Abstract
Purpose of Review
To examine and summarize the existing literature surrounding early stereotactic radiosurgery for hearing preservation in the treatment of vestibular schwannoma.
Recent Findings
There is no universally accepted definition of “early” stereotactic radiosurgery or “hearing preservation” within the vestibular schwannoma literature. Most recent studies originate from the University of Pittsburgh, where upfront stereotactic radiosurgery is routinely pursued in patients of all tumor sizes and hearing capabilities. The preliminary data suggests that short-term hearing preservation may be improved when radiating patients with smaller tumors and better hearing when compared to radiating those who present “later” in their disease course. Unfortunately, the existing literature suffers from significant limitations in study design such that proper conclusions about long-term hearing outcomes of “early” stereotactic radiosurgery and its comparison to observation or microsurgery cannot be drawn.
Summary
There is insufficient evidence in the current literature to support early stereotactic radiosurgery for hearing preservation in the treatment of vestibular schwannoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our understanding of the natural history of vestibular schwannoma (VS) and the principles of its management has changed dramatically in recent years. The incidence of VS is rising, which may be due to increased detection or possibly a true biological shift of unknown mechanism [1,2,3,4]. Regardless of the cause, patients on average are now presenting earlier in their disease course with better hearing and smaller tumors. Overall, there has been a recent trend towards favoring observation as the initial treatment of choice over treatment with stereotactic radiosurgery (SRS) or microsurgery [5,6,7]. However, some studies have explored early SRS for hearing preservation, theorizing a healthier cochlea may better tolerate radiation. In this article, we present our review of the existing literature on early SRS for hearing preservation for VS and summarize that it is currently insufficient to support early SRS as a superior treatment for preserving hearing.
Defining Early Stereotactic Radiosurgery
There are few articles in the literature that describe “early” SRS. “Early treatment” has been loosely defined in terms of time to treatment after initial tumor detection, time to treatment after initial consultation, timing relative to tumor size, and timing relative to ipsilateral hearing status. An inherent flaw with a “time to treatment” definition is that it ignores the amount of time the tumor may have existed prior to detection. Thus, a tumor which slowly grew over the course of 25 years but was treated immediately upon detection may be considered “early treatment,” whereas a tumor that was observed for 2 years prior to treatment would not be considered to receive “early treatment.” Therefore, its most sensible to define “early treatment” in terms of the natural history of the disease process, which is clearly difficult to define in VS. For this article, patients who are treated “early” are those with normal hearing or smaller tumors. In this context, treatment is “early” in that it takes place before there is progression in tumor size or deterioration of hearing.
Defining Hearing Preservation
Additionally, there is no standard definition for hearing preservation. Many studies, including all those reviewed in this article, define hearing preservation as remaining an American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) grade A/B or Gardner–Robertson (GR) grade II or better after treatment [8,9,10]. In other words, those patients that maintain serviceable hearing―i.e., the retained ability to derive benefit from a hearing aid, are considered to have their hearing preserved. This creates a simple binary distinction for the purposes of statistical analysis; however, its significant lack of granularity limits its interpretation in most settings. For example, a patient with normal hearing pre-treatment who declines to 50 dB PTA and 50% SDS is considered to have “hearing preserved.” However, a patient who starts at 50 dB PTA and declines to 51 dB PTA is no longer characterized as “hearing preserved.” The matter is further complicated by recent evidence that suggests there are biological differences in VS that cause hearing loss, theorizing that VS secrete ototoxic products that cause direct cochlear injury [11, 12]. It is not known how outcomes may differ between SRS for secreting and non-secreting VS. Two VS patients with (and therefore tumor secretion) and without hearing loss may both classify as GR I and therefore fail to be separated in statistical analysis of hearing preservation outcomes.
Further, the concept of hearing preservation can only be interpreted in the timeframe in which the study is conducted. Very few studies include audiometric data beyond 10 years post-treatment. Therefore, with VS commonly diagnosed around 60 years of age, and the average life expectancy around 80 years of age, there is a dearth of information on long-term hearing preservation outcomes following treatment.
