Abstract
Purpose of Review
Chronic rhinosinusitis (CRS) is a broad term used to describe a wide-ranging clinical entity driven by underlying inflammatory processes within the paranasal sinuses. Until recently, the condition was loosely classified into two phenotypical groups; CRS with and CRS without nasal polyps based on the clinical findings on nasal examination.
Recent Findings
Whilst this simplistic classification continues to hold some relevance in practice, recent evidence has raised the possibility of better defined subgroups, each promising potentially new therapeutic targets. This is beginning to direct both medical and surgical treatment of patients with CRS towards a more personalised approach with better overall outcomes.
Summary
This article will explore the latest evidence relevant to this topic and discuss factors reshaping the landscape of CRS and its management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
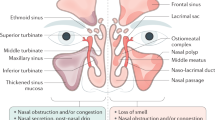
Since the publication of the European Position Paper on Chronic Rhinosinusitis in 2020 [1••], it is now widely acknowledged that CRS may be classified as a primary pathological process of inflammatory disease within the sinuses or secondary to underlying systemic disease or a condition outwith the sinuses and which requires treatment in itself as part of the management of the sinuses (Fig. 1a, b). As illustrated, CRS can also be subdivided into being either localised to one sinonasal compartment or diffusely affecting the whole sinonasal epithelium; localised disease often requires surgical intervention de novo, and recognition of this may help avoid unsuccessful attempts at medical treatment. Thus, it can be seen that the changes to classification of CRS may guide management decisions with the aim of improving outcomes.
a, b EPOS classification of CRS [1••]. Figures reproduced with permission from W. J. Fokkens 2023, copyright 2020 Rhinology
Traditional definitions of primary CRS based on the presence of absence of polyps allows simple dichotomisation of CRS in a wide range of clinical settings, without the need for imaging or investigations beyond visualising the nasal cavity. Inherent in classification systems is the assumption that they will identify groups that share common features; in the case of CRS, it should therefore follow that patients with and without polyps differ in their underlying inflammatory disease processes and therefore their natural history and response to different interventions. However, it is now recognised that these broad phenotypes do not fully account for differences in the underlying pathophysiology and therefore using this dichotomy to guide clinical algorithms [2] may lead to treatment failure.
It is now recognized that identification of the underlying cellular physiological mechanisms, or endotype, will allow better personalisation of treatment, increased response rates, and better outcomes [3]. Through the association between polyp disease and lower airway hypersensitivity and eosinophilia [4] and research in asthma and other atopic diseases [3], findings have been extrapolated to the management of CRS and have led to major breakthroughs in targeted immunotherapies, namely biologics for the treatment of CRS with a type 2 endotype (although currently restricted to patients with CRS with nasal polyps) [5,6,7]. Whilst knowledge on type 2-mediated CRS has gained significant strides, a similar level of understanding of non-type 2-mediated CRS and targeted interventions lags well behind. The dichotomy used in the main treatment algorithms in EPOS 2020 is therefore no longer based on the polyp/non-polyp phenotype of CRS but by the underlying endotype dominance of CRS as being either a result of type 2 inflammation or not [8••].
EPOS Classification
Primary CRS defines an intrinsic inflammatory condition of the respiratory epithelium that is limited to the upper and lower airways. This contrasts with secondary CRS whereby sinonasal inflammation is driven by an underlying systemic disease process such as immunodeficiency (e.g. selective immunoglobulin deficiencies), genetic conditions (e.g. cystic fibrosis) autoimmune conditions (e.g. granulomatosis with polyangitis) or as a concomitant effect of an underlying localised non-inflammatory disease process such as a sinonasal neoplasm or odontogenic sinusitis.
Both primary and secondary CRS can be further defined with regard to the anatomical distribution of affected sinuses into localised and diffuse disease. Localised disease occurs when sinonasal mucosal inflammation is limited to a discrete anatomical unit and typically follow patterns of functional sinus drainage. This includes an isolated sinusitis, which for example commonly occurs in fungal mycetomas (Fig. 2a). More than one sinus may be involved when the ostiomeatal unit is obstructed causing opacification of the maxillary, anterior ethmoid and frontal sinuses on the ipsilateral side, and is commonly seen in odontogenic sinusitis (Fig. 2b). Localised disease often requires surgical intervention; highlighting this within the classification may help avoid delays appropriate management.
