Abstract
Purpose of Review
Autoimmune encephalitis (AE) is an underrecognized and potentially curable disease, which has been the focus of intense neurologic research. In the present manuscript, we review recent updates and the current role of brain positron emission tomography imaging with 18F-fluorodeoxyglucose (FDG-PET) in the detection of AE. We appraise the many metabolic imaging manifestations described in this disease, the role of PET-FDG in its diagnosis and follow-up, and the possible relationship between some patterns and specific autoantibodies. We also briefly discuss recently recognized imaging patterns and the potential impact of new technologies in recognition of such metabolic imaging appearances.
Recent Findings
AE findings on FDG-PET may have various patterns, but three are dominant and can be summarized as follows: (1) hypermetabolism in cortical areas, mainly in mesial temporal regions and less frequently in basal ganglia and higher cortical regions, is a common pattern in early stages of the disease. Such pattern is highly suggestive of limbic AE, since it has not been described in many other entities, except for brain tumors and active epileptic foci. (2) Also common is a reduced metabolism in the regions described above, which could happen both in the detection of the disease or in previous hypermetabolic areas which changed their pattern during the course of illness. (3) Other areas with hypometabolism can also occur, especially the “diffuse whole-brain cortical hypometabolism” manifestation, which is unspecific and can have degenerative diseases and other conditions as differential diagnoses. Some antibodies are more related to specific metabolic imaging patterns, but others do not correlate closely with imaging appearances.
Summary
We consider that FDG-PET imaging can aid in the early diagnosis of AE and may also be helpful while accessing the disease longitudinally while showing functional changes that occur after therapy. In both situations it can provide valuable information that is not provided by anatomic imaging alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune encephalitis (AE) or encephalopathy is a condition caused by the production of antibodies against synaptic proteins, central nervous system (CNS) membrane antigens (cell surface antibodies), or intracellular neuronal antigens related to paraneoplastic syndromes [1, 2•]. In the last several years, AE has been progressively recognized as an underdiagnosed condition. Early diagnosis of AE is crucial as it may be a potentially reversible cause of encephalopathy and dementia [1, 3].
AE usually presents with acute symptoms such as altered sensorium, cognitive impairment, behavioral changes, or epilepsy and it typically has a fast progressive or fluctuating course. Treatment is based on immunomodulation and provides total or partial relapse of the symptoms in a considerable percentage of cases. Exceptions are AE of a paraneoplastic origin, in which additional treatment consists of the removal of the primary tumor [1, 4]. Diagnosis of AE is confirmed by immunoassays and isolation of specific antibodies in the plasma or cerebrospinal fluid (CSF) through different methods [5, 6•]. In many cases, however, the condition is detected at a late stage, which contributes to worse prognosis [7]. Additionally, in some cases with less acute presentations, autoantibodies are not always found.
Since very few cases fit in the classic “limbic encephalitis” syndrome (altered sensorium, seizures, rapid cognitive decline, altered mood and personality) and considering the spectrum of symptoms, it is often difficult to differentiate AE from other syndromes, such as status epilepticus of other etiologies, in which corticotherapy would be contraindicated [8, 9]. AE is also among the differential diagnoses of rapidly progressive dementia [1, 10]. In such cases, imaging methods such as magnetic resonance imaging (MRI) and positron emission tomography with [18F]fluorodeoxyglucose (FDG-PET) can be especially helpful for the diagnosis.
Most imaging physicians, however, are not aware of the potential clinical use of FDG-PET in this condition. It is of high importance for radiologists and nuclear medicine physicians to recognize the specific imaging patterns related to this condition and additional uncommon patterns, which would eventually help referring physicians in the differential diagnosis, including investigating the presence of these specific antibodies. FDG-PET may be of a particular help in the diagnosis of AE, since glucose uptake increases in active inflammation and is seen more easily in FDG-PET than in cerebral blood flow SPECT or anatomic MRI imaging [4].
