Abstract
Purpose of Review
The majority of individuals who have undergone Fontan palliation are surviving into adulthood, yet complications are common. This review will focus on late complications—cardiac and extra-cardiac—which may present insidiously.
Recent Findings
Recent meta-analysis of patients after Fontan operation suggests that over 80% will survive 20 years beyond surgery. This group is at risk for structural complications, arrhythmias, vascular complications, and heart failure. Not all forms of Fontan failure are the same, and categorization into separate entities such as failure with preserved versus reduced function may help guide therapies. Pulmonary vasodilators in particular may be useful to improve hemodynamics. Novel therapies aimed at lymphatic complications are emerging and promising. Late hepatic complications including ascites and liver cancer may be seen, and scoring systems may identify patients at higher risk.
Summary
Individuals who have undergone a Fontan surgery face challenges as they age which include both cardiac and non-cardiac systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1971, Dr. Francois Fontan successfully completed an operation that forever changed the lives of patients with congenital single ventricle heart disease. He connected the systemic venous return directly to the pulmonary arteries (PA), thereby bypassing the need for a subpulmonary ventricle. Fontan’s innovation made possible the long-term survival of patients with the most severe types of congenital heart disease. Since 1971, the number of patients who have undergone and survived Fontan palliation has steadily increased with most patients now surviving to adulthood. These patients increasingly seek care from general providers, cardiologists, and non-cardiac subspecialists for preventive, acute, and subspecialty care. These varied clinicians require a basic understanding of Fontan physiology in order to identify late complications of Fontan palliation and common illnesses and treatments that may be detrimental to Fontan physiology.
In this article, we will review the basics of single ventricle heart disease, modern day Fontan procedures, Fontan physiology, physiologic conditions detrimental to Fontan circulation, and long-term cardiac and non-cardiac complications of Fontan circulation.
The Physiology Behind Fontan Circulation
“Single ventricle heart disease” refers to a variety of cardiac defects including tricuspid atresia, unbalanced atrioventricular septal defects, hypoplastic left heart syndrome, double inlet left ventricle, and pulmonary atresia with a hypoplastic right ventricle. The term “single ventricle” is a misnomer, as most forms have a functionally univentricular heart with a dominant functional ventricle and one smaller rudimentary ventricle. Each of these defects precludes the heart’s ability to support biventricular cardiopulmonary circulation. “Single ventricle palliation” is a broad term encompassing both the Glenn procedure that connects the superior vena cava (SVC) to the PA’s and the Fontan procedure that connects the inferior vena cava to the PA’s. Ultimately, the Fontan procedure takes a functionally univentricular system and creates a circulation in series, separating the systemic and pulmonary venous return by (1) establishing passive pulmonary blood flow separate from the systemic circulation and (2) creating a functional single atrium and ventricle that receive the oxygenated pulmonary venous return and delivers it to the systemic circulation.
Fontan circulation depends on passive blood flow through the pulmonary vascular bed to the systemic ventricle. This is accomplished in part through sustained increase in central venous pressure that drives the systemic venous return back to the pulmonary arteries. This differs from biventricular circulation in which the systemic venous pressure is lower than the pulmonary venous pressure and pulmonary blood flow is maintained through the subpulmonary ventricle.
Although the Fontan circulation lacks a subpulmonary ventricular pump, it does benefit from two “pumps” that help maintain constant systemic venous return to the pulmonary vascular bed: (1) the “muscle pump,” or contraction of skeletal muscles, and (2) the “respiratory pump,” or negative intrathoracic pressure created by normal breathing and diaphragmatic excursion. Both of these pumps help deliver deoxygenated blood from the systemic venous system to the pulmonary arteries where it ideally flows unimpeded through the pulmonary capillaries to the single systemic ventricle.
Adequate pulmonary blood flow is critical to provide sufficient preload for a successful Fontan circulation, as the systemic ventricle alone is limited in its ability to increase cardiac output in the presence of systemic venous congestion and decreased preload. A number of factors may adversely affect Fontan circulation: intravascular volume depletion, loss of systemic venous tone, loss of the muscle and diaphragmatic pump, elevated pulmonary vascular resistance, and elevated cardiac filling pressures. Conditions such as dehydration, septic shock, use of paralytic agents for sedation, positive pressure ventilation, and pulmonary infections can decrease pulmonary blood flow and consequently cardiac preload and cardiac output [1••].