a Coronal CT scan demonstrating an isolated right maxillary sinus opacification containing hyperdense material, typical of a fungal mycetoma. b Odontogenic disease with bilateral maxillary and ethmoidal opacification secondary to an oroantral fistula on the right and a dental implant screw exposed within the sinus on the left (author’s image)
Diffuse disease refers to more generalised inflammation of the respiratory epithelium which can affect both the upper and lower airways and typically affects the sinuses on both sides to a varying degree. Where both upper and lower airways are involved, treatment of both in the context of the unified airway concept, which describes how the entire respiratory epithelium is affected by a common underlying pathophysiology, has been shown to achieve better outcomes.
Inflammatory Endotypes
Localised and diffuse disease can be subclassified further based on the underlying pathophysiological patterns of inflammation, again with the aim of understanding the natural disease process and directing treatment decisions. The respiratory epithelium lining the sinonasal mucosa, as well as the pulmonary system, is highly immunogenic, acting as the gatekeeper and protector against both commensal and pathological organisms and pollutants that enter the upper and lower airways. Typical patterns in T-cell mediated immune response have been described as the causal factor in inflammatory disease and are classified into type 1, type 2 and type 3 inflammation (Fig. 3) [9]. Within the context of CRS, type 2 inflammation is most widely understood in the development of nasal polyps as well as associated asthma [8••]. Epithelial dysbiosis triggers the release of thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, IL-1 and IL-33 leading to a differentiation of naive T cells into Th2 effector cells. Activation of these Th2 cells leads to upregulation of type 2 cytokines, notably IL-4, IL-5 and IL-13 and local B-cell immunoglobulin E (IgE). In addition, IL-4, dendritic cells and IL-C2 trigger changes in the IgE isotope and together with IL-13 stimulates fibrin cross linking that leads to the formation of polyps. Finally, IL-5 plays a key role in the recruitment and activation of eosinophils through mast cell degranulation, leading to profound eosinophilia (Fig. 4). Inhibition of these key immune mediators underlies the efficacy of biologic therapies such as dupilumab (anti-IL4 and IL13), benralizumab (anti-IL5R) omalizumab (anti-IgE) which has been proven in phase 3 trials for the treatment of chronic rhinosinusitis with nasal polyps [10,11,12].
Type 1 inflammation results from osteopontin stimulation in dendritic cells in response to an environmental stimulus or pollutant, which in turn induces TH1-cell differentiation and TH17-cell differentiation. This leads to an inflammatory cascade through interferon gamma (IFN-γ), tumour necrosis factor (TNF) and lymphotoxin α release, which ultimately results in neutrophil activation [13].
Type 3 inflammation also results in neutrophil proliferation, which is proposed to occur through selective activation of Th-17 cells inducing high volumes of IL-17A, IL-17F and IL-22 [14]. Studies from CRS patients suggest a combination of type 1 and type 3 inflammatory mechanisms [15]. The role of cytokines IL-6 and IL-8 is key, along with TNF, as they further perpetuate production of IFN-γ leading to cyclical IL-6 and IL-8 release as well as myeloperoxidase and further T-cell differentiation to TH1 cells [16]. The anti-IL-8 properties exhibited by macrolides are responsible for its immune modulating effect in the treatment of CRS [17].
Endotyping in Clinical Practice
There is an incomplete overlap between phenotype (polyp versus non-polyp) and endotype. (type 2 versus non-type 2). Cluster analysis based on a wide range of immune markers demonstrated 10 different clusters, with several demonstrating mixed endotypes. Four clusters were considered non-type 2 and included predominantly patients without nasal polyps or co-morbid asthma with low or undetectable IL-5. Cluster with moderate levels of IL-5 had a mixed with and without nasal polyp phenotype, while those with the highest levels of IL-5 also had greatly increased prevalence of nasal polyps and comorbid asthma [18]. In western populations, more than 85% CRSwNP demonstrate a type 2 predominant endotype [19]. It is estimated that 20–50% of CRSsNP also display a predominantly type 2 endotype [20].
