Abstract
Purpose of Review
The aim of this review is to present the current literature on pathophysiology, evaluation, and management of laryngeal dystonia.
Recent Findings
Recent evidence suggests loss of cortical inhibition, and sensory dysfunction plays an important role in the pathophysiology of laryngeal dystonia. New treatments addressing these changes include electrical stimulation for neuromodulation of the larynx, vibrotactile therapy, and sodium oxybate. Preliminary investigations are promising and these may impact the future of care for laryngeal dystonia patients.
Summary
The current literature emphasizes a new understanding of the pathophysiology of laryngeal dystonia which has led to investigation of novel therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laryngeal dystonia (LD), also known as spasmodic dysphonia, is a task-specific focal movement disorder primarily effecting voice production [1]. The dystonic movements of the vocal folds result in a varied phenomenology, typically hard vocal breaks and strain in the adductor-type laryngeal dystonia (ADLD), and breathy breaks or aphonia in the abductor-type laryngeal dystonia (ABLD). More than 80% of patients have suffered from ADLD [2]. By comparison, 17% of patients have suffered from ABLD [1]. In addition to these two main types of LD, there are also uncommon forms such as mixed adductor/abductor laryngeal dystonia, singer’s dystonia, and adductor respiratory dystonia.
The disease was first described 150 years ago and recognized as a psychogenic origin disorder. In 1980 Moore’s high-speed laryngeal imaging confirmed the vocal breaks in ADLD were due to irregular contractions of the vocal fold adductor muscles [3, 4].
Laryngeal dystonia is listed as a rare disease by the National Institutes of Health with an incidence of 1–4/100,000 primarily affecting women (2.5:1) [5, 6]. The average age of onset is 30–50 years old.
While a causative relationship has not been established, many environmental factors associated with the disease have been identified. Twenty-one percent of patients chronologically associate the onset of symptoms with a significantly stressful emotional event. It has been hypothesized this stress has a disease triggering neuroplastic effects on the brain. A recent upper respiratory tract infection is also one of the trigger events observed in 30% of patients [7]. Sixty-five percent of LD patients previously had measles or mumps in a survey study, compared with a national average of 15% at that time [7]. These findings raise the question of the role of viral infections in causing or triggering LD. While there is not a direct connection between viral infections and LD, it is well known they can cause significant neurologic insult and lead to peripheral and central nervous system disorders such as progressive multifocal leukoencephalopathy, subacute sclerosing panencephalitis, and multiple sclerosis.
Pathogenesis
The precise pathophysiology of LD is still unclear. The first report suggesting a neurological origin of the disease was in 1960 showing abnormalities on electroencephalography in the temporal area of patients with LD [8]. This was further confirmed by Dedo who demonstrated improvement of the voice after RLN sectioning [9]. As the abnormal motor activity was thought to be the cause of symptoms, further treatment efforts were targeted at the motor dysfunction. In 1988, this led to the treatment of LD by injections of botulinum toxin (BTX) into the laryngeal muscles which showed improvement in the voice [1]. Botulinum toxin remains the primary treatment for LD today. Recent studies confirm that LD is somatosensory disorder resulting from a neurologic network dysfunction along the pathways connecting the cortex, thalamus, basal ganglia, and cerebellum [10•]. These disparate abnormalities may be the reason for some of the differences in patients’ phenotypical presentations and may present opportunities for new treatments.
Neurologic Pathophysiology
Neural Network Dysfunction
The knowledge of the pathophysiology of LD has been evolving quickly over the last decade. Present evidence suggests dystonia as a functional network disorder [11]. Multiple structural abnormalities that underlie the speech sensorimotor network have been identified as possible contributors to the pathophysiology in patients with LD compared with healthy subjects. These structural changes exist in both white and gray matter in focal dystonias [12••, 13,14,15,16,17,18,19,20]. Gray matter anomalies in LD patients relative to healthy controls have been reported to include bilateral primary sensorimotor and premotor cortex, superior/medium temporal, supramarginal, inferior frontal gyri, inferior parietal lobule, insula, putamen, thalamus, and cerebellum whereas alterations in white matter include the genu of the internal capsule, inferior frontal gyrus and associative pathways, lentiform nucleus, thalamus, and cerebellum. In a recent study, Bianchi et al. analyzed phenotypes and genotype-specific structural differences in large samples of ADLD, ABLD, and sporadic versus familial LD using high-resolution MRI and diffusion-weighted imaging [12••]. Using these modalities, they showed that evaluation of structural abnormalities alone allows for the differentiation of ADLD from ABLD and sporadic from familial LD.