Many Fontan procedures will include the creation of a fenestration within the Fontan circuit. A fenestration is a surgically created shunt between the systemic venous return and the systemic arterial circulation, typically within the atrium. This allows deoxygenated blood returning from the body to “pop off” to the systemic arterial circulation and preserve cardiac output during times of suboptimal Fontan hemodynamics. In some patients, this fenestration may close spontaneously over time. In other patients, it may be electively closed through a future transcatheter procedure. If Fontan hemodynamics remain suboptimal, the fenestration may intentionally be left open to maintain adequate cardiac output. Although fenestrations in the peri-operative period decrease complications such as pleural effusions, they increase the risk for paradoxical embolism. Patients with persistent fenestrations should remain on anti-coagulation or anti-platelet therapy.
Survival Following Fontan Palliation
A recent meta-analysis by Poh et al. reviewed the late survival of 7536 Fontan patients with a mean follow-up duration of 114 ± 95 months [2••]. In this group, there were 688 (11%) late deaths. Including studies published between 1990 and 2015, the estimated mean survival at 5, 10, and 20 years post Fontan surgery were 95%, 91%, and 82%. Causes of death included late Fontan failure, sudden cardiac death, perioperative death, thromboembolism, sepsis, respiratory failure, multi-organ failure, bleeding, cancer, protein losing enteropathy (PLE), and liver failure. The authors identified a number of predictors of late death following Fontan palliation, the most significant of which were prolonged duration of pleural effusions following Fontan palliation, development of protein losing enteropathy, and need for permanent pacemaker.
Cardiac Complications After Fontan Palliation
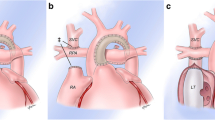
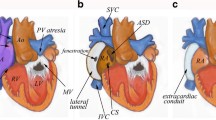
The American Heart Association/American College of Cardiology recently released guidelines for management of adults with congenital heart disease that highlight the need for close surveillance of cardiac complications following Fontan palliation [3••]. These complications include structural disease, arrhythmias, Fontan failure, lymphatic complications, and thromboembolic events (Fig. 1a, Table 1).
a Fontan palliation connecting the IVC and SVC to the pulmonary arteries. There is a fenestration (*) present. Potential cardiac complications include (1) anatomical obstruction or (2) thrombotic complications that may occur anywhere in the passive venous circuit; (3) elevated pulmonary vascular resistance; (4) arrhythmias; (5) valvular dysfunction; (6) ventricular dysfunction; and (7) lymphatic complications. b Extra-cardiac complications include (1) Fontan-associated liver disease, (2) pulmonary disease, and (3) developmental and neuropsychiatric complications
Structural Complications of Fontan Circulation
Structural complications following Fontan palliation include obstruction of the Fontan circuit, abnormal vascular formations, and valvular dysfunction. Fontan obstruction presents as systemic venous congestion which may be asymptomatic or manifest with edema or ascites, exercise intolerance, and sometimes hypoxemia. In the absence of a subpulmonary ventricle, even mild obstruction may cause significant symptoms [4]. While echocardiography is relatively inexpensive and easily employed, it has limited ability to detect obstructions, particularly in the distal Fontan conduit or branch pulmonary arteries. Diagnosis of obstruction typically requires three-dimensional imaging by CT, MRI, or direct angiography. Definitive management of obstruction can usually be accomplished through catheter-based procedures, although some patients may require surgery [1••].
Abnormal vascular connections include veno-venous collaterals, pulmonary arteriovenous malformations (PAVM), and aortopulmonary collaterals [5, 6]. Veno-venous collaterals and PAVM function as right-to-left shunts of deoxygenated blood from the systemic venous system directly to the pulmonary venous return. Patients present with new onset and often insidious hypoxemia. PAVM may be encountered more commonly in Fontan patients in whom IVC blood flows preferentially to one lung rather than equally to both lungs. PAVM more commonly form in the lung with diminished IVC flow [7]. Cardiac MRI flow mechanics identify IVC flow imbalance and are increasingly being used to guide surgical revision of Fontan connections to equalize distribution of hepatic flow to both lungs as treatment for PAVM [8].