Early Studies and the “Early Treatment” Hypothesis
In 2010, Régis et al. compared the hearing preservation rates of intracanalicular VS with those who pursued observation versus Gamma Knife SRS [13•]. All patients who received SRS had serviceable hearing at treatment initiation, although it is not specified whether they were treated upfront or after a period of observation. In their series, the observation group of 47 patients had a hearing preservation rate of 75%, 52%, and 41% at 3, 4, and 5 years. Further, within the observation group, 16 patients started with GR I hearing. Of these, 11 (69%) patients had no change in hearing during the follow-up period (median 26.3 months). By comparison, the SRS cohort of 34 patients had a hearing preservation rate of 77%, 70%, and 64% at 3, 4, and 5 years. The authors concluded that based on their data there should be a paradigm shift towards offering early Gamma Knife SRS to patients with serviceable hearing and intracanalicular tumors. There are significant limitations to this study, including small sample size, short duration of follow-up, and below average hearing preservation rate during observation when compared to the literature (54% at 5 years, Reznitsky et al.; 66% at 5 years, Hunter et al.) [14, 15]. Perhaps most importantly, those patients who pursued observation without hearing loss at the beginning of the study period had a significantly higher hearing preservation rate than the observation group as a whole. It is not clear that early SRS for these patients carries an advantage in short-term hearing preservation as compared to those patients who observed their VS. Despite these limitations, some centers began to transition towards early SRS.
A unique study by Yomo et al. aimed to compare the rate of hearing decline after Gamma Knife SRS to the natural rate of hearing decline of VS [16]. They specifically addressed the limitation of the binary definition of hearing preservation as “loss of serviceable hearing during the study period” by measuring the annual hearing decrease rate (AHDR) in decibels per year. While this study was not specifically aimed at patients treated with “early” SRS, there were a number of patients included in the analysis that could be considered “early” as they were GR I and/or graded Koos I (intracanalicular tumor) at initiation of treatment. The mean audiological follow-up before and after SRS were 22 and 52 months, respectively. They found a significant difference in mean AHDR in GR Class I patients pre- and post-GKS: −0.57 dB/year (95% CI −2.95 to 1.81 dB/year) and 3.59 dB/year (95% CI 2.52–4.65 dB/year), respectively (p = 0.007). Among patients who had already experienced hearing loss due to their VS, but still had serviceable hearing at initiation of treatment (GR II), there was no significant difference in AHDR: mean AHDR pre-GKS 5.09 dB/year (95% CI 1.36–8.82 dB/year) and post-GKS 4.98 dB/year (95% CI 3.86–6.10 dB/year) (p > 0.05). In grouping all patients, they reported no significant difference in AHDR overall: pre-GKS 5.39 dB/year (95% CI 3.31–7.47 dB/year) and post-GKS 3.77 dB/year (95% CI 3.13–4.40 dB/year) (p > 0.05). The authors concluded that Gamma Knife SRS does not increase the rate of hearing loss within the short term when compared to the natural progression of hearing loss from VS. However, their data indicate that this is not universally true as their patients who started as GR I had a significant increase in the rate of hearing loss following SRS. Unfortunately, individual pre-treatment hearing was not reported, so it is not possible to determine how many GR I patients had normal hearing at treatment initiation. Additionally, there are significant flaws in the calculation of AHDR that limit its interpretability. To start, the pre-treatment AHDR is guaranteed to be overestimated because it is impossible to know exactly when the patients developed a VS. Thus, the “pre-treatment AHDR” does not truly represent the annual rate of hearing decline in untreated VS, but rather the annual rate of hearing decline of VS under active surveillance. The post-treatment AHDR is limited by the short audiometric follow-up period. Despite its limitations, this data suggests that Gamma Knife SRS may increase the rate of hearing loss in patients who are GR I at initiation of treatment.
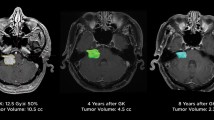
There is limited data on the long-term hearing outcomes of Gamma Knife SRS for patients with serviceable hearing. Watanabe et al. described their experience with up to 15 years of post-treatment audiometric data [17]. Among 66 patients with serviceable hearing and adequate follow-up, they estimated an actuarial hearing preservation rate (defined as maintenance of GR II or higher) of 49%, 24%, and 12% at 5-, 10-, and 15-years post-treatment. Additionally, they found that more advanced age (≥ 65 years), larger tumor volume (≥ 8 cm3), and higher cochlear dose (mean cochlear dose > 4.2 Gy) were unfavorable factors for hearing preservation. Pre-treatment hearing status (GR I versus GR II) did not meet statistical significance as an unfavorable factor for hearing preservation in this model. It is not reported how many patients had no hearing loss prior to treatment initiation, so the application of this model is limited in the context of hearing outcomes for “early” SRS. It remains a possibility that no patient included in this series had normal hearing at the time of treatment. However, assuming “early” patients are included in the sample, given an estimated 88% of patients will lose serviceable hearing at 15 years, it seems unlikely that early Gamma Knife SRS will have a significant protective effect on hearing preservation in the long term.