Moreover, it has been shown that abnormal functional connectivity within the sensorimotor and frontoparietal networks exists in LD patients relative to normals. Evaluation of these alterations allows for differentiation of LD patients from normal subjects with 71% accuracy. It also allows for discrimination of adductor from abductor types of LD with 71% accuracy [21, 22••].
These studies emphasizing the varying structural changes with these specific network dysfunctions could be used as a biological diagnostic tool for phenotype-based characterization of disease pathophysiology [22••].
It is possible that the structural changes related to LD are the result of neural network dysfunction [23••]. It remains unclear if these structural changes represent regional abnormalities or abnormal hubs of a large-scale structural dystonic network. A very recent study has tried to answer this pathophysiologic question examining inter-regional white matter connectivity of the whole-brain structural network in writer’s cramp and laryngeal dystonia, compared with healthy individuals [23••]. They discovered connectomes in both types of focal dystonia were defined by a series of unique changes of hubs and nodes. The most prominent regional abnormalities in the LD structural network were the supplementary motor area known to regulate the planning, initiation, and selection of actions during speech production [24,25,26]. As a result, this study offers new evidence that LD and other focal dystonias are network disorders at both the structural and functional levels.
Loss of Cortical Inhibition
Loss of cortical inhibition appears to occur in motor and sensory systems in dystonia patients. Transcranial magnetic stimulation can be used to evaluate cortical inhibition by measuring the cortical silent period (CSP) [27]. Compared with patients with muscle tension dysphonia and healthy controls, decreased CSPs in the masseter and first dorsal interosseous muscle have been shown in ADLD patients [28]. Shortened CSP indicates less cortical inhibition in phenotypically unaffected muscles. Decreased CSP is not specific to LD; it has been shown in other focal dystonias like cervical dystonia [29]. These changes in CSP show an association between the loss of cortical inhibition and focal dystonia. Yet, it is unclear if there is a causative relationship. It could be speculated to be a predisposing factor for the disease rather than a cause [10•]. The presence of a shorter CSP in unaffected muscles suggests less cortical inhibition suggests a global, GABA dysfunction [6]. This is further supported by the phenomenon of the alcohol responsive focal dystonia patients. Alcohol consumption, which is an indirect GABA agonist, has been shown to improve voice in more than 50% of LD patients [30]. Although the mechanism by how alcohol consumption improves the symptoms of LD is not yet known, it is postulated to be due to this modulation of GABAergic transmission [30].
Somatosensory Dysfunction
Evidence of somatosensory abnormalities is seen both in the central nervous system (CNS) and the periphery. The earliest suggestion of this peripheral proprioceptive dysfunction was in 1995 and was thought to be due to abnormal muscle spindle function [7]. This proprioceptive dysfunction is not limited to the area affected by focal dystonia but is global, including in LD [31,32,33]. Konczak et al. showed LD patients have impaired limb proprioception, relatively to healthy controls [31]. There are also abnormalities of tactile and visual temporal discrimination in focal dystonia [34, 35].
There is also radiologic evidence of abnormalities in cortical sensory areas. In a functional MRI study of ADLD patients, a positive correlation between symptom severity (i.e., number of voice breaks) and increased activation intensity in the left primary somatosensory cortex was shown [36]. A H215O PET study showed speech-related cortical blood flows in heteromodal sensory areas decreased significantly in people with ADLD relative to volunteers. After either unilateral or bilateral BTX injection, the blood flow in patients increases in unimodal and heteromodal sensory areas regions (left dorsal postcentral, left posterior supramarginal, left posterior middle temporal gyri) regardless of the injection side [37]. These positive changes correlate with clinical improvement. In the same study, there were changes in motor-associated regions too; however, these regions (left anterior cingulate, left dorsal precentral gyrus) are not typically associated with control of laryngeal muscles but oro-motor control [37].
