Abstract
Double-strand breaks (DSB) are considered to be the most relevant X-ray-induced DNA damages. γ-H2AX immunofluorescence microscopy is a sensitive and reliable method for the quantification of distinct DSB. The detectable amount of these DNA damages correlates well with the dose received. A protective effect of different antioxidants has been postulated. This review explains the principle of the γ-H2AX technique, describes important results concerning DSB in radiologic examinations, and provides an overview on studies evaluating the effect of antioxidants on radiation-induced DNA damages with a special focus on recent studies using the γ-H2AX method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shortly after the discovery of X-rays, their damaging effect on biological tissues was observed. The increasing number of X-ray examinations during the recent decades led to the focus on radiation protection and dosimetry. The determination of radiation exposure in diagnostic and interventional radiology is usually based on physical measurements or mathematical algorithms with standardized dose simulations. However, these dose estimations can assess the X-ray exposure but not the biological interaction of the X-rays within the body of the patients. Nowadays, it is well known that the biological radiation damage depends not only on dose but also on other individual factors which cannot be adequately ascertained by established techniques for dose measurement. Several biological dosimetry approaches failed due to the low sensitivity of the methods [1].
Double-strand breaks (DSB) are considered to be the most significant DNA lesions induced by ionizing radiation [2]. These DNA lesions are usually efficiently repaired, however DSB misrepair can lead to chromosomal translocations and therefore initiate carcinogenesis [3, 4].
A novel immunofluorescence microscopy approach, that is more sensitive than previous biological methods, allows the determination of distinct cytological foci representing DSB in peripheral blood lymphocytes and thus allows an accurate monitoring of radiation effects on DNA and an estimation of the biological radiation dose in diagnostic and interventional radiology, and cardiology procedures [1, 5, 6•, 7•]. Various studies evaluating radiation-induced DNA DSB after radiological examinations and interventions have been published during the last 10 years [3, 8–12].
A protective effect of several antioxidants against biological radiation damage has been previously reported. This review provides an overview on γ-H2AX immunofluorescence microscopy method and the recently published data about the effect of antioxidants on X-ray-induced DNA DSB.
γ-H2AX Immunofluorescence Microscopy
After the induction of DNA DSB, the phosphorylation of the histone variant H2AX is one of the earliest cellular responses. This phosphorylated histone (termed γ-H2AX) can be visualized by fluorescence microscopy as distinct fluorescent foci using specific primary and fluorescent secondary antibodies [2, 6•]. With this approach, lymphocytes, which are the most common white cells studied with radiation models, are isolated from blood samples using gradient centrifugation, and isolated cells are washed and resuspended in phosphate buffered saline, followed by fixation in 100 % methanol and permeabilization in 100 % acetone. After a repeat washing, staining is performed using specific γ-H2AX primary and Alexa Fluor 488-conjugated goat anti-mouse secondary antibodies. Finally, cells are mounted with Vectashield© mounting medium containing 4′,6-diamidino-2-phenylindole. Quantification of γ-H2AX foci is performed using a fluorescence microscope equipped with a 63× or a 100× magnification objective. The ratio of foci is related to the total number of enumerated cells. The numbers of excess foci representing distinct X-ray-induced DSB can be calculated by subtraction of corresponding non-irradiated (baseline levels) from irradiated samples. Additionally, lymphocytes can also be stained against 53BP1, which is another very sensitive marker for DNA DSB [13, 14]. This method of γ-H2AX and also 53BP1 immunofluorescence microscopy has been previously described in detail [2, 6•, 15•].
After X-ray exposure, DSB induction leads to a quick increase of foci and a peak value can be obtained within a few minutes after irradiation. It has been demonstrated in prior research studies that these yields of X-ray-induced DSB correlate linearly with the deposited radiation dose [6•]. In comparison to previous biological dosimetry approaches (e.g., pulsed gel electrophoresis or detection of chromosomal damages), this method is much more sensitive and enables detection of biological doses as low as 1 mGy in vitro and in vivo. Therefore, this method is applicable in dose ranges used in diagnostic and interventional radiological procedures.