Currently, there is no single biomarker that can be used to characterise individual endotypes with a high degree of sensitivity and specificity. A number of algorithms for the identification of T2 inflammation in CRSwNP without the need for biopsy and tissue analysis have been proposed, based on circulating biomarkers, cellular dominance (notably eosinophil levels), histopathology, cytokine profile and response to therapy [1••, 21,22,23]. These lack consensus with regards to the thresholds applied, particularly with regards to serum eosinophil levels (Table 1). Further complicating application of such criteria is the influence of lower airway disease on circulating levels of inflammatory cells and cytokines and their transient suppression by oral corticosteroids.
It is clear that further work is required to better characterise type 2 inflammation. Potential markers include the following: periostin, a matricellular protein encoded by the POSTN gene, which through systematic review demonstrated significantly higher serum levels and gene expression in those with CRSwNP regardless of underlying endotype, compared to CRSsNP and controls [24]; eosinophil cationic protein, a ribonuclease contained within eosinophils, but also some neutrophils, with potent cytotoxic and profibrotic qualities, which has been shown to be significantly elevated in patient with CRSwNP and was found to be the sole significant factor associated with early post operative polyp recurrence, regardless of atopic history or histological tissue eosinophil counts [25, 26]. It is possible that future practice may rely on several biomarkers to subcategorise patients and predict response to different treatment algorithms.
A number of clinical presentations have been shown to associate with different endotypes and can be applied in daily practice to help identify the underlying endotype. Patients with a type 2 endotype are more likely to have nasal polyps, asthma, loss of sense of smell and have ‘allergic’ mucin [9]. Indeed, if a patient has nasal polyps and comorbid asthma or allergy then nearly 95% display a type 2 predominant endotype, negating the need for any further investigations. In asthma studies, patients with increased markers of type 2 inflammation have been showed to be more responsive to treatment with corticosteroids [27]; similarly, markers of type 2 inflammation in nasal polyps have also been shown to associate with steroid responsiveness [28]. In contrast non-type 2 patients are often older and they do not have the same benefit from the use of corticosteroids [23]. The finding of pus on nasal examination also associates with a non-type 2 endotype [9].
Within daily practice, determining presence of loss of smell, comorbid type 2 diseases (including asthma, allergy, atopic dermatitis, eosinophil oesophagitis) and steroid responsiveness, coupled with a serum eosinophil count, at least 4 weeks after the last dose of systemic corticosteroids, will likely help to classify the majority of patients as type 2/non-type 2 (Fig. 4) [21].
Clinical Relevance and Potential Therapeutic Targets
With the availability of targeted therapies for CRS as described, there is a movement towards advocating early patient endotyping to guide directed treatment based on predicted response and likelihood of success. It is becoming more evident that there are a range of endotype subgroups who display different patterns of disease phenotypes. Between 20 – 50% of patients with CRS without nasal polyps (CRSsNP) display similar type 2 mediated inflammatory responses to CRSwNP, with characteristic tissue eosinophilia with formation of eosinophil extracellular traps (EET) and Charcot-Leyden crystals [20]. They have also been reported to demonstrate reduced quality of life scores and significantly higher rates of asthma and recurrence post-surgery, compared to non-eosinophilic CRSsNP [29]; however, this subgroup still display a more benign natural disease course compared to CRSwNP [30].
It is well recognised that those with a greater eosinophilic burden, that is type 2 disease, respond better to glucocorticoids. In one study, treatment with oral corticosteroids produced significantly greater reductions in polyp size, nasal congestion, total nasal symptom scores and nasal resistance in the eosinophilic predominant group than the neutrophilic group [31]. Adding to this complexity are the nasal polyp patients who conversely do not demonstrate elevated eosinophil counts which raises the possibility of another subgroup expressing non-type 2 inflammation who may demonstrate poor response to corticosteroids and certain biologics. Based on phenotypical features, the assumption is that many patients within these cohorts would have been classed as failing standard treatment or displaying ‘recalcitrant’ disease. Conversely, the indications for biologics in CRS are currently limited to patients with nasal polyps, and may deny the possibility of benefit to those CRSsNP patients with a type 2 endotype, which forms the basis of an active double-blind placebo-controlled RCT evaluating the efficacy of dupilumab in CRS patients without polyps [32].