Further support of somatosensory dysfunction in LD comes from the response to treatment from BTX or peripheral stimulation. The target of both therapies, whose manipulations result in symptomatic improvement, appears to be due to treatment of proprioceptive dysfunction, not motor dysfunction. Studies show direct effects on the muscle spindle as well as normalization of cortical sensory organization and function that parallel symptomatic improvement [37,38,39,40, 41•, 42].
Genetic
While 12% of LD patients have a family history of dystonia, a specific gene for LD has not been identified. Dystonia has had more than 20 genes associated with it but the genes shown to be LD-related are limited to TOR1A (DYT1), THAP1 (DYT6), and TUBB4A (DYT4) and GNAL (DYT25) [22••]. These genes mostly cause generalized or segmental familial dystonia associated with LD. THAP1 mutation is linked to various focal dystonias, including LD. Mutations in TUBB4A are linked to the autosomal dominant form of oro-lingual dystonia with a rare type of LD, whispering LD [22••, 43]. Mutations in the GNAL gene have been associated with cervical or cranio-cervical segmental dystonia including LD. A carrier mutation in this gene has been found in a patient with isolated ADLD [44, 45]. Not only are the mutations in specific genes linked with dystonia but polymorphisms also play a role. While mutations of the TOR1A gene are responsible for early onset segmental dystonia that rarely involves laryngeal dystonia, polymorphisms in the same gene have been associated with adult onset, primarily focal dystonia, including LD and even a decreased risk of developing dystonia [46,47,48].
Genotypic specific structural changes have also been identified in the extra-Sylvian regions and their connecting pathways. These findings suggest a possible role of the temporal lobe in pathophysiology of this subtype of LD [12••].
Although evidence of a clear link between specific genes and larynx-involving dystonia has been shown, the diagnostic and prognostic utility of genetic screening in clinical settings is still not clinically impactful [6]. These genotype-specific changes, however, can provide an important step toward future description of imaging markers and potential targets for new spasmodic dysphonia diagnostics and therapeutic interventions.
Treatment
Botulinum toxin has been used for the treatment of LD since 1988 [2, 49]. Since then, BTX injection of laryngeal musculature has been recognized as the gold standard treatment for LD. However, there are many undesirable side effects including breathy dysphonia and dysphagia. The procedures are unpleasant and need to be repeated approximately every 3 months. Optimal voicing is only achieved during 30% of each injection cycle due to the delayed onset of BTX effects and return of symptoms prior to repeated injection [50]. Due to these shortcomings, physicians continue to research new treatments.
Surgical Treatment
The first attempt at surgical treatment was described by Dedo in 1976. He was inspired by improvement in his patients’ symptoms after recurrent laryngeal nerve (RLN) block [9]. This led to him performing RLN section for ADLD. However, the long-term results for RLN sectioning have not been promising with a 64% failure rate. Despite this, the concept of mechanically preventing excessive glottal closure or inhibiting abnormal motor signals from reaching the laryngeal muscles has remained the goal of newer surgical treatments and BTX injections [51].
In 1999, selective laryngeal adductor denervation-reinnervation (SLAD-R) surgery was described for ADLD with 90% success rate in 3-year follow-up [52]. Their 7-year follow-up study reveals that while about 80% of the patients have decreased symptoms, 20% of the patients had unsatisfactory results with moderate to severe breathiness [52]. Despite the relative success, only few laryngologists routinely perform this technique for treatment of their LD patients.
Another surgical technique was reported by Isshiki et al. in 2000, midline lateralization thyroplasty (form II thyroplasty), for ADLD [53]. Type II thyroplasty is intended to prevent the spasmodic overclosure of the glottis during phonation. Substantial failure rates have been shown in the literature and may be associated with technical difficulty. Recently, the authors who described the technique have shared modifications of it [54]. New studies are needed for more conclusive results.