Technical and Clinical Development
The first studies evaluating AE with both MRI and FDG-PET reported findings that restricted to mesial temporal lobes (hypometabolism and abnormal hypersignal in T2-weighted images). Recent reports now recognize this condition as having a more diverse spectrum of manifestations, but diffuse global cortical hypometabolism has been suggested to be the most common FDG-PET finding. The second most common pattern would be focal hypometabolism or hypermetabolism in mesial temporal lobes, when limbic encephalitis and seizures are present [11], with hypermetabolism being more specific to AE [12]. Nevertheless, subsequent reports on FDG-PET abnormalities in more diverse areas shed light on the low specificity of some patterns such as “global cortical hypometabolism.” Rapidly progressive causes of dementia, such as Creutzfeldt–Jakob, Lewy body disease, or even other conditions such as severe Alzheimer’s disease, can have similar imaging patterns and clinical findings to AE [10, 12]. Therefore, we consider that a larger comprehension on the different FDG-PET imaging patterns of AE is still lacking. As we will describe below, a variable spectrum of findings can be seen, including impairment of deep gray matter structures.
When it comes to technical developments, the introduction to the clinical routine of different quantitative techniques such as 3D-stereotactic surface projection may have a substantial impact on the recognition of new imaging manifestations of AE in FDG-PET imaging. Such kinds of software also provide databases of subjects with “normal” brain metabolism for comparison. Focal hypermetabolism, for instance, may be hard to be correctly identified by nonexpert physicians. In some cases, global cortical hypometabolism may mimic hypermetabolism in primary sensory-motor cortices (such as paracentral gyri, visual and auditory cortices). This kind of pitfall can occur mostly due to normalization problems [13].
Another remarkable development is the clinical introduction of hybrid integrated PET/MRI machines, which offers the possibility of unifying positive attributes of both methods. Although a comprehensive investigation of AE with this modality has not been performed yet, some functional or metabolic MR sequences (i.e., spectroscopy, diffusion-weighted imaging, or perfusion) may provide additional information beyond the regular anatomic registration of both modalities separately. PET/MRI has been already used in a number of other neuropsychiatric conditions and could be useful to understand AE physiopathology better [14, 15].
Disease Detection
FDG-PET apparently increases the sensitivity of the traditional workup with MRI in the detection of AE [16], with a typical pattern of hypometabolism or hypermetabolism in mesial temporal regions, but also with extra-limbic abnormalities. Nevertheless, brain functional or structural damage in other areas (sometimes even with a reversion of the findings) have been described [2•, 4]. These extra-limbic findings could help to identify the extent of brain damage caused by the disease and could correlate with clinical symptoms [4, 6•, 12, 17]. Some correlation between different antibodies and specific metabolic imaging patterns is seen [2•, 5]. Also, approximately 17 autoantibodies that could cause AE are known to date [3]. In the next paragraphs, we will summarize the most relevant and recent reports of the literature regarding FDG-PET imaging and AE. We tried, whenever possible, to describe the most common imaging patterns for the most common entities and their variations (Table 1).
Anti-NMDA
The most common subtype of AE is the NMDA receptor-related. NMDA receptors (N-methyl-d-aspartate, a family of l-glutamate receptors) are ionotropic neuronal glutamatergic receptors present in higher rates in the hippocampi and amygdalae [18]. Young females are most commonly affected, and the condition may or may not be related to neoplastic states [19, 20]. Among them, ovarian and testicular teratomas are the most prevalent, but their relation with Hodgkin’s lymphoma, small cell lung carcinoma, and neuroblastoma has already been drawn [19–22].
Prodromal signs and symptoms include low-grade fever, headaches, and fatigue, with subsequent psychiatric disturbs such as agitation, psychosis, central hypoventilation, hallucinations, and memory impairment [19, 20]. In later phases, movement disorders such as orofacial dyskinesia, dystonia, autonomic instability, bradykinesia, dysarthria, seizures, and decreases in awareness and arousal can occur [19, 20]. Acute symptoms are mostly related to an increase of free synaptic glutamate (an excitatory neurotransmitter), which is secondary to the occupation of receptors due to anti-NMDA binding.