Some studies have also observed a mixed eosinophilic-neutrophilic inflammatory picture in some CRSwNP patients [33]. This subgroup of patients have been found to display more severe tissue inflammation, with higher Lund-Mackay scores and more pronounced olfactory dysfunction than classic type 2 or type 1 mediated CRS [34]. It has been suggested that this dual-mediated inflammatory picture has potentially occurred in response to the presence of biofilms that may trigger changes in local immune responses in otherwise eosinophilic patients. Sixty-four percent of CRSwNP sufferers reportedly exhibit nasal colonisation with S. aureus (vs 33% and 20% of CRSsNP and healthy control subjects, respectively) [35]. The underlying pathophysiology may lie in S. aureus enterotoxin simulating IL-33 release and inducing eosinophilic migration [36, 37].
The increasing understanding of endotype patterns in predicting polyp recurrence after endoscopic sinus surgery is likely to have significant influence in clinical decision making with regard to appropriateness for surgery, consent and treatment planning and the likelihood of utilising treatment adjuncts to achieve disease control. Evidence that patients with type 2 nasal polyposis are more likely to relapse after surgery than non-type 2 patients is now well documented. Raised total and specific IgE (including S. aureus enterotoxin-specific IgE) and IL-5 in tissue have been shown to predict more severe disease and correlates with the need for revision surgery [18, 38]. Similarly, IL-5 and IL-13 levels in sinonasal mucus were found to correlate with worse objective measures of disease severity and higher rates of revision surgery, obviating the need for tissue sampling to achieve the same purpose, and hence could be used to better assess a patient’s surgical candidacy and suitability from the outset [39]. Whilst such translational research has yet to be borne in practice, in time it is assumed that cytokine profiling will help equip clinicians in selecting the most appropriate biologic for a patient and enabling a tailored protocol based on monitoring cytokine levels during treatment. For now, the current evidence points to tissue eosinophil level as a more sensitive marker of severity and polyp recurrence than the more widespread practice of serum eosinophil count, advocating structured histological assessment [40, 41], as serum eosinophil levels lack both sensitivity and specificity to identify tissue eosinophilia. Whilst the practicalities of tissue sampling and histological analysis of tissue eosinophil levels does not make it an ideal prognostic marker for routine use in patients with mild disease managed medically, a histological specimen is advocated for all patients undergoing surgical intervention to aid prognostication and plan post operative follow up and treatment regimens. A metanalysis looked at correlations between tissue eosinophil counts and polyp recurrence post-surgery, with a cutoff value of > 55 eos/HPF as yielding the highest sensitivity and specificity for predicting recurrence [41]. Furthermore, patients with high tissue eosinophils (> 10/HPF) compared to those with low tissue eosinophil counts (≤ 10/HPF) were found to display better SNOT-22 and endoscopy outcomes with administration of high-volume corticosteroid irrigation in the post-operative setting [42].
With biologics acting on type 2 cytokine inhibition dominating the field, other cellular pathways implicated in CRS are also under review; elevated levels of certain mediators of the arachidonic acid pathway have been studied in nasal polyp tissue, especially in patients with aspirin-exacerbated respiratory disease [43], offering potential for more target therapies such as fibrinolytics and TH-1- and Th-17-mediated cytokines [44].
In contrast, there are currently no endotype-driven therapeutic agents for non-type 2-mediated CRS. Attempts to define the efficacy of macrolide therapy in patients with non-type 2 inflammation based on correlations with tissue neutrophilia have not revealed any significant findings other than a tendency towards a better response in those with low serum and tissue eosinophil counts [17, 45], further reinforcing the dichotomy between eosinophilic and non-eosinophilic CRS.
The Eosinophilic Paradox
Despite an abundance of research, no single biomarker has been identified to define CRS subpopulations and reliably predict disease control. To date, and as above, much focus has been placed on serum and tissue eosinophil counts as surrogate markers in type 2-mediated CRS, and a central role for eosinophils in type 2 inflammation has been assumed.
Similarly, studies investigating the drug demipramipexole (an anti-eosinophilic synthetic aminobenzothiazole) showed that despite depleting eosinophils in both blood and nasal polyps, the effect did not translate into clinical benefit in terms of reduction in nasal polyp size or symptom scores [46]. Pre-treatment serum eosinophilia does not predict the responsiveness with either mepolizumab, dupilumab or omalizumab and highlights that the local effects of systemic therapies such as biologics on nasal mucosa remains to be fully understood. This paradox is further reflected by the relatively poor treatment response to benralizumab in CRSwNP [47], and in a subset of patients treated with mepolizumab, despite the depletive effect on their serum eosinophil levels [48]. A proposed explanation may lie within a possible local negative feedback loop within nasal mucosa, which results in rebound increased IL-5 concentrations. This further begs the question of whether treatment efficacy lies in understanding the pharmacokinetic interplay at the mucosal level. While eosinophil levels remain central to current diagnostic algorithms, it is clear that the search for the perfect biomarker should continue.