Novel techniques of surgical treatment have not been limited to the transcervical approach. Su et al. described the transoral laser thyroarytenoid (TA) myoneurectomy in 2007 [55]. This surgery targets the end organ of ADLD by removing the muscle, terminal nerves, and neuromuscular junction of the thyroarytenoids. In an attempt to prevent muscle compensation causing a recurrence of symptoms, the surgeon resects most of the TA muscle, potentially resulting in a long-lasting effect [55]. While 92% of patients had benefit from the surgery in the original study, voice deterioration was observed in 45% of patients during follow-up after initial good short-term outcomes. A stable voice outcome was only achieved in 55% of patients after 12 months [56•]. No worsening of the symptoms or complications was reported in the study. TA myoneurectomy may be a potential treatment for ADLD, but long-term results and the outcome of revision TA myoneurectomy surgeries should be evaluated.
Although novel surgical techniques mostly focus on for ADLD, there are several surgical treatments suggested for ABLD as well. Surgical techniques proposed for ABLD are unilateral type 1 thyroplasty, bilateral medialization laryngoplasty, PCA myoplasty with medialization thyroplasty, and endoscopic partial posterior cricoarytenoid myectomy [57,58,59,60,61,62,63]. None of the surgical techniques has been widely accepted by laryngologists at this time. Botulinum toxin injection stills remain as the standard treatment for ABLD.
Novel Treatment Options
Multiple new treatments have been studied over the last few years. These novel approaches are either intended to cure the disease or aid in more effective treatment. These treatments can be divided into two main categories: those treating at the CNS or the end organ at the larynx.
Central Nervous System
Deep Brain Stimulation
Deep brain stimulation (DBS) is performed by surgically implanting a device into the brainstem that delivers electrical stimulation to modulate the neuronal circuits. Revolutionized in the last 30 years, DBS has been and effective treatment for severe movement disorders [64]. The FDA has approved it for Parkinson’s disease and essential tremor. It is approved for dystonia but only with special permission [65]. There have been several anecdotal reports indicating that basal ganglia DBS improves patients’ LD symptoms [66]. In these cases, the patients have confounding symptoms such as essential tremor, focal dystonia, or local dystonia. A clinical trial named Thalamic Deep Brain Stimulation for Spasmodic Dysphonia (DEBUSSY Trial) was recently launched; however, no report has been released [67]. If the findings are positive, such a trial could result in the incorporation of DBS as a treatment option for LD.
Pharmacological Treatment with Sodium Oxybate
Traditional pharmacological intervention for dystonia has been considered unsuccessful for task-specific dystonia. Recent investigations have focus on sodium oxybate, as alcohol consumption has long been known to improve with the symptoms of more than 50% patients with LD [30].
Sodium oxybate (Xyrem ®), the sodium salt of gamma hydroxybutyric acid (GHB), mimics some of the effects of alcohol. Sodium oxybate is quickly absorbed when ingested orally, crosses the blood-brain barrier, and transforms into GABA within the brain. Sodium oxybate has FDA approval for cataplexy and severe daytime narcolepsy sleepiness. In a recent open-label clinical trial of sodium oxybate for patients with alcohol-responsive LD with or without tremor, 82% had an improvement in their symptoms [68••]. The medicine’s effect started in less than 40 min and continued for approximately 3.5 h. Almost half of the patients experienced mild lightheadedness in the first hour after administration. A new randomized placebo-controlled double-blind clinical trial has recently been launched which is expected to be completed in August 2022. (ClinicalTrials.gov Identifier: NCT03292458) [69]. If the trial ends with encouraging results, patients with alcohol-responsive LD may have a therapy that can function alone as need or in conjunction with BTX injection.