Immunotherapy and tumor cytoreduction can lead to the improvement of the symptoms in up to 80 % of the cases, due to the reversion of the receptor/antibody binding [19, 20]. Correlation between antibodies levels and prognostic suggests its central role in the pathogenesis of this condition and the usually favorable response to immunotherapy [19]. Antibodies immunoassays in blood or CSF can be used to confirm this condition. CSF analysis may also indicate lymphocytic pleocytosis, and an elevation of blood proteins and oligoclonal bands may occur [20, 23].
MRI is a part of the initial investigation and may have a typical appearance or present subtle findings, such as increased signal in the medial temporal lobe in the fluid-attenuated inversion recovery sequence (FLAIR) and T2-weighted sequences, or focal hyperintensities in the frontal–parietal cortex. There is, however, a clinical–radiological paradox related to MRI, where only 23–55 % of the patients present MR findings and sometimes findings are more evident in less symptomatic patients [22].
FDG-PET imaging can show interesting findings even with normal MRI and CT scans. A classical finding is an abnormal medial temporal metabolism, in some cases associated with abnormalities in frontoparietal metabolism, varying from hypermetabolism in acute inflammation to hypometabolism in chronic cases, as shown in Fig. 1 [6•, 8, 24–28]. Occipital lobes, basal ganglia, cingulate gyrus, cerebellum, the pons, and even extensive hemispheric impairment are also reported, depending on the degree of inflammation [6•, 29–33]. Mesial temporal lobe and frontal hypermetabolism associated with reduction in occipital metabolism correspond to a big number of cases [17, 29, 31, 32].
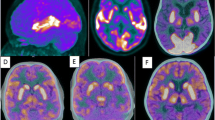
Female patient, 39 years old, with AE due to NMDA antibodies presenting with right temporal lobe hypermetabolism, namely in neocortical polar and lateral areas (a, b coronal and axial FDG-PET images, and d fused FDG-PET and MRI). MRI presented with unremarkable findings (c post-contrast T1-weighted MRI). Initial symptoms were mental confusion, aggressiveness, and visual hallucinations for 3 months before the scan
Anti-VGKC Complex Antibodies (LGI1 and CASPR2)
The anti-voltage-gated potassium channel (anti-VGKC) encephalitis group comprises antibodies to proteins complexes associated with VGKC: LGI1 (leucine-rich glioma inactivated 1, commonly related to limbic encephalitis) and CASPR2 (contactin-associated protein-like 2 which causes both CNS and peripheral nervous system symptoms). Both proteins are located in pre- and postsynaptic regions of the synaptic cleft and regulate neuronal transmission [21]. Approximately 50 % of the patients presenting with anti-VGKC positivity are also positive for anti-LGI1 or CASPR2 [18]. According to recent robust data, however, anti-VGKC positivity in the absence of antibodies to LGI1/CASPR2 is probably an unspecific finding and a nonreliable marker for AE [34]. Due to this, most of the FDG-PET findings in the literature are related to anti-LGI1 and anti-CASPR2 positivity (mostly the former than the latter) in addition to anti-VGKC.
This subtype of AE usually follows a typical clinical pattern of signs and symptoms. Anti-LGI1 encephalitis frequently begins with faciobrachial dystonic motor seizures (FBDS), followed by subacute loss of memory, changes in behavior and confusion, and hyponatremia, which can eventually lead to hippocampal atrophy detected by MRI [21, 35]. Seizures normally have a poor response to antiepileptic drugs but improve after immunotherapy. In anti-CASPR2-related encephalitis, sometimes Morvan syndrome and less frequently limbic encephalitis are also seen [2•].
