Abstract
Purpose of Review
Treatment for alopecia remains limited in terms of medication side effect profile, patient adherence to treatment, and clinical response. We sought to review the literature for burgeoning therapies affecting hair growth through regulation of paracrine signaling and its effect on dermal papilla cells.
Recent Findings
Newly proposed treatments for alopecia, including stem cell therapy derived from adipose tissue, hair follicles, umbilical cord blood, or bone marrow, and extracellular vesicles, such as exosomes, are tied to hair follicle regulation and regeneration through paracrine factor signaling, specifically through the Wnt/β-catenin signaling pathway.
Summary
Recent advances in hair follicle regeneration and regulation, including stem cell therapy or treatment with exosomes, modulate alopecia through dermal papilla cell regulation and promoting hair follicle growth through anagen phase induction. Randomized, high-quality studies are needed to determine safety, efficacy, and appropriate treatment protocols using these newest therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hair loss poses significant psychosocial sequelae in both men and women. The desire to pursue prevention and treatment is evident in the global valuation of the alopecia market, which was valued above $9.08 billion in 2019 and is expected to reach over $13.65 billion by 2027 [1]. Androgenetic alopecia (AGA) represents a vast majority of hair loss cases, affecting around 50 million men and 30 million women in the USA alone [2].
The most common types of non-scarring alopecia include involutional alopecia, that which occurs with age, AGA, telogen effluvium, and alopecia areata (AA). AGA, otherwise known as male or female pattern hair loss, is a hereditary condition causing progressive thinning of the hair secondary to increased androgen receptors and 5-alpha reductase resulting in diminution/miniaturization of dermal papilla cells (DPCs), which are mesenchymal stem cells of the hair follicle and play a major role in hair follicle morphogenesis and regeneration. A defect in conversion from stem cell to progenitor cell phenotype may play a role in patients suffering from AGA, as the amount of hair follicle stem cells remains stable while the number of proliferating progenitor cells decreases [3]. Telogen effluvium can be acute or chronic and most commonly secondary to acute severe illness, surgery, iron deficiency, thyroid pathology, malnutrition, chronic disease, and medications, such as oral contraceptives and lithium 4. AA is autoimmune inflammatory in etiology and most often results in non-scarring patches of hair loss, but may be diffuse in nature.
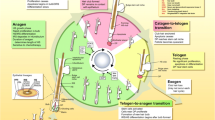
Hair follicles each have their own lifecycle divided into three phases occurring simultaneously: anagen, catagen, and telogen. The anagen phase is the active phase, during which time the cells within the hair bulb divide rapidly resulting in hair growth, which lasts 2–6 years, and 85% of hair on the head is in this phase. The catagen phase is a transitional phase that lasts 2–3 weeks wherein hair growth halts, as its blood supply is disconnected. The telogen phase is a resting phase lasting 2–3 months during which time the hair sheds as new hair replaces it within 2 weeks. Hair is in telogen for 10–15% of one’s life.
When evaluating patients with alopecia, it is important to obtain from the patient a thorough family and medical history, medication list, hair styling practices, hormonal imbalance history, menstrual cycle history, and menopausal symptoms. Depending upon history and presenting symptoms, laboratory work-up may be warranted, including hormonal profile, thyroid function testing, iron panels, vitamin D, and possibly skin biopsy to rule out underlying pathology as a cause for alopecia, such as polycystic ovarian syndrome, thyroid disease, or scarring alopecia.
Only two FDA-approved medications for hair loss exist: minoxidil and finasteride. These medications can be administered either topically or orally; however, both therapies may result in significant adverse effects, which may limit use or adherence to therapy. Although non-pharmacologic therapies, such as platelet-rich plasma and low-level laser therapy, may prove beneficial in the treatment of hair loss, the quality of evidence to support the use of these treatments is considered to be generally low [4, 5].
Emerging injectable therapies, such as stem cells and exosomes, are proposed to stimulate hair follicle regeneration and growth through the activation of specific signaling pathways, such as Wnt-mediating signaling. Herein, we will review these recent advances in hair restoration therapy and their proposed mechanism of action through paracrine signaling.