Laryngeal Treatments
Vibrotactile Stimulation of the Larynx
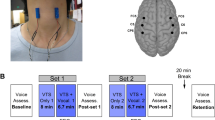
Abnormal proprioceptive muscle spindle activity of non-dystonic limbs has been observed in LD and other types of focal dystonia such as blepharospasm and cervical dystonia [70]. This suggests that somatosensory dysfunction could be a target for disease treatment, much like sensory tricks are found to relieve symptoms in patients with focal dystonia [70]. A recent study has shown that a one-time 40-min application of non-invasive laryngeal vibrotactile stimulation (VTS) resulted in a significant improvement in symptoms in 69% of patients with carryover effect lasting for at least 20 min after VTS was discontinued. This improvement was accompanied by positive changes in the somatosensory region of the motor cortex [71••]. Following these promising results, a new clinical trial was lunched to provide scientific evidence for assessing the appropriate dose of VTS therapy for effective improvement of voice symptoms in LD (ClinicalTrials.gov Identifier: NCT03746509) [72]. Successfully completing the planned study will be a significant step toward promoting laryngeal VTS as a therapeutic intervention. Theoretically, the developed neck collar could be worn to apply the treatment as need, resulting in a vocal “boost” for a meeting or phone call or social function.
Laryngeal Neuromodulation with Electrical Stimulation
Electrical stimulation of the larynx has been studied as a method of neuromodulation, treating the muscle spindle and associated proprioceptive and somatosensory abnormalities in LD. Previously, electrical stimulation was investigated for the treatment of focal dystonia in patients with writer’s cramp disease. Patients treated with transcutaneous electrical stimulation have significant improvement of their symptoms compared with placebo. The positive carry over effect persisted for 3 weeks after the treatment ended [73]. Similar results have also been shown in electrical stimulation for cervical dystonia [42]. In 2014, this was investigated in the larynx for ADLD [74••]. In this study, electrical stimulation was delivered to the left thyroarytenoid muscle by a hooked electrode. The stimulation was at below the level of motor neuron activation and was performed 1 h per day for five consecutive days. Outcome measures, including spasm counts, patient-reported outcomes, and the blinded evaluation by a speech language pathologist, showed significant patient improvement. In four of the five patients, improvement lasted 3 to 14 days after stimulation was discontinued. At present, a second and larger study is underway. If viable, an implanted electrical stimulator would allow for intermittent treatment as needed, by the patient instead of the physician. They would activate the stimulator when their voice began to deteriorate, allowing the patient to maintain a stable vocal improvement compared with the peaks and valleys of BTX injections.
Conclusions
The loss of sensorimotor inhibition and neural network abnormalities is crucial to the pathophysiology of LD and to the direction of future therapies. Studies focused on further elucidating these structural and functional abnormalities are essential to enhancing our understanding of the disease. Improved insight will allow for the development of novel treatments, some already under investigation, that will better address the needs of patients suffering from LD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108:1435–41.
Blitzer A, Brin MF, Fahn S, Lovelace RE. Localized injections of botulinum toxin for the treatment of focal laryngeal dystonia (spastic dysphonia). Laryngoscope. 1988;98:193–7.
Kaye R, Blitzer A. Chemodenervation of the larynx. Toxins (Basel). 2017;9(11):356. https://doi.org/10.3390/toxins9110356.
Murry T. Spasmodic dysphonia: let’s look at that again. J Voice. 2014;28:694–9.
Butler AG, Duffey PO, Hawthorne MR, Barnes MP. An epidemiologic survey of dystonia within the entire population of northeast England over the past nine years. Adv Neurol. 2004;94:95–9.
Hintze JM, Ludlow CL, Bansberg SF, Adler CH, Lott DG. Spasmodic dysphonia: a review. Part 1: pathogenic factors. Otolaryngol Head Neck Surg. 2017;157:551–7.
Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–3.
Robe E, Brumlik J, Moore P. A study of spastic dysphonia. Neurologic and electroencephalographic abnormalities. Laryngoscope. 1960;70:219–45.
Dedo HH. Recurrent laryngeal nerve section for spastic dysphonia. Ann Otol Rhinol Laryngol. 1976;85:451–9.