The University of Pittsburgh Early Treatment Experience
The largest series focused on early Gamma Knife SRS outcomes for VS is from the University of Pittsburgh. In 2016, they presented their hearing outcomes in treating patients with “normal hearing” with Gamma Knife SRS. Akpinar et al. compared hearing outcomes in 88 patients with GR I hearing at treatment initiation [18]. In this study, they defined “early” and “late” treatment based on time to treatment. Patients treated within 2 years of presentation were considered early, and the remainder late. This time frame was selected based on the median duration of observation in their cohort, which was 2 years. Their definition of “early” is thus arbitrary as it is reflective of their cohort but not necessarily of the disease process. For patients who received Gamma Knife SRS within 2 years of diagnosis, the 1-year, 3-year, 5-year, and 10-year rates of GR class I hearing preservation estimated rates were 95%, 89%, 77%, and 51%, respectively. The 1-year, 3-year, 5-year, and 10-year rates of GR class I hearing preservation estimated rates for patients treated after 2 years of observation were 84%, 65%, 33%, and 29%, respectively. The estimated serviceable hearing (GR I/II) preservation rate at 10 years was 64% in the early group and 55% in the late group. However, the late treatment group had a statistically significant difference in pre-treatment PTA (12 vs 17, p = 0.005) and slightly lower speech discrimination score (100% vs 96%, p = 0.57) than the early treatment group, which is a major confounder in this study. Given the difference in pre-treatment hearing, it is impossible to draw accurate conclusions about the effect of timing of treatment. It is a natural outcome that the group with worse pre-treatment hearing met hearing loss thresholds of progressing beyond GR I and II sooner than the better hearing group. Additionally, while this study considers its subjects to be patients with “normal hearing,” they do not delineate between patients with VS and no hearing loss from patients who have started to lose hearing. In the context of early Gamma Knife SRS, their data suggests that hearing preservation in the short term is more favorable in patients with less hearing loss; however, it is indeterminate whether this represents a true benefit of early treatment or simply lead time bias.
In another study from the University of Pittsburgh, Johnson et al. utilized the hearing outcomes data of 307 patients with serviceable hearing (GR I n = 233, GR II n = 74) to create a predictive scoring model for forecasting preservation of serviceable hearing called the Pittsburgh Hearing Preservation Score (PHPS) [19•]. Patients received 1 point if their age was < 45 years, 2 points if their age was 45–59 years, and 3 points if their age was ≥ 60 years. Patients received 1 point if tumor volume was ≥ 1.2 cm3 and 0 points if less. Finally, patients received 0 points for GR I hearing, and 1 point for GR II hearing. This created a 5-point scale with a minimum score of 1. Within their cohort, increasing PHPS score reduced the rate of serviceable hearing preservation. At the extremes, 92% of patients with a PHPS of 1 maintained serviceable hearing at 10 years. In contrast, no patient with a total PHPS score of 5 retained serviceable hearing at 10 years. The authors report a multi-center clinical trial is pending to determine the validity of the PHPS. This data seems to suggest that young patients with better hearing and smaller tumors (i.e. “early treatment”) have favorable hearing preservation outcomes at 10-years. However, this study is limited by its retrospective nature and small sample size at the 10-year mark. Further, we are unable to compare these outcomes to hearing preservation rates with observation or microsurgery. Additionally, being younger than 45 years of age prolongs the amount of time they are at risk for long-term hearing deterioration after Gamma Knife SRS. It is again impossible to determine if the short-term hearing preservation represents treatment benefit or simply lead time bias.