Conclusion
Endotyping has driven a rapid evolution in understanding the pathophysiology of CRS and its subtypes. Evidence borne from this is challenging the traditional view of classifying patients based on the presence or absence of nasal polyps and has been instrumental in both delivering new therapeutic targets and opening exciting avenues for further research. As knowledge on type 2-mediated eosinophilic CRS continues to strengthen, other inflammatory pathways implicated in CRS and its subgroups remain poorly understood but are likely to hold the key to novel therapeutic agents, as well as the answers behind non responders. A consequential shift towards personalised management with improved patient outcomes continues to drive research in the field.
Availability of Data and Materials
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. Updated landmark collaborative expert publication summarising evidence-based guidance on Chronic rhinosinusitis.
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists Rhinology. 2012;50(1):1–12.
Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–19.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25.
Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. The concepts of asthma endotypes and phenotypes to guide current and novel treatment strategies. Expert Rev Respir Med. 2018;12(9):733–43.
Chong LY, Piromchai P, Sharp S, Snidvongs K, Webster KE, Philpott C, et al. Biologics for chronic rhinosinusitis. Cochrane ENT Group, editor. Cochrane Database of Systematic Reviews [Internet]. [cited 2022 Nov 28]. 2021;2021(10). Available from: http://doi.wiley.com/10.1002/14651858.CD013513.pub3.
Xu X, Reitsma S, Wang DY, Fokkens WJ. Updates in biologic therapy for chronic rhinosinusitis with nasal polyps and NSAID -exacerbated respiratory disease. Allergy. 2022;77(12):3593–605.
•• Grayson JW, Hopkins C, Mori E, Senior B, Harvey RJ. Contemporary classification of chronic rhinosinusitis beyond polyps vs no polyps: a review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):831–8. Expert evidence- based review detailing the principles behind the changes to the definition and classification of CRS based on endotyping and the relevance to clinical practice.
Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations Between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019;7(8):2812–2820.e3.
Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595–605.
Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(10):1141–53.
Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–50.
Klingler AI, Stevens WW, Tan BK, Peters AT, Poposki JA, Grammer LC, et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol. 2021;147(4):1306–17.
Mucida D, Salek-Ardakani S. Regulation of TH17 cells in the mucosal surfaces. J Allergy Clin Immunol. 2009;123(5):997–1003.
Ahern S, Cervin A. Inflammation and endotyping in chronic rhinosinusitis—a paradigm shift. Medicina. 2019;55(4):95.
Hirschberg A, Kiss M, Kadocsa E, Polyanka H, Szabo K, Razga Z, et al. Different activations of toll-like receptors and antimicrobial peptides in chronic rhinosinusitis with or without nasal polyposis. Eur Arch Otorhinolaryngol. 2016;273(7):1779–88.
Oakley GM, Harvey RJ, Lund VJ. The role of macrolides in chronic rhinosinusitis (CRSsNP and CRSwNP). Curr Allergy Asthma Rep. 2017;17(5):30.
Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449-1456.e4.
Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124(4):318–25.
Delemarre T, Holtappels G, De Ruyck N, Zhang N, Nauwynck H, Bachert C, et al. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: Another relevant endotype. J Allergy Clin Immunol. 2020;146(2):337-343.e6.
Bachert C, Han JK, Wagenmann M, Hosemann W, Lee SE, Backer V, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol. 2021;147(1):29–36.
Agache I, Akdis CA, Akdis M, Canonica GW, Casale T, Chivato T, et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy. 2021;76(1):14–44.
Whats-new-in-GINA-2021_final_V2.pdf [Internet]. [cited 2023 Feb 26]. Available from: https://ginasthma.org/wp-content/uploads/2021/05/Whats-new-in-GINA-2021_final_V2.pdf.
Danielides G, Lygeros S, Kanakis M, Naxakis S. Periostin as a biomarker in chronic rhinosinusitis: A contemporary systematic review. Int Forum Allergy Rhinol. 2022;12(12):1535–50.