• Lungu C, Ozelius L, Standaert D, Hallett M, Sieber BA, Swanson-Fisher C, et al. Defining research priorities in dystonia. Neurology. 2020;94:526–37 A recent review from pioneering dystonia scientists discussing the future direction of research.
Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex. Oxford University Press. 2015;27:bhv313.
•• Bianchi S, Battistella G, Huddleston H, Scharf R, Fleysher L, Rumbach AF, et al. Phenotype-and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord. Wiley Online Library; 2017;32:560–8. It is one of the first studies to show association of structual changes in the CNS to LD.
Bianchi S, Fuertinger S, Huddleston H, Frucht SJ, Simonyan K. Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord. 2019;34:555–63.
Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Arch Neurol. 2009;66:502–8.
Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80.
Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–9.
Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, et al. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage. 2011;54:32–41.
Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord. 2014;29:1141–50.
Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–59.
Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22:417–25.
Kirke DN, Battistella G, Kumar V, Rubien-Thomas E, Choy M, Rumbach A, et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav. 2017;11:166–75.
•• Blitzer A, Brin MF, Simonyan K, Ozelius LJ, Frucht SJ. Phenomenology, genetics, and CNS network abnormalities in laryngeal dystonia: a 30-year experience. Laryngoscope. Wiley Online Library; 2018;128:S1–9. Comprehensive review of clinic and pathophysiological research studies from a cohort of 1400 LD patients.
•• Hanekamp S, Simonyan K. The large-scale structural connectome of task-specific focal dystonia. Hum Brain Mapp. 2020. https://doi.org/10.1002/hbm.25012Recommended paper for better understanding abnormal structural and functional neural network aspect of LD pathophsiology.
Fuertinger S, Horwitz B, Simonyan K. The functional connectome of speech control. PLoS Biol. 2015;13:1–31.
Bonini F, Burle B, Lieǵeois-Chauvel C, Reǵis J, Chauvel P, Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–91.
Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59:2860–70.
Chen M, Summers RLS, Prudente CN, Goding GS, Samargia-Grivette S, Ludlow CL, et al. Transcranial magnetic stimulation and functional magnet resonance imaging evaluation of adductor spasmodic dysphonia during phonation. Brain Stimul Elsevier BV. 2020;13:908–15.
Samargia S, Schmidt R, Kimberley TJ. Cortical silent period reveals differences between adductor spasmodic dysphonia and muscle tension dysphonia. Neurorehabil Neural Repair. 2016;30:221–32.
Pirio RS. Enhanced dorsal premotor-motor inhibition in cervical dystonia. Clin Neurophysiol. 2015;126:1387–91.
Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015;262:1548–56.
Konczak J, Aman JE, Chen YW, Li KY, Watson PJ. Impaired limb proprioception in adults with spasmodic dysphonia. J Voice. 2015;29:777.e17–23.
Yoneda Y, Rome S, Sagar HJ, Grünewald RA. Abnormal perception of the tonic vibration reflex in idiopathic focal dystonia. Eur J Neurol. 2000;7:529–33.
Grünewald RA, Yoneda Y, Shipman JM, Sagar HJ. Idiopathic focal dystonia: a disorder of muscle spindle afferent processing. Brain. 1997;120:2179–85.
Conte A, Rocchi L, Ferrazzano G, Leodori G, Bologna M, Li Voti P, et al. Primary somatosensory cortical plasticity and tactile temporal discrimination in focal hand dystonia. Clin Neurophysiol. 2014;125:537–43.
Termsarasab P, Ramdhani RA, Battistella G, Rubien-Thomas E, Choy M, Farwell IM, et al. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. NeuroImage Clin The Authors. 2016;10:18–26.
Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20:2749–59.
Ali SO, Thomassen M, Schulz GM, Hosey LA, Varga M, Ludlow CL, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–46.
Rosenkranz K, Butler K, Williamon A, Rothwell JC. Regaining motor control in musician’s dystonia by restoring sensorimotor organization. J Neurosci. 2009;29:14627–36.
