral edema, and normalize the vasculature bed, with some anecdotal evidence of efficacy in humans [19].
Fasting and Short-Term Starvation (STS)
Fasting and Short-Term Starvation (STS) have been practiced for millennia, but only recently have studies focused on their role in adaptive cellular responses that reduce oxidative damage and inflammation, optimize energy metabolism, and boost cellular protection. In some organisms, chronic or periodic fasting extends longevity, protects against diabetes, cancers, heart disease, and neurodegeneration. [20, 21, 22•] In humans, STS has the potential to delay aging and help prevent/reduce obesity, hypertension, asthma, and rheumatoid arthritis, minimizing the risk of malnutrition [22•]. Nutritional ketosis induced by fasting in humans promotes beneficial changes in metabolic pathways like stress resistance, lipolysis, and autophagy. It can be achieved by different fasting regimens. (See Fig. 2) Its beneficial effect could derive from reduction of IGF-1 and glucose levels (> 50%), increased growth factor inhibitor (IGFBP-1), and rapid return to normal weight after re-feeding [8].
Fasting Mimicking Diet (FMD)
Based on low consumption of proteins and simple carbohydrates and rich in healthy fats, FMD has been shown to promote stem cell-dependent regeneration in the immune and nervous systems (studies mainly performed in middle-age mice) when applied in intermittent periods of 5 consecutive days. During the effective fasting period, (> 3 days) the metabolism switches from a primarily sugar-burning mode to a fat-burning mode (mainly abdominal and visceral fat), and the organism exhibits several markers of a fasting-induced protective state such as (1) lower IGF-1 levels; (2) lower glucose levels; (3) higher ketone bodies levels; and (4) higher IGFBP1 levels [6].
Once the protective fasting state is attained, a beneficial destruction of damaged cell components and unnecessary cells is observed; the resultant by-products are used to re-build necessary proteins during starvation or period of scarcity (a process called autophagy). During the re-feeding period, there is a transitory, remarkable increase in number and function of circulating stem cells, which regenerates organs and systems, bringing characteristics of younger and more functional cells.
The effects of long-term fasting on autophagy and rejuvenation have been seen in humans under medical and nutritional supervision with similar effects [21]. Significantly, FMD has shown equivalent benefits, but with the possibility of being performed without strict supervision or the accompanied mental burden of complete abstinence from food, and with benefits that remain after returning to a normal diet [21]. The beneficial effects of fasting have mainly been seen in patients with high age-related risk factors like inflammatory processes, hypertension, hyperglycemia, and hyperlipidemia, but not in normal individuals.
A by-product of the fasting state, autophagy is an innate, complex, self-healing cellular process activated when the organism needs to save energy due to a scarcity of resources, which potentiates a destruction of defective cellular parts (to re-use them) and optimizes repair and rebuilding of cells once the resources are again available. This process is possibly less effective or dormant in humans due to our constant consumption of food.
In the case of the malignant gliomas, despite the observed benefits of TMZ treatment, a cure for GBM at this point is not realistic; almost all patients suffer recurrence, underlining the importance of augmenting the efficacy of existing treatments and the need for new therapeutic pathways. The metabolic effect of the KD, CR, and STS, reducing blood glucose levels and increasing blood ketones, has been shown to improve survival in animal models with these brain cancers and can significantly enhance the anti-tumor effect of chemotherapies and radiation treatment.
Pre-clinical Models
The available literature investigating the therapeutic benefit of metabolic modulation in malignancy has been steadily growing during the last decade. Studying different kinds of cancers, at least six different laboratories have shown a protective effect in animal models attained with FMD, with reduction in incidence and severity of side effects of chemotherapeutic drugs. Similar protective results have been replicated in three small clinical trials [21, 23, 24]. Most of the trials are focused in proving the safety of and tolerance for the diet during the peri-chemotherapy period.
The effects of STS on tumor growth and efficacy of antineoplastic therapy have been demonstrated in multiple pre-clinical models across a wide range of tumor types, including CNS malignancies. A 2012 study demonstrated that starvation conditions improved sensitivity to chemotherapy in different mammalian cell lines, including glioma and neuroblastoma. More significantly, when fasting was evaluated in murine models with these cancers, fasting alone delayed tumor growth, though a combination with chemotherapy produced a higher and more consistent anti-tumor effect [17]. These findings have been confirmed by other groups, particularly with respect to starvation’s ability to sensitize primary gliomas to chemoradiotherapy [7].