There is a consistent sequence of reports of metabolism abnormalities on FDG-PET in mesial temporal structures (basically the hippocampus and amygdala) and also in the basal ganglia in this class of antibodies. In a series of cases of 10 anti-LGI1-positive patients and FDG-PET/CT images, 7 had temporal lobe hypometabolism (3 of each being bilateral), 7 presented bilateral basal ganglia hypermetabolism, and only 1 patient had no findings on FDG-PET [36]. In this group of patients, sensitivity for detection of the condition was 90 % for PET/CT, 76.9 % for electroencephalography (abnormal pattern in 10 out of 14 cases), and 71.4 % for MRI (mesial temporal T2 hypersignal being the most common pattern) [36]. Hypermetabolism in the striatum was reported in five anti-LGI1-positive patients, two of whom had restricted putaminal hypermetabolism and three with caudate hypermetabolism. Additionally, four of the five patients with unilateral FBDS had hypermetabolism in the contralateral motor cortex, and three patients with temporal lobe epilepsy presented with mesial temporal lobe hypermetabolism [35]. Bilateral mesial temporal lobe and basal ganglia hypermetabolism were also consecutively related in case reports of patients with anti-LGI1, one of whom showed metabolism normalization after 5 months of therapy [37, 38]. An example of anti-LGI1 AE with many simultaneous imaging manifestations can be seen in Fig. 2.
Female, 75 years old, with sudden executive dysfunction and memory impairment associated with epileptic seizures. Isolation of anti-LGI-1 in CSF confirmed an AE. Axial images show the FDG-PET images before treatment (a1–3) and after beginning of the treatment (b1–3). Column a shows a diffuse whole-brain cortical hypometabolism, associated with right caudate and right occipital focal hypometabolism (a2), and a focal hypermetabolic area in the posterior aspect of the right mesial temporal lobe (a3). After therapy with corticosteroids (column b), there is an improvement of whole-brain cortical glucose uptake, except in the right caudate and in the focal right occipital cortex (ischemic lesions confirmed in MRI images), as seen in b2. There is also a focal hypometabolic area in the posterior mesial right temporal lobe, where the hypermetabolic focus was seen before treatment (lower rows a3 and b3)
Anti-AMPAr
AE related to AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors (anti-AMPAr) are rare and usually have a clinical manifestation of limbic encephalitis [39, 40]. They are far more prevalent in women, and approximately two-thirds are related to tumors (mainly breast, lung, and thymus) and tend to relapse [40, 41•]. AMPAr is a subtype of glutamate receptor related to neural plasticity mechanisms, memory, and learning [9]. Autoantibodies against glutamate receptor subunits GluA1 and GluA2 can occasionally cause a sensible reduction of AMPAr levels through a cross-linking mechanism, leading to its internalization and changing its location in the synapses [9, 40].
PET imaging patterns are variable, and there are only a few reports on the literature on this issue. Caudate hypometabolism associated with multiple cortical areas of hypometabolism in frontal, temporal, and occipital areas were already described in a pregnant patient with positive anti-AMPAr after immunotherapy [42]. Bilateral hippocampal hypermetabolism was also seen in a patient with anti-AMPAr isolated in the CSF after 3 months of symptoms (seizures and memory deficit). This hypermetabolism progressively returned to normal levels after 10–25 months of treatment with immunotherapy and antiepileptic drugs [41•]. A third report found no brain metabolism abnormalities in a patient with thymoma and myasthenia gravis associated with positive anti-AMPAr. In this last report, PET was important in the detection of the primary tumor [43].
Anti-GABA
Neuroreceptors such as GABA-B are linked to the inhibition of epileptogenic stimuli, while GABA-A plays a significant role in fast inhibitory synaptic transmission [44]. Antibodies against subunits of the inhibitory GABA receptors lead to its dysfunction and numeric reduction [45]. Thus, patients with anti-GABA-B AE present with limbic encephalitis symptoms and have a higher risk of associated neoplastic diseases, especially small lung cancer, and thymoma. Patients with anti-GABA-A tend to show refractory seizures, episodic memory loss, and changes in behavior [2•, 44]. On the other hand, anti-GABA-A encephalitis has no higher risk of paraneoplastic origin. Both subtypes tend to have a good response to immunotherapy as well as a better improvement when a neoplastic tissue is found in anti-GABA-B AE [45].
Only a few reports of the FDG-PET imaging pattern of anti-GABA-B AE are found. They comprise unilateral or bilateral mesial temporal lobe hypermetabolism [46, 47], which could be associated with global cortical hypometabolism, the whole hippocampus and amygdaloid body [46], and isolated global hypometabolism and even normal scans [47]. In the biggest series of cases, four out of five patients presented with small cell lung cancer [47].