DNA Double-Strand Breaks After X-ray Exposure in Radiology
The initial experience studying γ-H2AX foci in lymphocytes of patients undergoing CT scans was published in 2005 [2]. In all of the patients, a significant increase of γ-H2AX foci was observed 30 min after CT, and subsequently the DSB values dropped rapidly due to repairing processes and at 24 h near-baseline values were reached. The number of CT-induced DSB correlated well with the dose length product, which is an established exposure parameter in CT. An interesting observation in that study was that at a comparable dose length product, an excessive increase of CT-induced γ-H2AX foci was present in one patient with a DNA repair defect, compared to the remaining subjects, that was considered to reflect that the individual radiation damage depended not only on dose but also on individual factors such as the repair capacity [2].
Several studies evaluated the effect of the scan protocols on CT-induced γ-H2AX foci in cardiac CT. A significant correlation between radiation induced γ-H2AX foci and the dose length product was observed. Prospectively, ECG-triggered sequential and high-pitch helical scans led to a significant reduction of γ-H2AX foci levels compared to low-pitch helical scans [11, 12, 16]. A reduction of the tube voltage to 100 kV led to significantly lower excess foci levels compared to 120 kV protocols [16]. Interestingly, in one report, excess foci, normalized to the dose length product, showed a significant correlation to the attenuation of the blood in the heart and in the great thoracic vessels, indicating that iodinated contrast media may increase radiation-induced damage levels [12].
The effect of contrast media on radiation-induced DSB was evaluated in detail in a study published in Radiology in the year 2009. Both in vitro experiments and in vivo studies with patients undergoing chest CT have shown that at the same radiation dose, significantly more DNA DSB were measured when iodinated contrast media were administered. The cause for the increased induction of DSB in the presence of contrast medium can be explained with a greater photoelectric absorption in the iodine atoms of the contrast agent and the consequent greater exposure of neighboring lymphocytes [17].
Various authors described γ-H2AX foci after angiographic examinations. In patients undergoing percutaneous transluminal angioplasty of lower extremity arteries, a correlation between DSB induction and the dose area product, an established exposure parameter for fluoroscopy and angiography, was found [9]. In another study, an increase of DSB was observed in children following cardiac catheterization, but the in vivo biological dose was not linear in this low dose range [18]. An explanation or confirmation of this finding has not as yet been established. Two other studies have shown that the radiation-induced DSB did not reveal good correlation with the dose area product in patients undergoing angiography, but the separate analysis of the various body regions showed good correlation coefficients (e.g., r = 0.71 for pelvis-leg angiography and r = 0.96 for abdominal angiography). These differences can be explained by the different blood volumes of the various body regions and the associated variable number of exposed lymphocytes [10, 19].
Most recently, a study has described a slight but significant increase of γ-H2AX foci after full-field digital mammography. In this study, a biological phantom model was created that allowed generation of an estimation of the DNA damage level in the exposed organ [20].
Effect of Antioxidants on X-ray-Induced DNA Damages
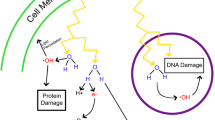
DNA damage induction is based on the formation of free radicals. A free radical is an atom or molecule that contains one or more unpaired electrons in an outer orbital. Free radicals are extremely reactive and induce oxidative stress in tissues. Therefore, theoretically an antioxidant should protect the body from free radicals and oxidative stress by donating electrons. The protective effect of antioxidants against radiation damages has been previously postulated [1, 21–23].
Some of these substances have been reported to be able to reduce X-ray-induced DSBs in vitro or in animal studies. Reliene et al. have described that N-acetylcysteine protects against radiation-induced DNA damages in cell cultures with human lymphoblastoid cells and in blood lymphocytes in a mouse model. This effect was dependent on the concentrations of N-acetyl cysteine and the radiation dose, and achieved a reduction of up to 80 % of DNA damage [24]. Mansour et al. determined DNA damages in rats by agarose gel electrophoresis after daily intraperitoneal injection of N-acetylcysteine (1 g/kg) for 7 consecutive days and gamma irradiation with 6 Gy. Pre-treatment led to a significant decrease of DNA damage compared to controls [25]. Tiwari et al. observed a concentration-dependent protective effect of N-acetylcysteine, glutathione, and thioproline against DNA damage in irradiated human lymphocytes (2–4 Gy) determined by micronucleus assay (MN) [26]. Other antioxidants, vitamin E and selenium, have demonstrated protective effects against radiation damage in animal experiments [21]. Another study reported protection against radiation-induced oxidative stress by various substances including vitamin C, N-acetyl cysteine, sodium ascorbate, alpha-lipoic acid, l-selenomethionine, and vitamin E in cell cultures of human breast epithelial cells. In this report, the protection after X-ray exposure reached up to 87 % using individual substances, and 94 % using a combination of five compounds [27]. None of the above-mentioned studies evaluated the effect after administration of these substances to humans.