Aortopulmonary collaterals (APCs) are left-to-right shunts that recirculate oxygenated blood from the aorta to the pulmonary arteries. Up to 30% of individuals post-Fontan may have persistent significant APCs [9]. Significant APCs present as volume overload, pulmonary congestion, or peripheral edema. In rare cases, hemoptysis may be a life-threatening presentation of APCs and may require urgent intervention. Advances in MRI imaging increasingly allow for quantification of the volume of flow through collateral vessels, helping guide treatment decisions for APCs [10, 11].
Most abnormal vascular connections (veno-venous collaterals, PAVM, and APCs) can be embolized in the cardiac catheterization lab, although each carries a high likelihood of recurrence after embolization. Patient symptoms and the hemodynamic effect of the abnormal vascular connections drive decisions to intervene.
Valvular dysfunction includes atrioventricular (AV) valve regurgitation and stenosis and semilunar valve insufficiency and stenosis. AV insufficiency in particular is poorly tolerated and is associated with increased morbidity and mortality in Fontan patients [12]. Valvular dysfunction presents with volume overload and exercise intolerance. Medical therapy may be attempted for symptom management but does not address the underlying structural problem. Refractory symptoms or progressive cardiac dysfunction may necessitate surgical and possibly transcatheter interventions [3••]. In some cases if AV valve regurgitation is accompanied by significant ventricular dysfunction or other concerns, such patients may benefit from consideration of cardiac transplantation rather than high-risk AV valve surgery.
Arrhythmia
Arrhythmias, especially atrial arrhythmias, are a frequent late complication in Fontan patients. Lasa et al. evaluated a contemporary cohort of 434 Fontan patients and found an overall prevalence of late arrhythmias of 31%, with bradyarrhythmias being more common (30%) that tachyarrhythmias (4%) [13]. Tachyarrhythmias commonly encountered include atrial flutter (also called intra-atrial reentrant tachycardia), atrial fibrillation, ectopic atrial tachycardia, and re-entrant supraventricular tachycardia. Although rare, sudden cardiac death due to ventricular arrhythmias does occur in 5–10% of Fontan patients, occurring at a mean age of 20 years in independent studies [14].
Arrhythmias may be insidious in Fontan patients. For example, many patients adapt to sinus node dysfunction and underreport symptoms of exercise intolerance and bradycardia. Similarly, tachyarrhythmias (especially intra-atrial reentrant tachycardias) may be mistaken for sinus rhythm as patients often have slower heart rates than those reported in non-Fontan patients due to the macro re-entrant circuit associated with the flutter. Routine surveillance for arrhythmia complications includes annual or biennial periodic outpatient rhythm monitoring (e.g., Holter) and exercise stress testing as well as a low threshold to assess for occult arrhythmias [3••]. When present, arrhythmia symptoms include thromboembolic complications, exercise intolerance, syncope, or new onset heart failure including new onset ascites. Providers should conduct a thorough arrhythmia evaluation in any patient who presents with any of these unexplained symptoms. Treatment options for arrhythmias include anti-arrhythmic medications, catheter-based ablation, or surgical ablation (usually done at the time of a concomitant surgery). In many cases, several treatment modalities are required to control the arrhythmia burden over the patient’s lifespan.
Fontan Failure
Shortly after the advent of Fontan palliation, Dr. Fontan warned that even the best Fontan procedure imposed on patients “a gradually declining functional capacity and premature late death after an initial period of often excellent palliation” [15]. A 2014 report from the Australia and New Zealand Fontan registry of over 1000 patients found that freedom from Fontan failure, defined as NYHA class III/IV symptoms, lymphatic failure, death, transplant, or need for Fontan takedown or conversion, was 83% at 15 years, 70% at 20 years, and 56% at 25 years [16]. Fontan failure can present with a variety of clinical symptoms including exercise limitation, pulmonary or systemic congestion, and lymphatic abnormalities.