Demonstration of such effects has led investigators to delve into the mechanistic underpinnings by which fasting delays tumor growth and sensitizes tumors to antineoplastic therapy. In vitro studies have demonstrated an additive effect of STS conditions and doxorubicin on DNA damage in tumor cells [17]. These findings have been supported in animal models of both locally advanced and metastatic brain tumors, in which fasting causes reduction of tumor growth in both cases by sensitizing malignant cells and simultaneously protecting normal cells from the toxicity inherent in systemic chemotherapy [25].
Considering the significant, demonstrated effects on metabolic pathways, investigators have attempted to combine such dietary regimens with agents altering host and/or tumor metabolism. STS/CR has been successfully combined with inhibitors of glycolysis as well as with hyperbaric oxygen therapy—HBOT (aimed at minimizing tumor-promoting hypoxia and utilization of glycolysis for energy) [26]. The KD and HBOT prolonged survival in mice with systemic metastatic cancer [27]. In both cases, the combination of a KD/CR and metabolic modulation resulted in synergistic improvements in survival in mice with advanced primary brain tumors.
Evidence in Humans
In the light of these striking findings in animal models, several groups have utilized KD/STS in the treatment of patients with advanced brain tumors. While much of the existing evidence supporting such practices derives from case studies, numerous active clinical trials are aiming to prospectively evaluate the efficacy of a KD/STS on tumor growth and responsiveness to antineoplastic therapies.
The first use of the KD for the treatment of human malignant brain tumors was reported in 1995 by Nebeling and colleagues [28]. She treated two female children diagnosed with unresectable advanced stage brain tumors (stage IV anaplastic astrocytoma and stage III cerebellar astrocytoma) after failure with extensive standard radio/chemotherapy. Both children tolerated the diet quite well for 8 weeks and demonstrated decreases in tumor glucose uptake. Indeed, the diet was associated with a halt in tumor progression, stabilizing the disease over a period of 12 months in one of the patients.
Two additional cases of adults with progressive GBM treated with KD as monotherapy demonstrated stabilization of disease for only a short period of time (4 week and 6 weeks, respectively), suggesting that KD enjoyed limited efficacy as monotherapy in adult patients with aggressive primary brain tumors [29]. However, a 2010 study reported a case of a 65-year-old woman with multifocal GBM, treated with a calorie-restricted KD (600 kcal/day), in addition to standard chemoradiotherapy. The patient tolerated the diet and treatment regimen well for two months, at which time follow-up imaging with MRI and FDG-PET demonstrated a complete radiographic response. Unfortunately, the patient developed a recurrence 10 weeks after stopping the KD and was placed on irinotecan and bevacizumab before subsequently dying of her disease [30]. Still, this study pointed to the value of a KD as part of multimodal therapy in treatment of primary brain tumors.
One larger study has evaluated the safety and tolerability of a KD in patients with recurrent GBM. Of 20 patients enrolled, three discontinued the diet due to an inability to tolerate the regimen, though these patients did not demonstrate tumor progression at the time of diet discontinuation. The author noted a trend toward longer progression-free survival in patients with sustained ketosis as well as an increase response rate in patients who received bevacizumab for treatment of progressive disease [31].
More recently, several prospective clinical trials have been initiated [ERGO (NCT01754350), KETONES (NCT01535911), KGDinGBM (NCT01865162)]. Majority of these studies focus on safety and efficacy of the use of a KD or STS in combination with chemotherapy and/or radiation in treatment of recurrent or treatment-refractory GBM. In addition, one study is actively evaluating the use of KD as an adjunct to first line chemoradiotherapy in treatment of GBM (NCT02046187). Of note, this study initially employs a KD during chemoradiotherapy before transitioning patients onto a modified Atkins diet (MAD) during subsequent chemotherapy, a diet that has been proposed as a more tolerable and practical alternative to the conventional KD [32].
The Challenges
Using fasting and/or specific tailored diets like the KD to strategically induce metabolic changes as part of an anti-cancer regimen holds much promise; however, significant challenges and questions remain:
-
(1)
Optimal targets for blood glucose and ketone levels to obtain optimal therapeutic benefit remain unclear; more data are needed.
-
(2)
It remains unclear which nutritional formula(s) to use for different individuals.
-
(3)
Possible resistance to this concept in the medical community, as current popular belief is to avoid weight loss in cancer patients and perceived threats such us increased fatigue and weakness.
-
(4)
Questions regarding anticipated reduced quality of life by imposing periodic fasting regimen on patients.
-
(5)
Some medications like steroids can make compliance more challenging as they can increase hunger and blood glucose levels.
-
(6)
The beneficial effect seen with STS-induced DSR may be specific to different cancer cell types.