In a study published in Radiology, a proprietary composition of antioxidants and glutathione-elevating agents containing calcium ascorbate (vitamin C), d-alpha tocopheryl succinate (vitamin E), natural mixed carotenoids (primarily beta-carotene), N-acetylcysteine, alpha-lipoic acid, and l-selenomethionine was tested in in vitro and combined in vivo/in vitro experiments (15). In this study, blood samples were obtained from 25 healthy volunteers (age 23–55 years; 12 female and 13 male). Exclusion criteria were chemotherapy or radiation therapy within the last 6 months, X-ray exposure within the last 3 days, and history of leukemia or lymphoma.
Non-irradiated samples were used for the determination of baseline levels in all experiments and ranged from 0.050 to 0.101/cell.
For in vitro experiments, samples were pre-incubated for 15 and 60 min with the antioxidant agent in solution (final concentrations: 33 µg/ml calcium ascorbate, 0.027 IU/ml d-alpha tocopheryl succinate, 1 µg/ml carotenoids, 16.7 µg/ml N-acetylcysteine, 2 µg/ml R-Alpha-lipoic acid, and 6.7 ng/ml l-selenomethionine) and irradiated with a dose of 10 mGy at room temperature. Blood samples irradiated with 10 mGy without antioxidants served as controls. Cells were cultivated for 5-15 min at standard conditions before staining against γ-H2AX. Five minutes after irradiation, mean X-ray-induced foci (excess foci) were 0.145 ± 0.021/cell without and 0.112 ± 0.019/cell after a 15-min pre-incubation with radioprotective agents (p < 0.0001). 15 min after irradiation, excess foci levels were lower due to DSB repair and the mean was 0.116+/0.016/cell in non-treated and 0.088 ± 0.018/cell in treated cells (p < 0.0001). Pre-incubation of in vitro samples for 60 min led to a further reduction of γ-H2AX foci levels.
Additional samples were administered radioprotectants immediately after irradiation, incubated for 5–15 min and examined again for γ-H2AX foci. This did not lead to a reduction of X-ray-induced γ-H2AX excess foci 5 (0.149 ± 0.007/cell without vs. 0.147 ± 0.010/cell with, p = 0.6905) and 15 min after irradiation (0.096 ± 0.015/cell without vs. 0.094 ± 0.017/cell with, p = 0.8413). This provides evidence that the observed effect of antioxidants on radiation-induced DNA damages is caused by a reduction of DSB induction and not by accelerated DSB repair.
In order to exclude that the radioprotective substances had a direct effect on foci levels, non-irradiated samples were incubated with antioxidants. There was no significant difference between non-treated and pre-treated control samples.
In a further step, correlation of the findings was made with 53BP1, another independent marker of DSB (n = 10). This approach showed comparable results to the γ-H2AX experiments.