Tinazzi M, Zarattini S, Valeriani M, Stanzani C, Moretto G, Smania N, et al. Effects of transcutaneous electrical nerve stimulation on motor cortex excitability in writer’s cramp: neurophysiological and clinical correlations. Mov Disord. 2006;21:1908–13.
Trompetto C, Currà A, Buccolieri A, Suppa A, Abbruzzese G, Berardelli A. Botulinum toxin changes intrafusal feedback in dystonia: a study with the tonic vibration reflex. Mov Disord. 2006;21:777–82.
• Rosales RL, Arimura K, Takenaga S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin-A injection. Muscle Nerve. 1996;19:488–96 This paper suggests BTX mitigates the symptoms of dystonia via treatment of the intrafusal fibers of the muscle spindle.
Leis AA, Dimitrijevic MR, Delapasse JS, Sharkey PC. Modification of cervical dystonia by selective sensory stimulation. J Neurol Sci. 1992;110:79–89.
Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. Ovid Technologies (Wolters Kluwer Health). 2010;74:229–38.
Fuchs T, Saunders-Pullman R, Masuho I, San Luciano M, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet Nature Publishing Group. 2013;45:88–92.
Putzel GG, Fuchs T, Battistella G, Rubien-Thomas E, Frucht SJ, Blitzer A, et al. GNAL mutation in isolated laryngeal dystonia. Mov Disord Wiley Online Library. 2016;31:750–5.
Clarimon J, Asgeirsson H, Singleton A, Jakobsson F, Hjaltason H, Hardy J, et al. Torsin A haplotype predisposes to idiopathic dystonia. Ann Neurol Wiley. 2005;57:765–7.
Hague S, Klaffke S, Clarimon J, Hemmer B, Singleton A, Kupsch A, et al. Lack of association with TorsinA haplotype in German patients with sporadic dystonia. Neurology. Ovid Technologies (Wolters Kluwer Health). 2006;66:951–2.
Sharma N, Franco RA, Kuster JK, Mitchell AA, Fuchs T, Saunders-Pullman R, et al. Genetic evidence for an association of the TOR1A locus with segmental/focal dystonia. Mov Disord Wiley. 2010;25:2183–7.
Blitzer A, Lovelace RE, Brin MF, Fahn S, Fink ME. Electromyographic findings in focal laryngeal dystonia (spastic dysphonia). Ann Otol Rhinol Laryngol. 1985;94:591–4.
Novakovic D, Waters HH, D’Elia JB, Blitzer A. Botulinum toxin treatment of adductor spasmodic dysphonia: longitudinal functional outcomes. Laryngoscope. 2011;121:606–12.
Aronson AE, De Santo LW. Adductor spastic dysphonia: three years after recurrent laryngeal nerve resection. Laryngoscope. 1983;93:1–8.
Berke GS, Blackwell KE, Gerratt BR, Verneil A, Jackson KS, Sercarz JA. Selective laryngeal adductor denervation-reinnervation: a new surgical treatment for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108:227–31.
Isshiki N, Tsuji DH, Yamamoto Y, Iizuka Y. Midline lateralization thyroplasty for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109:187–93.
Matsushima K, Isshiki N, Tanabe M, Yoshizaki N, Otsu K, Fukuo A, et al. Operative procedure of anterior commissure for type II thyroplasty. J Voice Elsevier BV. 2018;32:374–80.
Su CY, Chuang HC, Tsai SS, Chiu JF. Transoral approach to laser thyroarytenoid myoneurectomy for treatment of adductor spasmodic dysphonia: short-term results. Ann Otol Rhinol Laryngol. 2007;116:11–8.
• Schuering JHC, Heijnen BJ, Sjögren E V, Langeveld APM. Adductor spasmodic dysphonia: Botulinum toxin a injections or laser thyroarytenoid myoneurectomy? A comparison from the patient perspective. Laryngoscope. Wiley; 2020;130:741–6. First comparative study between the gold standard BTX therapy and surgergical intervention for ADLD.