A recent review by Book and colleagues introduced the concept that not all forms of Fontan failure are the same and categorized failure into four distinct phenotypes: (1) Fontan failure with reduced ejection fraction, (2) Fontan failure with preserved ejection fraction, (3) Fontan circulatory failure, and (4) lymphatic failure [17••, 18••]. Once a patient’s particular phenotype of Fontan failure is identified, providers should institute therapies that target the underlying pathophysiology of that type of failure. For example, Fontan failure with reduced ejection fraction is more likely to benefit from diuretics and afterload reduction whereas Fontan circulatory failure may be more likely to benefit from pulmonary vasodilators.
Many evidence-based treatments for heart failure management in non-Fontan patient populations either have not been well studied or have not been shown to be effective in slowing or preventing Fontan failure. For example, ACE inhibitors and beta-blockers are a cornerstone of heart failure management in patients with systolic ventricular dysfunction. For Fontan patients, however, studies have not shown a conclusive benefit of either medication class in improving or slowing the progression of Fontan failure with reduced systolic function [17••, 18••, 19, 20]. Pulmonary vasodilator therapy is drawing increasing attention as a potentially effective therapy for Fontan failure [21]. A recent meta-analysis by Wang et al. reviewed the results of nine randomized controlled trials of pulmonary vasodilators in Fontan patients. Overall, they found that pulmonary vasodilators improved Fontan hemodynamics and NYHA class [22].
Present management of Fontan failure consists of optimizing hemodynamics by addressing structural Fontan complications, eliminating arrhythmias, and attempting medical therapy targeted at the particular features of dysfunction. Failing this, patients should be referred for advanced heart failure therapies such as mechanical support and heart transplant when feasible.
Lymphatic Failure
Although not traditionally thought of as a manifestation of heart failure in other disease states, lymphatic complications are a worrisome presenting sign of Fontan failure. These complications are thought to be due to the increased central venous pressure of Fontan circulation that in turn triggers increased liver and central lymphatic flow. Lymphatic complications include protein losing enteropathy (PLE), chylous effusions, and plastic bronchitis. The reported incidence of lymphatic complications varies by clinical series with plastic bronchitis reported to occur in 0.5–4% of Fontan patients and PLE occurring in 5–15% of patients [23,24,25,26].
Plastic bronchitis is the result of abnormal pulmonary lymphatic flow that leads to extravasation of proteinaceous material into the airways. This material solidifies into casts of the airways and leads to cough and airway obstruction. Symptoms can be missed until a patient expectorates a cast. Diagnosis is established through bronchoscopic visualization of casts in the airway or expectoration of typical airway casts. One recent case control analysis found that patients with plastic bronchitis were more likely to have a prior history of chylothorax at prior surgery or have required diaphragm plication [26]. Although a number of medical treatment options have been tried, the most successful targeted therapy to date has come from Dori et al. who have performed selective lymphatic embolization of abnormal lymphatic networks identified through MR lymphangiography [27]. In a series of 18 patients with plastic bronchitis referred for embolization, 16 of the 18 were found to have abnormal lymphatic flow from the thoracic duct to the lung parenchyma. Of the 17 that underwent intervention, 88% had significant symptom improvement at a median follow-up around 1 year [28•].
PLE is a condition in which there is excessive lymphatic drainage to the intestines that results in protein wasting. Patients present with hypoalbuminemia, ascites, and edema due to low oncotic pressure. They may also develop immunodeficiency due to loss of gamma globulin and hypercoagulability due to the loss of anti-thrombotic proteins. Although Fontan obstruction should always be considered as a cause of new onset PLE, it is rarely found in patients with PLE outside of the perioperative period. Instead, causes of late-onset PLE are thought to include abnormal hepatoduodenal lymphatic connections, altered mesenteric hemodynamics, and gastrointestinal inflammation. A variety of treatments are available including pulmonary vasodilators, oral budesonide, and embolization procedures, each of which has some documented success. Prior to modern treatment strategies, the 5-year survival of Fontan PLE patients was estimated to be 50%; with modern therapies, this has improved to 88% [29]. Ultimately, however, if lymphatic complications cannot be overcome, the definitive treatment is heart transplantation.