Another Pittsburgh-based study by Ogino et al. described the difference in hearing preservation rate between patients who were GR I at treatment initiation versus patients who have deteriorated to GR II by the initiation of treatment [20•]. In this study, hearing preservation was defined as deterioration to GR III or worse in follow-up. They identified 100 patients who initially presented as GR I, with 67 remaining GR I at GKS initiation, forming the hearing maintenance (HM) group and the remaining 33 deteriorated to GR II, forming the hearing deterioration (HD) group. The median interval between initial diagnosis and SRS was 17.4 months (range, 3.1–190.2 months). Median follow-up after SRS was 4.4 years (range, 0.5–21.0 years). The interval between diagnosis and treatment as well as subsequent follow-up time were not significantly different between HM and HD groups. There was a significant difference in PTA at presentation between the HM and HD group such that the HM group presented with better hearing. There was additionally a larger proportion of intracanalicular tumors in the HM group. The authors reported the serviceable hearing preservation rates of HM patients were 79.9% at 3 years, 63.4% at 5 years, and 51.2% at 10 years. In contrast, the serviceable hearing preservation rates of HD patients were 40.0% at 3 years, 32.7% at 5 years, and 19.6% at 10 years. The authors conclude that initiation of Gamma Knife SRS prior to hearing loss improves long-term maintenance of hearing preservation. Unfortunately, there are several issues with the study design that complicate this conclusion. A more reasonable conclusion from this study is that among patients who will have SRS, initiation prior to hearing deterioration may have short-term benefits to hearing preservation. Additionally, the significant difference in tumor size and PTA at presentation between groups are obvious confounders. We cannot separate the effect of radiation prior to hearing decline from the effect of pre-treatment hearing status and tumor size in this study. There is also significant limitation due to lead time bias—if HM patients are treated earlier than HD patients, they should then require a longer period of follow-up than HD patients to prove the treatment influenced the natural history of the disease process. Instead, the HM and HD groups had no significant difference in follow-up post-treatment. Finally, this study again is limited by lack of long-term follow-up. It is intuitive that in the short term a patient with GR II hearing will progress to GR III faster than a GR I patient by the simple fact that their hearing is poorer. It is not clear from this study that there is a long-term benefit to hearing preservation for Gamma Knife SRS prior to hearing deterioration.
In an attempt to control for tumor size, a separate study examined hearing outcomes after early treatment specifically in patients with intracanalicular tumors [21]. In this series, 120 patients with intracanalicular VS were identified who were GR I or II at treatment initiation. The serviceable hearing preservation rates of GR I patients were 88.1% at 3 years, 77.9% at 5 years, and 38.1% at 10 years, whereas those for GR II were 57.0% at 3 years, 39.7% at 5 years, and 9.6% at 10 years. While this study controls for tumor size, many of the previously mentioned limitations to the other studies still apply. There are very few patients with 10-year data, and even this can be considered short term relative to the patient’s lifespan. Comparing GR I patients to GR II patients is vulnerable to lead time bias. Further, there is no clear distinction among the GR I patients as to whether they had started to experience hearing decline but remained GR I at initiation of treatment. This data highlights that the majority of patients will eventually lose serviceable hearing after SRS regardless of their hearing status and tumor size at the time of intervention.
Conclusion
Based on data from a few centers, short-term hearing preservation is favorable in early Gamma Knife SRS for young patients with small tumors and good hearing. The absence of long-term data leaves uncertainty whether this is a true benefit of early treatment or simply lead time bias. In the absence of a prospectively designed clinical trial, it is not clear if early Gamma Knife SRS has better hearing outcomes than observation or microsurgery. The existing literature has significant limitations in the study designs that preclude meaningful conclusions about the true effect of early Gamma Knife SRS on hearing preservation in VS.
References
Papers of particular interest, published recently, have been highlighted as: • Of major importance
Marinelli JP, Lohse CM, Carlson ML. Incidence of vestibular schwannoma over the past half-century: a population-based study of Olmsted County, Minnesota. Otolaryngology - Head and Neck Surgery (United States). 2018;159:717–23.
Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence of vestibular schwannomas in the United States. J Neurooncol. 2015;124:223–8.
Koo M, Lai JT, Yang EYL, Liu TC, Hwang JH. Incidence of vestibular schwannoma in Taiwan from 2001 to 2012: a population-based national health insurance study. Annals of Otology, Rhinology and Laryngology. 2018;127:694–7.
Marinelli JP, Lohse CM, Grossardt BR, Lane JI, Carlson ML. Rising incidence of sporadic vestibular schwannoma: true biological shift versus simply greater detection. Otol Neurotol. 2020;41:843–7.
Carlson ML, van Gompel JJ, Wiet RM, Tombers NM, Devaiah AK, Lal D, et al. A cross-sectional Survey of the North American Skull Base Society: current practice patterns of vestibular schwannoma evaluation and management in North America. Journal of Neurological Surgery, Part B: Skull Base. 2018;79:289–96.
Marinelli JP, Lees KA, Lohse CM, Driscoll CLW, Neff BA, Link MJ, et al. Natural history of growing sporadic vestibular schwannomas: an argument for continued observation despite documented growth in select cases. Otol Neurotol. 2020;41:e1149–53.