Koufman JA. Management of abductor spasmodic dysphonia by endoscopic partial posterior cricoarytenoid (PCA) myectomy. Phonoscope-San Diego. Singular Publishing Group Inc; 1999;2:159–66.
Dewan K, Berke GS. Bilateral vocal fold medialization: a treatment for abductor spasmodic dysphonia. J Voice. Elsevier BV. 2019;33:45–8.
Ludlow CL, Naunton RF, Terada S, Anderson BJ. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngol Head Neck Surg. 1991;104:849–55.
Eller RL, Miller M, Weinstein J, Sataloff RT. The innervation of the posterior cricoarytenoid muscle: exploring clinical possibilities. J Voice. 2009;23:229–34.
Benito DA, Ferster APO, Sataloff RT. Bilateral posterior cricoarytenoid myoneurectomy for abductor spasmodic dysphonia. J Voice. Elsevier BV. 2020;34:127–9.
Shaw GY, Sechtem PR, Rideout B. Posterior cricoarytenoid myoplasty with medialization thyroplasty in the management of refractory abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2003;112:303–6.
Postma GN, Blalock PD, Koufman JA. Bilateral medialization laryngoplasty. Laryngoscope. 1998;108:1429–34.
Bari AA, Thum J, Babayan D, Lozano AM. Current and expected advances in deep brain stimulation for movement disorders. Prog Neurol Surg. 2018;33:222–9.
Dougherty DD. Deep brain stimulation. Psychiatr Clin North Am Elsevier BV. 2018;41:385–94.
Lyons MK, Boucher OK, Evidente VGH. Spasmodic dysphonia and thalamic deep brain stimulation: long-term observations, possible neurophysiologic mechanism and comparison of unilateral versus bilateral stimulation. J Neurol Neurophysiol. 2010;1:106. https://doi.org/10.4172/2155-9562.1000106.
NCT02558634 @ clinicaltrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT02558634. Accessed 5/10/2020.
•• Rumbach AF, Blitzer A, Frucht SJ, Simonyan K. An open-label study of sodium oxybate in spasmodic dysphonia. Laryngoscope. 2017;127:1402–7 First trial of a promising therapeutic for alcohol responsive LD patients.
NCT03292458 @ clinicaltrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03292458. Accessed 5/10/2020.
Cakmur R, Donmez B, Uzunel F, Aydin H, Kesken S. Evidence of widespread impairment of motor cortical inhibition in focal dystonia: a transcranial magnetic stimulation study in patients with blepharospasm and cervical dystonia. Adv Neurol. 2004;94:37–44.
•• Khosravani S, Mahnan A, Yeh IL, Aman JE, Watson PJ, Zhang Y, et al. Laryngeal vibration as a non-invasive neuromodulation therapy for spasmodic dysphonia. Sci Rep. 2019;9:17955 This study shows non-invasive laryngeal vibrotactile stimulation improving symptoms of ADLD by positive changes in the somatosensory region of the motor cortex.
NCT03746509 @ clinicaltrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03746509. Accessed 5/10/2020.
Tinazzi M, Farina S, Bhatia K, Fiaschi A, Moretto G, Bertolasi L, et al. TENS for the treatment of writer’s cramp dystonia: a randomized, placebo-controlled study. Neurology. Ovid Technologies (Wolters Kluwer Health). 2005;64:1946–8.
•• Pitman MJ. Treatment of spasmodic dysphonia with a neuromodulating electrical implant. Laryngoscope. Wiley; 2014;124:2537–43. First study showing a positive effect of neuromodulation with electrical stimulation on laryngeal dystonia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Necati Enver declares no conflict of interest.
Michael J. Pitman has a royalty and patent interest with MedEl in the use of electrical stimulation for laryngeal and focal dystonia.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Neurolaryngology
Rights and permissions
About this article
Cite this article
Enver, N., Pitman, M.J. What Is New in Laryngeal Dystonia: Review of Novel Findings of Pathophysiology and Novel Treatment Options. Curr Otorhinolaryngol Rep 8, 209–215 (2020). https://doi.org/10.1007/s40136-020-00301-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-020-00301-x