Despite the above caveats, pre-clinical data, indicating increased anti-tumor efficacy and effectiveness of other standard therapies when combined with some form of fasting, indicate further controlled clinical trials are warranted. Specific areas of interest holding potential promise and questions include, but not limited to, the following:
-
(1)
How does KD/STS interact with different therapies and what are the mechanisms of synergistic benefits of this interaction?
-
(2)
Significant anti-inflammatory effects of fasting observed in vivo hold particular potential in reducing side effects of chemotherapy and enhancing efficacy of other anti-cancer treatments and should be explored further.
Current recommendations are based on the understanding that FMD is in an experimental stage as an adjunct therapy in the management of cancer and is not intended to be used as a monotherapy or outside of clinical trials. General considerations or recommendations include administering the FMD concurrently with chemotherapy; for patients with higher caloric needs, a higher caloric FMD (without the caloric restriction) may be indicated; limiting physical activity during the diet period; and returning to baseline bodyweight before a second cycle of FMD.
Future Research
The unique metabolism of malignancy has been demonstrated to be a potentially powerful therapeutic target. The fact that STS, KD, and/or CR simultaneously target multiple aspects of tumor biology, including energy metabolism, angiogenesis, and inflammation, highlights their potential for incorporation into several avenues of future research. For example, can we mimic the effects of some current chemotherapies using metabolic alteration? Can the use of KD/STS mitigate the tumorigenic effects of abnormal metabolic processes underlying brain tumors? Can the neuro-protective actions of metabolic therapies that increase blood ketones help reduce the deleterious side effects of current therapies? Can the use of conventional therapies (i.e., chemotherapy and radiation) be augmented by exploiting aspects of tumor cell metabolism? Answering such questions can help delineate the role of dietary metabolic alteration in treatment of primary brain tumors and how to optimally incorporate such regimens into current treatment algorithms.
In addition to the aforementioned lines of investigation, clinical studies must address four key issues precluding widespread adoption and interest in dietary glycemic modulation in neuro-oncology: (1) the lack of a defined nutritional goal or prescribed “dose” of dietary glycemic modulation, (2) concerns regarding associated weight loss in patients with increased metabolic activity as a result of their malignancy, (3) the lack of clearly defined optimal endpoints for response and efficacy with such metabolic interventions, and (4) the safety and efficacy of combination strategies incorporating glycemic modulation into other antineoplastic treatment regimens. Effectively addressing these issues will likely allay concerns and mitigate skepticism regarding the use of glycemic modulation in neuro-oncology and foster greater interest in larger-scale investigation into its optimal manner of use.
Conclusions
Survival rates of GBM have been improved through various combinations of current standard therapies like surgical resection, chemo- and radiotherapy. Nonetheless, recurrence and rapid progression in adults are the norm, toxic side effects and high rates of resistance to chemotherapy remain problematic, and the uncertainty, expense, and time required in the drug development process highlight the pressing need for viable alternatives.
In vitro studies and some small human case studies incorporating KD, CR, and/or STS have shown significant promise as anti-tumor potentiators, with the concomitant ability to induce a protective response to various toxic treatments in normal, healthy cells. While fasting may provide benefit as a stand-alone option in patients who cannot receive conventional therapy, it is especially exciting for its potential synergistic and enhancing effect on current standard therapies. Preliminary observations show remarkable effects of the fasting state which exploit metabolic vulnerabilities in cancer cells and enhance protective and regenerative characteristics in healthy cells. Further research is needed, but the potential for a significant step forward in our therapeutic strategies is apparent and compelling.
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Olar A, Aldape KD. Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol. 2014;232(2):165–77. https://doi.org/10.1002/path.4282.
Muggeri A, Vago M, Pérez S, Rubio M, González C, Magariños C, et al. A randomized, open-label, two-way crossover, single-dose bioequivalence study of temozolomide 200 mg/m(2) (Dralitem(®) vs Temodal(®) capsules) in patients with primary tumors of the central Nervous system under fasting conditions. Drugs R&D. 2017;17(3):427–34. https://doi.org/10.1007/s40268-017-0199-3.
Ballesta A, Zhou Q, Zhang X, Lv H, Gallo JM. Multiscale design of cell-type-specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors. CPT. 2014;3:e112. https://doi.org/10.1038/psp.2014.9.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. https://doi.org/10.1016/s1470-2045(09)70025-7.