Since in the in vivo setting the oral bioavailability and catabolism of the radioprotectants plays an important role, in vitro tests may reflect only partially in vivo situations. There this study also examined a combination of in vivo treatment of volunteers with in vitro irradiations of blood samples obtained at different time points before and after administration of radioprotectants. Blood samples were obtained from 17 healthy volunteers before, 15, 30, and 60 min and 2, 3, and 5 h after oral ingestion of one dosage of the radioprotective agent (resulting in a total of 500 mg calcium ascorbate, 400 IU d-alpha tocopheryl succinate, 15 mg natural mixed carotenoids, 250 mg N-acetylcysteine, 30 mg alpha-lipoic acid, and 100 µg l-selenomethionine). Each sample was also irradiated in vitro with a dose of 10 mGy and incubated for 5 min before staining against γ-H2AX. In these in vivo/in vitro experiments, mean excess foci levels, measured 5 min after 10 mGy X-ray exposure, were 0.140 ± 0.012/cell prior to radioprotectants, 0.102 ± 0.013/cell 15 min, 0.078 ± 0.015/cell 30 min, 0.058 ± 0.012/cell 60 min, 0.055 ± 0.010/cell 120 min, 0.061 ± 0.018/cell 180 min, and 0.060 ± 0.018/cell 300 min after oral pre-treatment with of antioxidant pills. Statistic analyses revealed significantly lower foci levels after all of the time points between 15 min and 5 h after pre-treatment compared to the control values obtained before treatment with antioxidants (p < 0.01 each). Between 2 and 5 h after treatment, foci levels were at similar low levels compared to the values obtained 60 min after pre-treatment. Therefore, a pre-treatment time of 60 min was considered ideal [15•].
A follow-up study evaluated the in vitro effect of distinct antioxidants and described the greatest effect on γ-H2AX foci reduction for N-acetylcysteine, comparable to the effect of the combination tested in the study published in Radiology. Other tested substances (calcium ascorbate, d-alpha tocopheryl succinate, beta-carotene, and l-selenomethionine) also led to a reduction of X-ray-induced γ-H2AX foci formation with a lower effect compared to N-acetylcysteine, whereas no significant γ-H2AX foci decrease was found with zinc and lipoic acid regardless of the concentration and the pre-incubation time [28].
Two more current in vivo studies evaluating the protective effect of antioxidants merit description. A pilot clinical study examined the ability of N-acetylcysteine to confer protection against radiation-induced chromosomal DNA damage during cardiac catheterization procedures using MN in peripheral human lymphocytes as a biomarker of chromosomal damage and intermediate endpoint of carcinogenesis [29]. In this study, MN in peripheral human lymphocytes was performed before and after radiation exposure in patients receiving clinically driven N-acetylcysteine to prevent contrast-induced nephropathy compared to patients receiving standard hydration therapy without N-acetylcysteine. Eligible patients were classified into two groups: 35 patients (26 males, age 63.4 ± 11.1 years) receiving the standard hydration protocol consisting of intravenous isotonic saline for 12 h after catheterization and 30 patients (26 males, age 65.5 ± 12.9 years) at risk for radiocontrast nephropathy with preexisting renal insufficiency receiving a double intravenous dose of N-acetylcysteine (6 mg/kg/h diluted in 250 ml of NaCl 0.9 %) for 1 h before and a standard dose (6 mg/kg/h diluted in 500 ml of NaCl 0.9 %) for 12 h following catheterization. MN frequency was 13.7 ± 4.7 % at baseline and showed a significant rise at 2 h (18.0 ± 6.8, p = 0.01) and 24 h (17.6 ± 5.9, p = 0.03) in the group without treatment. Importantly, there was no significant increase of MN in the treatment group (13.7 ± 7.0, 15.5 ± 6.0 and 14.9 ± 6.3 for baseline, 2 and 24 h, respectively, p = 0.4), showing that N-acetylcysteine treatment reduced radiation-induced chromosomal DNA damage in human lymphocytes after interventional catheterization procedures [29].
In a recently published single-center, double-blinded, placebo-controlled study, the in vivo effect of N-acetylcysteine and vitamin C was tested [30]. In this study, 30 patients undergoing low-dose coronary CT angiography (<3 mSv) and 29 patients undergoing catheter-based cardiac interventions (>9 mSv) were enrolled. They were compared to 29 controls who did not undergo any X-ray examination. Exclusion criteria were smoking, leukemia, lymphoma, radio- or chemotherapy, and X-ray-based examinations within the last three days. Blood collections were performed before intravenous infusion of 1.2 g N-acetylcysteine, 3 g vitamin C or saline and within minutes after X-ray exposure. DSB were quantified using γ-H2AX immunofluorescence microscopy. As expected, substantially higher DSB induction was found after high-dose exposure compared to low-dose exposure. In the control group, the antioxidants did not show any effect on DSB values. In the low exposure group, coronary CT angiography led to 0.15 excess foci/cell after placebo and to 0.07 excess foci/cell after antioxidant treatment (not significant). In the high exposure group, excess foci were significantly lower in the antioxidant group (0.1 excess foci/cell) compared to the placebo group (0.3 excess foci/cell). Subgroup analysis showed that the effect of vitamin C (−87 %) was significantly higher than that of N-acetylcysteine (−43 %, p = 0.005). Additionally performed flow cytometry showed also a significant effect of the antioxidants (403 FACS units) compared to placebo (1091 FACS units) in the high-radiation exposure group [30].