Anti-GAD
Anti-glutamic acid decarboxylase (GAD) AE is associated with GAD enzymes, which are located in the presynaptic GABAergic terminals [48•]. Despite being an intracellular enzyme, as most of the onconeural antibodies, the neurologic symptoms mediated by anti-GAD are usually unrelated to malignant tumors, and symptoms may include cerebellar ataxia, seizures, “stiff-person syndrome,” and limbic encephalitis [48•, 49]. Concomitant type 1 diabetes mellitus was also reported [48•, 50].
The etiology of the anti-GAD neurologic syndrome remains unclear. A T cell mechanism against the limbic system causing cytotoxicity and local inflammation was described as a possible origin of the tissue damage/repair and gliosis [51]. However, there is no correlation between antibodies titers and severity of the disease, there is lower inflammation than in AE related to other autoantibodies, and response to immunotherapy is usually worse than in other neurological syndromes caused by antibodies against surface antigens [51–53].
There are few FDG-PET image studies on AE due to anti-GAD published in literature, and they mostly describe limbic hypermetabolism [6•, 29, 54, 55], usually associated with T2-FLAIR hyperintensity in the medial temporal lobes on cerebral MRI studies [2•]. In a case report of a 66-year-old female, a subtle onset of seizures progressing within 1 week to aphasia, confusion, weakness, and status epilepticus was reported, in addition to multiple hypermetabolic lesions in bilateral frontal, right temporal lobe, and left anterior superior frontal gyrus on FDG-PET [54]. Another study described mesial temporal hypermetabolism on FDG-PET performed in a 43-year-old female with hallucinations and nausea [6•]. FDG-PET images showed hypermetabolism in the pallidus, midbrain, brainstem, lateral parietotemporal and orbitofrontal cortex bilaterally, cerebellar tonsillae, and culmen in a 23-year-old female patient with subtle onset of psychosis-like syndrome, epilepsy, and dyskinesia, with both anti-GAD and anti-NMDA-positive serology [29]. In a group of nine anti-GAD-positive patients, one presented with right hippocampal hypermetabolism, while the eight remaining presented with mesial temporal hypometabolism [55]. Figures 3, 4, and 5 show different manifestations of anti-GAD AE. In one of those patients, anti-GAD was isolated in conjunction with anti-AMPA and anti-GABA-B.
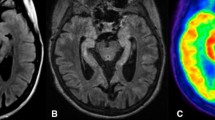
Female, 56 years old, patient presenting with cerebellar ataxia and weight loss, and anti-GAD positivity confirmed an AE. Images after 3 months of the symptoms onset show hypermetabolism in the right hippocampus, parahippocampal gyrus, and amygdala (a fused FDG-PET and T1-weighted MRI, and c FDG-PET only). Anatomic T1-weighted MRI images showed no abnormalities (b middle row) in the corresponding structures
Male, 49 years old, with suspicion of limbic encephalitis after two epileptic seizures. MRI shows an enlarged left hippocampus with high signal in T2-FLAIR acquisitions (middle column b) and focal hypermetabolism in the left hippocampus and parahippocampal gyrus (a, c). AE with concomitant anti-GABA-B, anti-AMPAr, and anti-GAD was confirmed. Column a fused MRI and FDG-PET images in the axial, coronal, right sagittal, and left sagittal planes. Column b FLAIR MRI images in axial, coronal, right sagittal, and left sagittal planes. Column c FDG-PET images in the axial, coronal, right sagittal, and left sagittal planes
Male, 28 years old, with febrile seizure since 13 years old. At the time of the scans, he presented with motor seizures four times a week. T2-weighted FLAIR images with high signal in left hippocampus and enlarged left amygdala (left column). FDG-PET images demonstrated focal hypermetabolism restricted to the posterior component of the left hippocampus and mild hypometabolism in its anterior component (right column). Left column FLAIR MRI images, and right column FDG-PET images, both in axial, sagittal, and coronal planes). The isolation of anti-GAD in peripheral blood confirmed the diagnosis of an AE
Whole-Body FDG-PET Imaging
AE or rapidly progressive dementia may occur as an aberrant by-product of an immune response directed against nonneurologic cancers [10]. In this case, AE would be due to paraneoplastic autoantibodies, most commonly onconeural antibodies. In such situations, whole-body FDG-PET/CT imaging may be included in the workflow of cancer investigation, in addition to conventional anatomic modalities. Nevertheless, the different imaging patterns seen on the numerous types of cancers related to paraneoplastic AE is beyond the scope of this manuscript.