Importance of Wnt-Mediated Signaling in Hair Growth
The Wnt signaling pathway is a primary player in the regulation of hair morphogenesis, cycling, and regeneration, promoting hair follicle growth by advancing the hair follicle from telogen to anagen phase and increasing hair-related and anagen gene expression [6, 7, 8••, 9–14, 14•, 16–19]. Various Wnt proteins promote hair cycling and regeneration through the activation of β-catenin signaling, thereby inducing anagen and new hair follicles [8••, 17, 20–23]. Wnt-mediated signaling also plays a significant role in the maintenance and proliferation of stem cell reservoirs; Wnt/β-catenin signaling is paramount to the growth and maintenance of DPCs [10, 24, 25].
Circulating androgens have been proposed to inhibit canonical Wnt-β-catenin pathway causing hair loss in AGA [26]. Downregulated genes in AGA belong to that of the Wnt and TGF-β signaling pathways, further implicating the importance of the Wnt signaling pathway in alopecia [27]. Furthermore, the aging process causes a gradual loss of sex hormones, by which the hair follicle is negatively impacted. This is perhaps best realized in menopausal females, wherein substantial decreases in hair density and diameter are seen, as is decreased anagen phase and transition to greater amounts of finer vellus hair, likely secondary to lack of ovarian estrogen production [28–30]. Likewise, when treating osteoporosis in menopausal women, the primary therapies induce osteoblast differentiation from bone marrow stem cells via Wnt/ β-catenin signaling, an element necessitating further consideration when evaluating response of alopecia in menopausal women during treatment with these therapies [31, 32].
Newly proposed treatments for alopecia, including stem cell therapy and exosomes, are tied to hair follicle regulation and regeneration through paracrine factor signaling, specifically affecting the Wnt/β-catenin pathway, and may prove to be exciting treatment options for patients with alopecia.
Stem Cell Therapy
Stem cells secrete molecules, such as nucleic acids, extracellular vesicles, and proteins, which play a role in paracrine factor signaling, thereby regulating hair follicle cycles and regeneration [15•, 33, 34]. Stem cells may be derived from adipose tissue, bone marrow, hair follicles, or umbilical cord blood [19, 35–38]. Patients who underwent one treatment of intradermal injection of autologous stem cells, either from follicular or bone marrow-derived stem cells, demonstrated significant improvement in both AA and AGA [36]. Furthermore, stem cells derived from hair follicles have been shown to increase hair density in patients with AGA [37].
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells (MSCs) found in subcutaneous adipose tissue. MSC-derived signaling and growth factors stimulate hair follicle development through β-catenin 39. ADSCs increase proliferation of DPCs and have been shown to decrease healing time in transplant-induced wounds, shorten telogen phase, and improve hair growth following hair transplantation [35, 39,40,41,42]. When combined with microneedling, ADSCs increased both hair density and thickness in women [43]. Response to ADSCs may be augmented by its surrounding environment. For example, when placed in conditioned medium (CM), a nutrient-rich medium with signaling molecules including nucleic acids, extracellular vesicles, and proteins from stem cells, ADSCs have been shown to improve hair growth and hair numbers in both men and women, increase anagen hair rate and human follicular cell proliferation, improve hair growth, and protect human DPCs against cytotoxic injury by androgen and reactive oxygen species [44–50]. Additionally, when combined with nappage mesotherapy, multiple treatments with ADSC-CM demonstrated increased hair numbers without reported complications [46]. Similarly, adipose-derived stromal vascular cells demonstrated improvement in hair thickness in 19/20 patients and increase in hair density and decrease in hair-pull test scores in 18/20 patients, while adipose-derived regenerative cells increased mean hair counts following injection into the subcutaneous scalp in patients with AGA [51, 52].