Marcucci F, Sensi LG, Migali E, Coniglio G. Eosinophil cationic protein and specific IgE in serum and nasal mucosa of patients with grass-pollen-allergic rhinitis and asthma. Allergy. 2001;56(3):231–6.
Lu PC, Lee TJ, Huang CC, Chang PH, Chen YW, Fu CH. Serum eosinophil cationic protein: a prognostic factor for early postoperative recurrence of nasal polyps. Int Forum Allergy Rhinol. 2021;11(4):766–72.
Busby J, Khoo E, Pfeffer PE, Mansur AH, Heaney LG. The effects of oral corticosteroids on lung function, type-2 biomarkers and patient-reported outcomes in stable asthma: a systematic review and meta-analysis. Respir Med. 2020;173:106156.
Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155(1):126–35.
Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142(1):64–71.
Calus L, Van Bruaene N, Bosteels C, Dejonckheere S, Van Zele T, Holtappels G, et al. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy. 2019;9:30.
Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129(6):1522-1528.e5.
Sanofi. A randomized, double-blind, placebo-controlled, 2-part study to evaluate the efficacy and safety of dupilumab in patients with uncontrolled, chronic rhinosinusitis without nasal polyposis (CRSsNP) [Internet]. clinicaltrials.gov; 2022 May [cited 2022 Nov 28]. Report No.: NCT04678856. Available from: https://clinicaltrials.gov/ct2/show/NCT04678856.
Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–9.
Succar EF, Li P, Ely KA, Chowdhury NI, Chandra RK, Turner JH. Neutrophils are underrecognized contributors to inflammatory burden and quality of life in chronic rhinosinusitis. Allergy. 2020;75(3):713–6.
Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114(4):981–3.
Teufelberger AR, Nordengrün M, Braun H, Maes T, De Grove K, Holtappels G, et al. The IL-33/ST2 axis is crucial in type 2 airway responses induced by Staphylococcus aureus –derived serine protease–like protein D. J Allergy Clin Immunol. 2018;141(2):549-559.e7.
Bachert C, Holtappels G, Merabishvili M, Meyer T, Murr A, Zhang N, et al. Staphylococcus aureus controls interleukin-5 release in upper airway inflammation. J Proteomics. 2018;180:53–60.
Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28(3):192–8.
Turner JH, Li P, Chandra RK. Mucus T helper 2 biomarkers predict chronic rhinosinusitis disease severity and prior surgical intervention. Int Forum Allergy Rhinol. 2018;8(10):1175–83.
Snidvongs K, Lam M, Sacks R, Earls P, Kalish L, Phillips PS, et al. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol. 2012;2(5):376–85.
McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis: Predicting ECRS recurrence. Int Forum Allergy Rhinol. 2018;8(12):1421–9.
Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(5):415–21.
Kowalski ML, Pawliczak R, Wozniak J, Siuda K, Poniatowska M, Iwaszkiewicz J, et al. Differential metabolism of arachidonic acid in nasal polyp epithelial cells cultured from aspirin-sensitive and aspirin-tolerant patients. Am J Respir Crit Care Med. 2000;161(2 Pt 1):391–8.
Shaghayegh G, Cooksley C, Ramezanpour M, Wormald PJ, Psaltis AJ, Vreugde S. Chronic rhinosinusitis, S. aureus biofilm and secreted products, inflammatory responses, and disease severity. Biomedicines. 2022;10(6):1362.
Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long-term, low-dose macrolide administration for chronic sinusitis. Rhinology. 2009;47(1):66–71.
Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–6.
Bachert C, Han JK, Desrosiers MY, Gevaert P, Heffler E, Hopkins C, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immunol. 2022;149(4):1309-1317.e12.
Walter S, Ho J, Alvarado R, Smith G, Croucher DR, Liang S, et al. Mepolizumab decreases tissue eosinophils while increasing type-2 cytokines in eosinophilic chronic rhinosinusitis. Clin Exp Allergy. 2022.
Author information
Authors and Affiliations
Contributions
NH primary manuscript author; CH assisted with literature review, editing the final manuscript and supplying the figures.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haloob, N., Hopkins, C. Moving the Classification of Chronic Rhinosinusitis Away from Polyp/No Polyps. Curr Otorhinolaryngol Rep 11, 414–421 (2023). https://doi.org/10.1007/s40136-023-00488-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-023-00488-9