• Laviano A, Rossi Fanelli F. Toxicity in chemotherapy–when less is more. N Engl J Med. 2012;366(24):2319–20. https://doi.org/10.1056/nejmcibr1202395. This comment explains how chemotherapy-induced oxidative stress shrinks the tumor volume and also negatively impacts normal cells; however, if you induce short-term fasting before or after chemotherapy, the fasting state results in a protective differential stress resistance in normal cells that is not seen in cancer cells.
Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105(24):8215–20. https://doi.org/10.1073/pnas.0708100105.
Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS ONE. 2012;7(9):e44603. https://doi.org/10.1371/journal.pone.0044603.
Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Can Res. 2010;70(4):1564–72. https://doi.org/10.1158/0008-5472.can-09-3228.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–33. https://doi.org/10.1126/science.1160809.
•• Strickland M, Stoll EA. Metabolic reprogramming in glioma. Front Cell Dev Biol. 2017;5:43. https://doi.org/10.3389/fcell.2017.00043. This article is a complete review of glioma tumor cells’ metabolism, from their shift into aerobic glycolysis to provide energy, regardless of the availability of oxygen, to other various genetic and mitochondrial defects.
Woolf EC, Scheck AC. The ketogenic diet for the treatment of malignant glioma. J Lipid Res. 2015;56(1):5–10. https://doi.org/10.1194/jlr.R046797.
Woolf EC, Curley KL, Liu Q, Turner GH, Charlton JA, Preul MC, et al. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS ONE. 2015;10(6):e0130357. https://doi.org/10.1371/journal.pone.0130357.
Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122. https://doi.org/10.3389/fnmol.2016.00122.
Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 1914;20(5):433–51.
Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–96. https://doi.org/10.1038/ejcn.2013.116.
Branco A, Ferreira A, Simões R, Novais S, Zehowski C, Cope E, et al. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Investig. 2016;46(3):285–98.
Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2013;4(124):124ra27. https://doi.org/10.1126/scitranslmed.3003293.
Eslami S, Barzgari Z, Saliani N, Saeedi N, Barzegari A. Annual fasting; the early calories restriction for cancer prevention. BioImpacts. 2012;2(4):213–5. https://doi.org/10.5681/bi.2012.028.
Nogueira LM, Lavigne JA, Chandramouli GV, Lui H, Barrett JC, Hursting SD. Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med. 2012;1(2):275–88. https://doi.org/10.1002/cam4.23.
Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–18. https://doi.org/10.1016/j.cell.2015.02.020.
Longo V. The longevity diet: discover the new science behind stem cell activation and regeneration to slow aging, fight disease and optimize weight. Penguin. 2018.
• Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–92. https://doi.org/10.1016/j.cmet.2013.12.008. This manuscript reviews multiple experiments in animals, and reports on humans, to describe the effects of fasting on different metabolic health parameters and aging.
de Groot S, Vreeswijk MPG, Welters MJP, Gravesteijn G, Boei JJWA, Jochems A, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. https://doi.org/10.1186/s12885-015-1663-5.
Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16(1):360. https://doi.org/10.1186/s12885-016-2370-6.
Mathews EH, Liebenberg L. Short-term starvation for cancer control in humans. Exp Gerontol. 2013;48(11):1293. https://doi.org/10.1016/j.exger.2013.08.008.
Marsh J, Mukherjee P, Seyfried TN. Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-d-glucose and the restricted ketogenic diet. Nutr Metab. 2008;5:33. https://doi.org/10.1186/1743-7075-5-33.
Poff AM, Ari C, Seyfried TN, D’Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE. 2013;8(6):e65522. https://doi.org/10.1371/journal.pone.0065522.
Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202–8.
Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3. https://doi.org/10.1186/s40170-015-0129-1.
Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab. 2010;7:33. https://doi.org/10.1186/1743-7075-7-33.
Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, Brucker D, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–52. https://doi.org/10.3892/ijo.2014.2382.
Strowd RE, Cervenka MC, Henry BJ, Kossoff EH, Hartman AL, Blakeley JO. Glycemic modulation in neuro-oncology: experience and future directions using a modified Atkins diet for high-grade brain tumors. Neuro-Oncol Pract. 2015;2(3):127–36. https://doi.org/10.1093/nop/npv010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Carla Venegas-Borsellino, Sonikpreet, and Neal Bhutiani declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Nutrition, Metabolism, and Surgery.
Rights and permissions
About this article
Cite this article
Venegas-Borsellino, C., Sonikpreet & Bhutiani, N. Fasting and its Therapeutic Impact in Brain Tumors. Curr Surg Rep 6, 12 (2018). https://doi.org/10.1007/s40137-018-0208-7
Published:
DOI: https://doi.org/10.1007/s40137-018-0208-7