Future Challenges and Conclusions
Studies with larger samples and prospective cohorts still lack in the literature. Such systematic investigations would be useful to demonstrate changes in brain imaging patterns through time (before and after therapy), the clinical meaningfulness of different imaging findings during early diagnosis and also possible prognostic implications. It is still an open question whether or not treatment of patients with hypermetabolic cortical areas (which could mean acute inflammation or active epileptic foci) has a better outcome than subjects with hypometabolic lesions.
Overall, after reviewing the most relevant reports in the literature, it can be concluded that AE findings on FDG-PET may have various patterns, but three are dominant and can be summarized as follows: (1) hypermetabolism in cortical areas, mainly in mesial temporal regions and less frequently in basal ganglia and higher cortical regions, is a common pattern in early stages of the disease [4, 6•, 17]. As noted by Fisher et al. [12], this pattern is highly suggestive of limbic AE, since it has not been described in many other entities, except for brain tumors and active epileptic foci [12], (2) also common is a reduced metabolism in the regions described above, which could happen both in the detection of the disease or in previously hypermetabolic areas which changed their pattern during the course of illness, (3) other areas with hypometabolism can also occur, especially the “diffuse whole-brain cortical hypometabolism” manifestation, which is unspecific and can have neurodegenerative diseases and other conditions as differential diagnoses. As shown above, some antibodies are more related to specific metabolic imaging patterns, but others do not correlate closely with imaging appearances.
In conclusion, we consider that FDG-PET imaging can aid in the early diagnosis of AE and may also be helpful while accessing the disease longitudinally while showing functional changes that occur after therapy. In both situations, it can provide valuable information that is not found in anatomic imaging alone.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McKeon A. Autoimmune encephalopathies and dementias. Contin Lifelong Learn Neurol. 2016;22:538–58.
• Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis—relevance for clinical practice and hippocampal function. Neuroscience 2015; 309:68–83. Well-illustrated update on the state-of-the-art of FDG-PET and AE.
Vollmer T, McCarthy M. Autoimmune encephalitis: a more treatable tragedy if diagnosed early. Neurology. 2016. doi:10.1212/WNL.0000000000002641.
Sekigawa M, Okumura A, Niijima S, Hayashi M, Tanaka K, Shimizu T. Autoimmune focal encephalitis shows marked hypermetabolism on positron emission tomography. J Pediatr. 2010;156:158–60.
Sinmaz N, Amatoury M, Merheb V, Ramanathan S, Dale R, Brilot F. Autoantibodies in movement and psychiatric disorders: updated concepts in detection methods, pathogenicity, and CNS entry. Ann NY Acad Sci. 2015;1351:22–38.
• Baumgartner A, Rauer S, Mader I, Meyer P. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 2013;260:2744–53. One of the studies which tried to correlate imaging appearances with antibodies.
Wingfield T, McHugh C, Vas A, Richardson A, Wilkins E, Bonington A, Varma A. Autoimmune encephalitis: a case series and comprehensive review of the literature. QJM. 2011;104:921–31.
Probasco J, Benavides D, Ciarallo A, Sanin B, Wabulya A, Bergey G, Kaplan P. Electroencephalographic and fluorodeoxyglucose-positron emission tomography correlates in anti-N-methyl-d-aspartate receptor autoimmune encephalitis. Epilepsy Behav Case Rep. 2014;2:174–78.
Höftberger R. Neuroimmunology: an expanding frontier in autoimmunity. Front Immunol. 2015;206:1–6.
Geschwind M. Rapidly progressive dementia. Contin Lifelong Learn Neurol. 2010;16:31–56.