Tan D, Killeen DE, Kutz JW. The natural history of vestibular schwannoma and when to intervene. Curr Otorhinolaryngol Rep [Internet]. 2021;9:134–8. Available from: https://springerlink.bibliotecabuap.elogim.com/10.1007/s40136-021-00337-7.
Committee on Hearing and Equilibrium Guidelines for the Evaluation of Hearing Preservation in Acoustic Neuroma (Vestibular Schwannoma): Committee on Hearing and Equilibrium. Otolaryngology–Head and Neck Surgery [Internet]. 1995;113:179–80. Available from: http://journals.sagepub.com/doi/10.1016/S0194-5998%2895%2970101-X.
Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Annals of Otology, Rhinology & Laryngology [Internet]. 1988;97:55–66. Available from: http://journals.sagepub.com/doi/10.1177/000348948809700110.
Tolisano AM, Hunter JB. Hearing preservation in stereotactic radiosurgery for vestibular schwannoma. Journal of Neurological Surgery, Part B: Skull Base. 2019;80:156–64.
Gan J, Zhang Y, Wu J, Lei D, Zhang F, Zhao H, et al. Current understanding of hearing loss in sporadic vestibular schwannomas: a systematic review. Front Oncol [Internet]. 2021;11. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2021.687201/full.
Celis-Aguilar E, Lassaletta L, Torres-Martín M, Rodrigues FY, Nistal M, Castresana JS, et al. The molecular biology of vestibular schwannomas and its association with hearing loss: a review. Gene Res Intern [Internet]. 2012;2012:1–10. Available from: https://www.hindawi.com/journals/gri/2012/856157/.
• Régis J, Carron R, Park MC, Soumare O, Delsanti C, Thomassin JM, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010;113(Suppl):105–11. This was the first manuscript to suggest early radiosurgery may be beneficial to hearing preservation.
Hunter JB, Dowling EM, Lohse CM, O’Connell BP, Tombers NM, Lees KA, et al. Hearing outcomes in conservatively managed vestibular schwannoma patients with serviceable hearing. Otol Neurotol [Internet]. 2018;39:e704–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30036205.
Reznitsky M, Cayé-Thomasen P. Systematic review of hearing preservation in observed vestibular schwannoma. Journal of Neurological Surgery, Part B: Skull Base. 2019;80:165–8.
Yomo S, Carron R, Thomassin JM, Roche PEH, Régis J. Longitudinal analysis of hearing before and after radiosurgery for vestibular schwannoma. J Neurosurg. 2012;117:877–85.
Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg. 2016;125:64–72.
Akpinar B, Mousavi SH, McDowell MM, Niranjan A, Faraji AH, Flickinger JC, et al. Early radiosurgery improves hearing preservation in vestibular schwannoma patients with normal hearing at the time of diagnosis. Intern J Rad Oncol Biol Phys [Internet]. Elsevier Inc. 2016;95:729–34. Available from: https://doi.org/10.1016/j.ijrobp.2016.01.019.
• Johnson S, Kano H, Faramand A, Niranjan A, Flickinger JC, Dade LL. Predicting hearing outcomes before primary radiosurgery for vestibular schwannomas. J Neurosurg. 2020;133:1235–41. This manuscript is interesting as they provide a scoring rubric to identify patients who might be ideal candidates to receive radio surgery early in their disease course.
• Ogino A, Long H, Johnson S, Faramand A, Niranjan A, Flickinger JC, et al. Useful hearing preservation is improved in vestibular schwannoma patients who undergo stereotactic radiosurgery before further hearing deterioration ensues. J Neuro-Oncol [Internet]. Springer US; 2021;152:559–66. Available from: https://doi.org/10.1007/s11060-021-03726-6. This paper indirectly suggests that patients who receive radiosurgery prior to hearing deterioration may have short term benefits to hearing preservation.
Ogino A, Lunsford LD, Long H, Johnson S, Faramand A, Niranjan A, et al. Stereotactic radiosurgery as the first-line treatment for intracanalicular vestibular schwannomas. J Neurosurg. 2021;135:1051–7.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Otology
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, D., Hunter, J.B. Early Gamma Knife Radiosurgery for Hearing Preservation in Vestibular Schwannoma. Curr Otorhinolaryngol Rep 10, 365–369 (2022). https://doi.org/10.1007/s40136-022-00423-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-022-00423-4