Thromboembolic Complications
Fontan circulation increases the risk of venous and arterial thromboembolic complications. Passive flow through the Fontan circuit is low velocity and predisposes patients to venous thrombosis. This risk increases if the patient has underlying arrhythmias, liver dysfunction, residual cyanosis that causes reactive polycythemia, or inherited hypercoaguable states [30•]. The reported incidence of thromboembolism ranges from 3 to 33% [30•, 31, 32]. Symptoms of thromboembolism depend on the location and effect of the thrombus. Venous thrombi may be asymptomatic or may cause obstruction to pulmonary flow leading to systemic venous congestion and reduced cardiac output. In patients with residual intracardiac shunts, venous thromboembolism may result in paradoxical emboli to the arterial circulation. Primary arterial thrombi are less common. Risk factors for arterial thrombi include sustained arrhythmias and decreased cardiac function. Arterial thrombi can be silent or may present as stroke or systemic emboli.
Fontan patients treated with either aspirin or warfarin have fewer thromboembolic events than those who do not receive any anticoagulation or antiplatelet treatment. No study to date has shown superiority of either aspirin or warfarin in the prevention of thromboembolic complications [33]. Appropriate anticoagulation decisions are based on individual patient risk factors. Once a patient develops a thrombus or atrial arrhythmias, however, indefinite treatment with warfarin is typical [3••]. The use of novel oral anticoagulants is an area of current investigations with early reports suggesting these may be an alternative agent for Fontan patients [34•, 35].
Extra-Cardiac Fontan Complications
As long-term survival of individuals following Fontan surgery has become expected, it has been increasingly recognized that clinicians must be aware of extra-cardiac complications of this circulation in addition to the aforementioned cardiac issues. There are a number of organ systems that may be affected including the liver, lungs, and long-term neuro-cognitive effects among others (Fig. 1b, Table 2).
Fontan-Associated Liver Disease
Hemodynamic studies of post-Fontan circulation estimate that central venous pressure rises 2–6 times above baseline values immediately following Fontan completion causing post-sinusoidal portal hypertension, liver congestion, and fibrosis. This condition is known as Fontan-associated liver disease (FALD). While the majority of Fontan patients develop hepatic fibrosis, some patients will progress to decompensated cirrhosis with refractory ascites, varices, and even hepatocellular carcinoma [36•]. In light of this, the American College of Cardiology released a consensus statement recommending baseline screening for FALD beginning 5 years after Fontan palliation [37••]. This screening includes both laboratory assessment and imaging evaluation (primarily done by ultrasound) with further testing if significant abnormalities are found.
The detection and management of FALD presents numerous clinical challenges [38]. Typical physical exam findings, lab abnormalities, and imaging findings used in the identification and staging of non-FALD hepatic disease do not reliably identify FALD or its progression. For example, physical exam findings of advancing cirrhosis, such as jaundice, gynecomastia, palmar erythema, and spider angiomata are typically only seen in Fontan patients once end-stage FALD is present. Similarly, unlike other forms of non-FALD liver disease, synthetic liver function is typically preserved until the final stages of liver disease making hypoalbuminemia and coagulopathy unreliable markers of hepatic dysfunction. Lab abnormalities frequently seen in FALD include mild hyperbilirubinemia, mild transaminitis, elevations in GGT, and thrombocytopenia. However, the degree of these lab abnormalities does not correlate with severity of underlying FALD [39].
Two scoring systems have been developed to assess the severity of portal hypertension and liver disease in Fontan patients: the VAST score and the MELD-XI score. Both of these scores identify patients at increased risk of death and transplant. The VAST score uses a 4-point system with 1 point being given for the presence of varices, ascites, splenomegaly, and thrombocytopenia. Patients with a score of 2 or greater are at increased risk of adverse cardiovascular outcomes [40]. The MELD-XI score is a variation of the model for end-stage liver disease that does not include a measurement of INR. It is calculated using a patient’s creatinine and bilirubin. This score has been shown to correlate with severity of liver fibrosis and adverse cardiac events [41].