Bu et al. through the use of CK15 expression, demonstrated that hair follicle cells can be differentiated from umbilical cord blood MSCs [53]. Human umbilical cord blood–derived MSCs prevent hair regression resulting from dexamethasone in mouse catagen induction models and increase proliferation of human DPCs [54].
Although reported side effects have been minimal when using stem cells, with the exception of procedural pain affecting patient compliance, CM preparation and contents vary widely, and degradation of CM factors may require both frequent administration and large quantities for effect thereby limiting clinical application [47, 55, 56].
Exosome Therapy
Exosomes are 30–150-nm extracellular vesicles responsible for transmission of transcription factors, cytokines, mRNA, and microRNA [57–60]. Exosomes transport Wnt proteins, which induce activation of β-catenin signaling pathways [7, 13, 14, 61]. Studies have shown that exosomes promote hair follicle stem cell proliferation and differentiation and cell migration and angiogenesis and aid in tissue repair [9, 19, 62–65].
Exosomes derived from MSCs increase proliferation, migration, and growth factor expression and release in DPCs66 and, thus, have been evaluated for their role in hair follicle regeneration and growth. MSC-derived extracellular vesicles both activate DPC hair inductivity and regulate DPC proliferation and have been shown to convert hair follicles from telogen to anagen phase [61, 64, 66].
DPC-derived exosomes may augment hair follicle regeneration through regulation of hair follicle growth via paracrine mechanisms [64, 67, 68]. DPC-derived exosomes regulate growth and development of hair follicles through proliferation of DPCs, hair matrix cells, and outer root sheath cells and increase growth factors in DPCs [67, 69, 70]. Additionally, DPC-derived exosomes have also been shown to prolong anagen phase and increase hair shaft elongation [67, 69, 70]. For example, human DP exosomes, when injected into mouse skin, promote hair growth through an induction of β-catenin and Shh levels [67].
Although there are no published clinical trials evaluating exosomes in hair restoration, exosomes have been shown to stimulate hair follicle proliferation with an increased hair density and thickness in patients with AGA after 12 weeks of treatment without reported serious adverse reactions [71]. Although no significant adverse events are cited, there are potential risks of transferring genetic information and immune responses [72–74]. See Figs. 1, 2, and 3 for clinical examples of patients treated with exosomes.
Gene Engineering
Wnt protein expression can be modified to positively impact the hair cycle. CM derived from gene-engineered retroviral-mediated Wnt1a-overexpressing bone marrow MSCs has been shown to result in hair regrowth through effect on DPCs when injected intradermally, while Wnt7a-MSC-CM induces more hair follicle regeneration when compared to MSC-CM, further demonstrating the importance of Wnt signaling in hair growth [19, 75, 76].
Choi et al. introduced genes of three trichogenic platelet-derived growth factor-A, SOX2, and β-catenin to ADSCs and demonstrated that these ADSCs with trichogenic factors were similar to DPCs in terms of mRNA expression and have enhanced hair-regenerative potential, as they accelerate the telogen to anagen transition [77].
Conclusion
Recent advances in hair follicle regeneration and regulation, including stem cell therapy or treatment with exosomes, modulate alopecia through DPC regulation and promoting hair follicle growth through anagen phase induction. Wnt-mediating signaling seems to play an important role in the response of DPCs to stem cell and exosomal therapies. Burgeoning therapies using stem cells or exosomes for alopecia still require randomized, double-blinded, high-quality human studies with adequate power to determine safety, efficacy, and appropriate treatment protocols. Further data collection is also needed to ensure appropriate preparation and administration of product and gather information pertaining to side effects and expected treatment response in patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global Alopecia Market is Forecast to Deliver a CAGR of 5.1% Between 2019 and 2027. Available at: https://www.globenewswire.com/en/news-release/2020/12/09/2142566/28124/en/Global-Alopecia-Market-is-Forecast-to-Deliver-a-CAGR-of-5-1-Between-2019-and-2027.html. Accessed June 15 2021.
Androgenetic alopecia. Available at: https://medlineplus.gov/genetics/condition/androgenetic-alopecia/#frequency. Accessed June 15 2021.
Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22.
Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144–51.
Gupta AK, Mays RR, Dotzert MS, Versteeg SG, Shear NH, Piguet V. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112–25.
Mounsey AL, Reed SW. Diagnosing and treating hair loss. Am Fam Physician. 2009;80:356–62.
Dey-Rao R, Sinha AA. Genome-wide gene expression dataset used to identify potential therapeutic targets in androgenetic alopecia. Data Brief. 2017;13:85–7.
•• Choi BY. Targeting Wnt/beta-catenin Pathway for developing therapies for hair loss. Int J Mol Sci 2020;21. Important article summarizing the importance of Wnt/beta-catenin signaling in hair loss.
Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20.
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45.
Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53.
Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–24.
Li J, Ji L, Chen J, Zhang W, Ye Z. Wnt/beta-catenin signaling pathway in skin carcinogenesis and therapy. Biomed Res Int 2015;2015:964842.
Reddy S, Andl T, Bagasra A, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82.
• Yuan AR, Bian Q, Gao JQ. Current advances in stem cell-based therapies for hair regeneration. Eur J Pharmacol 2020;881:173197. Summary of stem cell-based therapies in the treatment of alopecia.
Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–5.
Li YH, Zhang K, Yang K, et al. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J Invest Dermatol. 2013;133:42–8.
Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin Exp Dermatol. 2011;36:534–40.
Dong L, Hao H, Xia L, et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci Rep. 2014;4:5432.
Ohnemus U, Uenalan M, Conrad F, et al. Hair cycle control by estrogens: catagen induction via estrogen receptor (ER)-alpha is checked by ER beta signaling. Endocrinology. 2005;146:1214–25.
Narhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135:1019–28.
Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–99.
Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14.
Xiong Y, Liu Y, Song Z, Hao F, Yang X. Identification of Wnt/beta-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J Dermatol. 2014;41:84–91.
Tsai SY, Sennett R, Rezza A, et al. Wnt/beta-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol. 2014;385:179–88.
Leiros GJ, Attorresi AI, Balana ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/beta-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035–42.
Premanand A, Rajkumari BR. In silico analysis of gene expression data from bald frontal and haired occipital scalp to identify candidate genes in male androgenetic alopecia. Arch Dermatol Res. 2019;311:815–24.
Pierard-Franchimont C, Pierard GE. Alterations in hair follicle dynamics in women. Biomed Res Int 2013;2013:957432.
Mirmirani P. Managing hair loss in midlife women. Maturitas. 2013;74:119–22.
Mirmirani P. Hormonal changes in menopause: do they contribute to a “midlife hair crisis” in women? Br J Dermatol. 2011;165(Suppl 3):7–11.
Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–32.
Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66:1139–46.
Beer L MM, Ankersmit HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Annals of Translational Medicine 2017;5.
Vizoso FJEN, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852.
Zanzottera F LE, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. Journal of Cosmetics, Dermatological Sciences and Applications 2014;4:268–274.
Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat. 2018;29:431–40.
Gentile P, Cole JP, Cole MAet al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci 2017;18.
Yoo BY, Shin YH, Yoon HH, Seo YK, Song KY, Park JK. Application of mesenchymal stem cells derived from bone marrow and umbilical cord in human hair multiplication. J Dermatol Sci. 2010;60:74–83.
Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells 2019;8.
Won CH, Yoo HG, Kwon OS, et al. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci. 2010;57:134–7.
Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34.
Choi H CE, Yoon S, et al. Effect of exosomes from human adipose-derived stem cells on hair growth. J Extracell Vesicles 2019;8:PF08.02.
Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol. 2015;54:730–5.
Kim HO CS-M, Kim H-S. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Engineering and Regenerative Medicine 2013;10:93–101.
Egger A, Tomic-Canic M, Tosti A. Advances in stem cell-based therapy for hair loss. CellR4 Repair Replace Regen Reprogram 2020;8.
Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12:531–4.
Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty 2015;15:e10.