Despite the promising results of these studies, some limitations must be mentioned. Only a few studies with small subject numbers have been published to date. However, despite the limited number of studies, the observed effects were sufficiently large that they achieved statistical significance. Only limited data exist about the effect of individual substances, and it remains unclear if there is an additive effect of several antioxidants. Little is known about the biodistribution of the radioprotectants, and the concentration in tissue cells was not evaluated. Furthermore, it was not tested whether the antioxidants interfere with the phosphorylation or dephosphorylation of the histone variant H2AX, which could affect the number of detectable γ-H2AX foci. Therefore, further experiments using other methods like chromosomal analyses are mandatory.
Conclusion
γ-H2AX immunofluorescence microscopy is a sensitive method for measurement of radiation-induced DNA DSB. With this technique, it is possible to reliably estimate radiation-induced DNA DSB in the dose range of diagnostic and interventional radiology procedures. The DSB induction correlates well with the dose delivered, but also reflects the influence of patients’ individual factors (e.g., repair capacity and application of iodinated contrast agent etc.).
Using this technique, a significant reduction of radiation-induced DNA DSB by antioxidants was observed in lymphocytes, both after in vitro and in vivo radiation exposure. In these studies, lymphocytes were chosen because they are the conventional cell to be used in this type of model, they can be easily obtained from venous blood samples, and γ-H2AX immunofluorescence microscopy is well established in lymphocytes. It is important to note that damages to lymphocytes assessed by using the laboratory approach empolyed in the referenced studies is neither the same as cancer induction, nor cancer induction in a variety of other cell types. It should therefore be surmised that despite the clear reduction of X-ray-induced γ-H2AX foci by these radioprotective agents, the effect on prevention of cancer induction risk remains unclear and further studies are required in the future [15•].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Brand M, Uder M, Kuefner MA. DNA double-strand breaks as biological markers for radiation-induced DNA damages in radiology. In: Semelka RS, Elias J, editors. Health care reform in radiology. 1st ed. Hoboken: Wiley Blackwell; 2013. p. 183–90.
Lobrich M, Rief N, Kuhne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci USA. 2005;102(25):8984–9.
Jeggo PA, Lobrich M. DNA double-strand breaks: their cellular and clinical impact? Oncogene. 2007;26(56):7717–9.
Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7(11):861–9.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–68.
• Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci USA. 2003;100(9):5057–62. In vitro study describing interesting and important aspects of DSB induction and repair kinetics in different cell lines.
• Lobrich M, Shibata A, Beucher A, et al. GammaH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2010;9(4):662–9. Review providing a well-founded overview of the principle, strength and limitations of the γ-H2AX immunofluorescence microscopy method.
Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242(1):244–51.
Geisel D, Heverhagen JT, Kalinowski M, Wagner HJ. DNA double-strand breaks after percutaneous transluminal angioplasty. Radiology. 2008;248(3):852–9.
Kuefner MA, Grudzenski S, Schwab SA, et al. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest Radiol. 2009;44(8):440–6.
Kuefner MA, Hinkmann FM, Alibek S, et al. Reduction of X-ray induced DNA double-strand breaks in blood lymphocytes during coronary CT angiography using high-pitch spiral data acquisition with prospective ECG-triggering. Invest Radiol. 2010;45(4):182–7.
Kuefner MA, Grudzenski S, Hamann J, et al. Effect of CT scan protocols on X-ray-induced DNA double-strand breaks in blood lymphocytes of patients undergoing coronary CT angiography. Eur Radiol. 2010;20(12):2917–24.