FALD predisposes Fontan patients to the development of hepatocellular carcinoma (HCC). A recent multicenter case series found a 1.3% incidence of HCC in Fontan patients [42]. The median age of diagnosis was 30 years and ranged from 12 to 52 years. Accordingly, the ACC guidelines recommend that HCC screening begin 5 years after Fontan completion. Symptoms of HCC include abdominal pain, jaundice, ascites, shortness of breath, and fever, though in the early stages HCC may be asymptomatic. Screening for HCC should consist of yearly imaging such as ultrasound or three-dimensional imaging that may be combined with measurement of alpha-fetoprotein in order to increase the sensitivity of HCC screening. AFP is elevated in 80% of HCC cases. Clinical or pathologic diagnosis is made by CT, MRI, or biopsy. Treatment of HCC depends on stage of disease and may include chemotherapy, radiation, surgical resection, and liver transplant.
Management of FALD consists of primary prevention of liver disease through optimization of Fontan circulation, vaccination against viral hepatitis, surveillance for HCC, and avoidance of hepatic toxins. Limited or no alcohol use may be prudent. If significant liver disease is identified, patients should be referred for cardiac catheterization to evaluate for Fontan obstruction and suboptimal hemodynamics contributing to increased post-sinusoidal portal hypertension. Patient with significant liver disease should also be referred to a hepatologist who is familiar with Fontan physiology and can guide decisions about invasive evaluations such as screening for varices and liver biopsy [37••]. In some cases, progressive liver disease may necessitate heart or combined heart and liver transplant.
Pulmonary Disease
Fontan circulation functions best in patients with healthy lung parenchyma, normal respiratory mechanics, and low pulmonary vascular resistance. Unfortunately, this triad of pulmonary health is rarely present in Fontan patients. Recent studies have reported that the incidence of significant pulmonary dysfunction in Fontan patients is as high as 40–50% [43]. Opotowsky et al. found that 46% of pediatric Fontan patients ages 6–18 had reduced forced vital capacity, and these patients had a higher incidence of decreased exercise capacity [44]. Pulmonary dysfunction may be due to a patient’s underlying cardiac disease, surgical history, or primary pulmonary disease [43, 45, 46]. Cardiac causes of pulmonary disease include restrictive lung disease due to multiple thoracic surgeries, plastic bronchitis (detailed above), and iatrogenic diaphragmatic paralysis. Primary pulmonary disease in Fontan patients includes obstructive pulmonary disease, frequent pulmonary infections, and sleep-disordered breathing. In addition, clinicians should be aware of the co-existence of primary ciliary dyskinesia with congenital heart disease, especially in individuals with heterotaxy syndrome [47]. Disorders of ciliary motility may be identified through ciliary biopsy.
Evaluation and aggressive management of pulmonary disease is critical to the maintenance of a healthy Fontan circulation [48]. In particular, consideration should be given to respiratory training to improve cardiorespiratory performance with exercise, as some studies indicate this improves aerobic capacity in Fontan patients [48, 49].
Neurodevelopment and Mental Health
Critical congenital heart disease increases patients’ risk for neurodevelopmental disabilities, and neurodevelopmental screening is an essential component of routine well patient care in all single ventricle patients [50]. A prospective study recently showed that Fontan patients score lower than healthy peers on neuropsychological tests and have a higher incidence of structural brain abnormalities detected by MRI [51]. Besides neurodevelopmental abnormalities, Fontan patients also have an increased risk for and undertreatment of psychiatric disease [52, 53]. Adolescents with single ventricle heart disease have a higher lifetime incidence of anxiety, depression, and psychosocial concerns than peers without congenital heart disease (65% vs. 22%) [54]. A study by the Pediatric Heart Network found that more than 50% of parents of Fontan patients report that their children struggle with anxiety, depression, and behavior problems [55]. Given this, screening and early intervention for mental health disorders should be included in routine Fontan follow-up care.
Transitions to Adult Care
Transition from pediatric to adult care should include education regarding age-appropriate awareness of one’s health condition and the skills needed for self-care. Current studies indicated that gaps of care occur in 40–60% of congenital heart disease patients, with the first gap occurring around age 19 [56]. Over 25% of patients with complex congenital heart disease had at least a 3-year gap in care. Patients with significant gaps in care are more likely to require acute interventions at the time of presentation to adult care [57]. In order to prevent these gaps and provide age-appropriate care that addresses the medical and psychosocial aspects of transition, the American Heart Association has published best practice guidelines [58]. The guidelines suggest specific transition preparation begin at age 12 and include attention to medical, psychosocial, emotional, educational/vocational, and family needs.