McEvoy LK, Fennema-Notestine C, Roddey JC. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment 1 [Internet]. Radiology. 2009. doi:10.1148/radiol.2511080924.
Fisher R, Patel N, Lai E, Schulz P. Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med. 2012;37:e213–8.
Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz H-G, Peters J, Bartenstein P, Lieb K, Schreckenberger M. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2008;44:43–50.
Catana C, Guimaraes A, Rosen B. PET and MR imaging: the odd couple or a match made in heaven? J Nucl Med. 2013;54:815–24.
Catana C, Drzezga A, Heiss D, Rosen B. PET/MRI for neurologic applications. J Nucl Med. 2012;53:1916–25.
Scheid R, Lincke T, Voltz R, Cramon D, Sabri O. Serial 18F-fluoro-2-deoxy-d-glucose positron emission tomography and magnetic resonance imaging of paraneoplastic limbic encephalitis. Arch Neurol Chic. 2004;61:1785–9.
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C, Lewerenz J. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-d-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–6.
Kumar A. NMDA receptor function during senescence: implication on cognitive performance. Front Neurosci. 2015;9:1–15.
Titulaer M, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig L, Benseler S, Kawachi I, Martinez-Hernandez E, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65.
Dalmau J, Gleichman A, Hughes E, Rossi J, Peng X, Lai M, Dessain S, Rosenfeld M, Balice-Gordon R, Lynch D. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8.
Irani S, Michell A, Lang B, Pettingill P, Waters P, Johnson M, Schott J, Armstrong R, Zagami A, Bleasel A, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900.
Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi J, Voloschin A, Baehring J, Shimazaki H, Koide R, King D, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
Ances B, Vitaliani R, Taylor R, Liebeskind D, Voloschin A, Houghton D, Galetta S, Dichter M, Alavi A, Rosenfeld M, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77.
Chanson J-B, Diaconu M, Honnorat J, Martin T, Seze J, Namer I-J, Hirsch E. PET follow-up in a case of anti-NMDAR encephalitis: arguments for cingulate limbic encephalitis. Epileptic Disord Int Epilepsy J Videotape. 2012;14:90–3.
Lee E, Kang J, Oh J, Kim J, Shin Y-W, Kim C-Y. 18F-fluorodeoxyglucose positron-emission tomography findings with anti-N-methyl-d-aspartate receptor encephalitis that showed variable degrees of catatonia: three cases report. J Epilepsy Res. 2014;4:69–73.
Wegner F, Wilke F, Raab P, Tayeb S, Boeck A-L, Haense C, Trebst C, Voss E, Schrader C, Logemann F, et al. Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-d-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol. 2014;14:136.
Morooka M, Kubota K, Minamimoto R, Furuhata M, Abe T, Ito K, Okasaki M, Ishii K, Ishiwata K. 18F-FDG and 11C-methionine PET/CT findings in a case with anti-NMDA (NR2B) receptor encephalitis. Clin Nucl Med. 2012;37:400–2.
Greiner H, Leach J, Lee K-H, Krueger D. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure. 2011;20:266–70.
Cistaro A, Caobelli F, Quartuccio N, Fania P, Pagani M. Uncommon 18F-FDG-PET/CT findings in patients affected by limbic encephalitis: hyper–hypometabolic pattern with double antibody positivity and migrating foci of hypermetabolism. Clin Imaging. 2015;39:329–33.
Tobin WO, Strand EA, Clark HM, Lowe VJ. NMDA receptor encephalitis causing reversible caudate changes on MRI and PET imaging. Neurol Clin Pract. 2014;4:470–73.
Yuan J, Guan H, Zhou X, Niu N, Li F, Cui L, Cui R. Changing brain metabolism patterns in patients with ANMDARE: serial 18F-FDG PET/CT findings. Clin Nucl Med. 2016;41:366–70.
Mohr B, Minoshima S. F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. Clin Nucl Med. 2010;35:461–3.