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151(7):1381–90.
Ward IM, Minn K, Jorda KG, Chen J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J Biol Chem. 2003;278(22):19579–82.
• Kuefner MA, Brand M, Ehrlich J, Braga L, Uder M, Semelka RC. Effect of antioxidants on X-ray-induced γ-H2AX foci in human blood lymphocytes: preliminary observations. Radiology. 2012;264(1):59–67. Experimental study describing that a formulation of various antioxidants leads to a clear reduction of radiation induced γ-H2AX foci both in in vitro and combined in vivo/in vitro experiments.
Brand M, Sommer M, Achenbach S, Anders K, Lell M, Löbrich M, Kuefner MA. X-ray induced DNA double-strand breaks in coronary CT angiography: comparison of sequential, low-pitch helical and high-pitch helical data acquisition. Eur J Radiol. 2012;81(3):357–62.
Grudzenski S, Kuefner MA, Heckmann MB, Uder M, Lobrich M. Contrast medium-enhanced radiation damage caused by CT examinations. Radiology. 2009;253(3):706–14.
Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. Gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120(19):1903–9.
Kuefner MA, Grudzenski S, Schwab SA, et al. X-ray-induced DNA double-strand breaks after angiographic examinations of different anatomic regions. Rofo. 2009;181(4):374–80.
Schwab SA, Brand M, Schlude IK, Wuest W, Meier-Meitinger M, Distel L, Schulz-Wendtland R, Uder M, Kuefner MA. X-ray induced formation of γ-H2AX foci after full-field digital mammography and digital breast-tomosynthesis. PLoS One. 2013;8(7):e70660.
Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189(1–2):1–20.
Prasad KN, Cole WC, Haase GM. Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol. 2004;77(914):97–9.
Dardano A, Ballardin M, Ferdeghini M, et al. Anticlastogenic effect of Ginkgo biloba extract in Graves’ disease patients receiving radioiodine therapy. J Clin Endocrinol Metab. 2007;92(11):4286–9.
Reliene R, Pollard JM, Sobol Z, Trouiller B, Gatti RA, Schiestl RH. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat Res. 2009;665(1–2):37–43.
Mansour HH, Hafez HF, Fahmy NM, Hanafi N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem Pharmacol. 2008;75(3):773–80.
Tiwari P, Kumar A, Balakrishnan S, Kushwaha HS, Mishra KP. Radiation-induced micronucleus formation and DNA damage in human lymphocytes and their prevention by antioxidant thiols. Mutat Res. 2009;676(1–2):62–8.
Wan XS, Ware JH, Zhou Z, Donahue JJ, Guan J, Kennedy AR. Protection against radiation-induced oxidative stress in cultured human epithelial cells by treatment with antioxidant agents. Int J Radiat Oncol Biol Phys. 2006;64(5):1475–81.
Brand M, Vogt S, Engert C, Uder M, Küfner M. Auswirkungen von verschiedenen Antioxidantien auf strahleninduzierte DNA-Doppelstrangbrüche. RoFo. 2013;185:S215–6.
Andreassi MG, Cioppa A, Manfredi S, Neri MG, Foffa I, Picano E. N-acetyl cysteine reduces chromosomal DNA damage in circulating lymphocytes during cardiac catheterization procedures: a pilot study. Int J Cardiol. 2012;161(2):93–6.
Stehli J, Fuchs TA, Ghadri JR, Gaemperli O, Fiechter M, Kaufmann PA. Antioxidants prevent DNA double-strand breaks from X-ray-based cardiac examinations: a randomized, double-blinded, placebo-controlled trial. J Am Coll Cardiol. 2014;64(1):117–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Radiation Exposure and Reduction.
Rights and permissions
About this article
Cite this article
Kuefner, M.A., Brand, M., Andreassi, M.G. et al. Chemoprevention of Radiation-Induced DNA Double-Strand Breaks with Antioxidants. Curr Radiol Rep 3, 81 (2015). https://doi.org/10.1007/s40134-014-0081-9
Published:
DOI: https://doi.org/10.1007/s40134-014-0081-9