Conclusion
Despite the comorbidities faced by single ventricle patients following Fontan palliation, the long-term survival of these patients is a testament to the dedication of the many physicians and families who have cared for them. As we look towards the next 50 years of Fontan management, patients, families, and physicians must continue to work together to improve our understanding of the challenges facing patients. This article has reviewed not only modern Fontan palliation and its underlying physiologic implications, but also the current understanding and management of late Fontan complications. With ongoing research and multi-disciplinary approaches, the next 50 years of Fontan care will undoubtedly improve therapy for the late complications of Fontan palliation and a better quality of life for Fontan patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–6 This paper details the current understanding of Fontan physiology including optimal and suboptimal hemodynamic conditions.
•• Poh CL, d’Udekem Y. Life after surviving fontan surgery: a meta-analysis of the incidence and predictors of late death. Heart Lung Circ. 2018;27(5):552–9 One of the largest series detailing the long-term outcomes in Fontan patients.
•• Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;S0735-1097(18):36845–1 The guidelines detail the anatomic and physiologic classification of congenital heart disease, general guidelines for the care of adult congenital heart disease, and lesion-specific guidance.
Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98(14):1098–104.
McElhinney D, Reddy V, Hanley F, Moore P. Systemic venous collateral channels causing desaturation after bidirectional cavopulmonary anastomosis: evaluation and management. J Am Coll Cardiol. 1997;30(3):817–24.
Lluri G, Levi DS, Aboulhosn J. Systemic to pulmonary venous collaterals in adults with single ventricle physiology after cavopulmonary palliation. Int J Cardiol. 2015;189:159–63.
Hoffman J. Normal and abnormal pulmonary arteriovenous shunting: occurrence and mechanisms. Cardiol Young. 2013;23(5):629–41.
Sundareswaran KS, de Zelicourt D, Sharma S, Kanter KR, Spray TL, Sotiropoulos F, et al. Correction of pulmonary artery malformation using image-based surgical planning. JACC Cardiovasc Imaging. 2009;2(8):1024–30.
Triedman JK, Bridges ND, Mayer JE, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. JACC. 1993;22(1):207–15.
Grosse-Wortmann L, Al-Otay A, Yoo S-J. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion quantification with MRI. Circulation. 2(3):219–25.
Wang R-P, Liang C-H, Huang M-P, Liu H, Deng Q-P, Yang M-F. Assessment of aortopulmonary collateral flow and pulmonary vascular growth using a 3.0 T magnetic resonance imaging system in patients who underwent bidirectional Glenn shunting. Eur J Cardiothorac Surg. 2012;41(6):e146–53.
Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, et al. 40-year follow-up after the Fontan operation long-term outcomes of 1,052 patients. JACC. 2015;66(15):1700–10.
Lasa J, Glatz AC, Daga A, Shah M. Prevalence of arrhythmias late after the Fontan operation. Am J Cardiol. 2014;113(7):1184–8.
Pundi KN, Johnson JN, Dearani JA, Li Z, Driscoll DJ, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017;12(1):17–23.
Fontan F, Kirkin JW, Fernandez G, Costa F, Naftel DC, Tritto F, et al. Outcome after a perfect Fontan. Circulation. 1990;81(5):1520–36.
d’Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, et al. Redefining expectations of long-term survival after the Fontan procedure. Circulation. 2014;130(11 suppl 1):S32–8.
•• Hebson C, Book W, Elder RW, Ford R, Jokhadar M, Kanter K, et al. Frontiers in Fontan failure: a summary of conference proceedings. Congenit Heart Dis. 2017;12(1):6–16 This document introduced the use of a standardized classification system for Fontan failure including the clinical findings associated with different phenotypes of Fontan failure.
•• Book WM, Geradin J, Saraf A, Valente AM, Rodriguez F. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis. 2016;11:296–308 A document summarizing phenotype-specific management strategies for Fontan failure.
Wilson TG, Iyengar AJ, d’Udekem Y. The use and misuse of ACE inhibitors in patients with single ventricle physiology. Heart Lung Circ. 2016;25(3):229–36.
Kouatli A, Garcia J, Zellers TM, Weinstein E, Mahony LM. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation. 1997;96(5):1507–12.