Fukuoka HSH, Narita K, Watanabe R, Shintani S. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. Am J Cosmet Surg. 2012;29:273–82.
Narita K, Fukuoka H, Sekiyama T, Suga H, Harii K. Sequential scalp assessment in hair regeneration therapy using an adipose-derived stem cell-conditioned medium. Dermatol Surg. 2020;46:819–25.
Won CH, Park GH, Wu X, et al. The basic mechanism of hair growth stimulation by adipose-derived stem cells and their secretory factors. Curr Stem Cell Res Ther. 2017;12:535–43.
Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9:141.
Perez-Meza D, Ziering C, Sforza M, Krishnan G, Ball E, Daniels E. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017;10:1–10.
Bu ZY, Wu LM, Yu XH, Zhong JB, Yang P, Chen J. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303–7.
Bak DH, Lee E, Choi MJ, et al. Protective effects of human umbilical cord bloodderived mesenchymal stem cells against dexamethasoneinduced apoptotic cell death in hair follicles. Int J Mol Med. 2020;45:556–68.
Teixeira FG, Carvalho MM, Panchalingam KM, et al. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s disease. Stem Cells Transl Med. 2017;6:634–46.
Khosravi A, Cutler CM, Kelly MH, et al. Determination of the elimination half-life of fibroblast growth factor-23. J Clin Endocrinol Metab. 2007;92:2374–7.
Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888–93.
Liu Y, Wang H, Wang J. Exosomes as a novel pathway for regulating development and diseases of the skin. Biomed Rep. 2018;8:207–14.
Maguire G. Stem cell therapy without the cells. Commun Integr Biol 2013;6:e26631.
Liu R, Liu J, Ji X, Liu Y. Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases. Metab Brain Dis. 2013;28:551–62.
Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–45.
Carrasco E, Calvo MI, Blazquez-Castro A, et al. Photoactivation of ROS production in situ transiently activates cell proliferation in mouse skin and in the hair follicle stem cell niche promoting hair growth and wound healing. J Invest Dermatol. 2015;135:2611–22.
Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122:448–54.
Taghiabadi E, Nilforoushzadeh MA, Aghdami N. Maintaining hair inductivity in human dermal papilla cells: a review of effective methods. Skin Pharmacol Physiol. 2020;33:280–92.
Yan H, Gao Y, Ding Q, et al. Exosomal micro RNAs derived from dermal papilla cells mediate hair follicle stem cell proliferation and differentiation. Int J Biol Sci. 2019;15:1368–82.
Rajendran RL, Gangadaran P, Bak SS, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560.
Zhou M, Hu M, He S, et al. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit. 2018;24:7841–9.
le Riche A, Aberdam E, Marchand L, et al. Extracellular vesicles from activated dermal fibroblasts stimulate hair follicle growth through dermal papilla-secreted norrin. Stem Cells. 2019;37:1166–75.
Chen Y, Huang J, Chen R, et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454–78.
Kwack MH, Seo CH, Gangadaran P, et al. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp Dermatol. 2019;28:854–7.
Huh C-H KS. Exosome for hair regnereation: from bench to bedside. J Am Acad Dermatol 2019.
Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–94.
Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191:5515–23.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Dong L, Hao H, Liu J, et al. A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int. 2017;2017:3738071.
Dong L, Hao H, Liu J, et al. Wnt1a maintains characteristics of dermal papilla cells that induce mouse hair regeneration in a 3D preculture system. J Tissue Eng Regen Med. 2017;11:1479–89.
Choi N, Choi J, Kim JH, et al. Generation of trichogenic adipose-derived stem cells by expression of three factors. J Dermatol Sci. 2018;92:18–29.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on FACIAL PLASTICS: Facial Skin Rejuvenation
Rights and permissions
About this article
Cite this article
Krane, N.A., Christofides, E.A. & Halaas, Y. Advances in Hair Restoration. Curr Otorhinolaryngol Rep 9, 436–441 (2021). https://doi.org/10.1007/s40136-021-00368-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-021-00368-0