Endres D, Perlov E, Stich O, Rauer S, Maier S, Waldkircher Z, Lange T, Mader I, Meyer P, Elst L. Hypoglutamatergic state is associated with reduced cerebral glucose metabolism in anti-NMDA receptor encephalitis: a case report. BMC Psychiatry. 2015;15:186.
Sonderen A, Schreurs M, Bruijn M, Boukhrissi S, Nagtzaam M, Hulsenboom E, Enting R, Thijs R, Wirtz P, Smitt P, et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology. 2016. doi:10.1212/WNL.0000000000002637.
Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, Psimaras D, Habert M-O, Baulac M, Delattre J-Y, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139:1079–93.
Shin Y-W, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, Kim T-J, Lee K-J, Kim Y-S, Park K-I, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. 2013;265:75–81.
Kamaleshwaran K, Iyer R, Antony J, Radhakrishnan E, Shinto A. 18F-FDG PET/CT findings in voltage-gated potassium channel limbic encephalitis. Clin Nucl Med. 2013;38:392–4.
Park S, Choi H, Cheon G, Kang K, Lee D. 18F-FDG PET/CT in anti-LGI1 encephalitis: initial and follow-up findings. Clin Nucl Med. 2015;40:156–8.
Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–89.
Lai M, Hughes E, Peng X, Zhou L, Gleichman A, Shu H, Matà S, Kremens D, Vitaliani R, Geschwind M, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34.
• Spatola M, Stojanova V, Prior J, Dalmau J, Rossetti A. Serial brain 18FDG-PET in anti-AMPA receptor limbic encephalitis. J Neuroimmunol. 2014;271:53–55. Elegant investigation on anti-AMPA AE and prospective imaging.
Wei Y-C, Liu C-H, Lin J-J, Lin K-J, Huang K-L, Lee T-H, Chang Y-J, Peng T-I, Lin K-L, Chang T-Y, et al. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol. 2013;261:129–33.
Li X, Mao Y-T, Wu J-J, Li L-X, Chen X-J. Anti-AMPA receptor encephalitis associated with thymomatous myasthenia gravis. J Neuroimmunol. 2015;281:35–7.
Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason W, Kruer M, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–86.
Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen M, Grisold W, Kimura A, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2009;9:67–76.
Su M, Xu D, Tian R. 18F-FDG PET/CT and MRI findings in a patient with anti-GABAB receptor encephalitis. Clin Nucl Med. 2015;40:515–17.
Kim T-J, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, Shin Y-W, Lee K-J, Jung K-H, Kim Y-S, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. 2014;270:45–50.
• Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90. Concise and easy to read review on clinical AE pathogenesis.
Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–63.
Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509–17.
Bien C, Vincent A, Barnett M, Becker A, Blümcke I, Graus F, Jellinger K, Reuss D, Ribalta T, Schlegel J, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–38.
Ariño H, Gresa-Arribas N, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau J, Saiz A, et al. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71:1009–16.
Rakocevic G, Raju R, Dalakas M. Anti-glutamic acid decarboxylase antibodies in the serum and cerebrospinal fluid of patients with stiff-person syndrome: correlation with clinical severity. Arch Neurol Chic. 2004;61:902–4.
Kojima G, Inaba M, Bruno M. PET-positive extralimbic presentation of anti-glutamic acid decarboxylase antibody-associated encephalitis. Epileptic Disord. 2014;16:358–61.
Malter M, Helmstaedter C, Urbach H, Vincent A, Bien C. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–8.
Acknowledgments
We would like to thank Kimberly Stephens for her kind help with English language issues. AMNC would like to acknowledge the financial support of Sociedade Beneficente Hospital Sirio Libanes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Marianne Kimura Soriano, Carla Rachel Ono, and Artur M. N. Coutinho each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on PET/CT Imaging.
Rights and permissions
About this article
Cite this article
Soriano, M.K., Ono, C.R. & Coutinho, A.M. Positron Emission Tomography with 18F-Fluorodeoxyglucose Imaging Patterns in Autoimmune Encephalitis. Curr Radiol Rep 4, 47 (2016). https://doi.org/10.1007/s40134-016-0174-8
Published:
DOI: https://doi.org/10.1007/s40134-016-0174-8