Hebert A, Mikkelsen U, Thilen U, Idorn L, Jensen A, Nagy E, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) Study. Circulation. 2014;130(23):2021–30.
Wang W, Hu X, Liao W, Rutahoile WH, Malenka DJ, Zeng X, et al. The efficacy and safety of pulmonary vasodilators in patients with Fontan circulation: a meta-analysis of randomized controlled trials. Pulmonary Circulation. 2019;9(1).
Van der Ven J, Van den Bosch E, Bogers AJCC, Helbing WA. State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Research. 2018;935:1–14.
Feldt R, Driscoll D, Offord K, Cha RH, Perrault J, Schaff HV, et al. Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 1996;112(3):672–80.
Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. J Thorac Cardiovasc Surg. 1998;115(5):1063–73.
Schumacher KR, Stringer KA, Donohue JE, Yu S, Shaver A, Caruthers RL, et al. Fontan-associated protein-losing enteropathy and plastic bronchitis. J Pediatr. 2015;166(4):970–7.
Dori Y, Keller MS, Fogel MA, Rome JJ, Whitehead KK, Harris MA, et al. MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. Am J Roentgenol. 2014;203(2):426–31.
• Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–70 The authors report on the success of a novel percutaneous intervention for plastic bronchitis.
John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014;64(1):54–62.
• Egbe AC, Connolly HM, Niaz T, Yogeswaran V, Taggart NW, Qureshi MY, et al. Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J. 2017;183:10–7 Extensive retrospective review of thromboembolic complications in 387 adults with a Fontan procedure.
Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. 2001;71(6):1990–4.
Dennis M, Zannino D, du Plessis K, Bullock A, Disney P, Radford DJ, et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71(9):1009–17.
Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101(21):1731–7.
• Georgekutty J, Kazerouninia A, Want Y, Ermis P, Parekh D, Franklin W, et al. Novel oral anticoagulant use in adult Fontan patients: a single center experience. Congenital Heart Dis. 2018;1–7. This study reports the outcomes of novel anticoagulant use in Fontan patients for the prevention of thromboembolic complications.
Yang H, Bouma BJ, Mulder BJ. Vitamin antagonist anticoagulants for prevention in adult congenital heart disease investigators NK. Is initiating NOACs for atrial arrhythmias safe in adults with congenital heart disease? Cardiovasc Drugs Ther. 2017;31(4):413–7.
• Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O’Byrne ML, Lin HC, et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc. 2017;6(5):e004809-9 This study details the universal presence of hepatic fibrosis following Fontan palliation and correlates this fibrosis with clinical and hemodynamic outcomes.
•• Daniels CJ, Bradley EA, Landzberg MJ, Aboulhosn J, Beekman RH, Book W, et al. Fontan-associated liver disease proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol. 2017;70(25):3173–94 This document provides the first set of suggested guidelines for the screening and management of Fontan-associated liver disease.
Lemmer A, VanWagner LB, Ganger D. Assessment of advanced liver fibrosis and the risk for hepatic decompensation in patients with congestive hepatopathy. Hepatology. 2018;68(4):1633–41.
Wu FM, Earing MG, Aboulhosn JA, Johncilla ME, Singh MN, Odze RD, et al. Predictive value of biomarkers of hepatic fibrosis in adult Fontan patients. J Heart Lung Transplant. 2017;36(2):211–9.
Elder RW, McCabe NM, Hebson C, Veledar E, Romero R, Ford RM, et al. Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol. 2013;168(4):3764–9.
Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–6.
Egbe AC, Poterucha JT, Warnes CA, Connolly HM, Baskar S, Ginde S, et al. Hepatocellular carcinoma after Fontan operation. Circulation. 2018;138(7):746–8.
Liptzin DR, Maria MV, Younoszai A, Narkewicz MR, Kelly SL, Wolfe KR, et al. Pulmonary screening in subjects after the Fontan procedure. J Pediatr. 2018;199:140–3.
Opotowsky AR, Landzberg MJ, Earing MG, Wu FM, Triedman JK, Casey A, et al. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol. 2014;307(1):H110–7.
Fredriksen P, Therrien J, Veldtman G, Warsi M, Liu P, Siu S, et al. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart. 2001;85(3):295–9.
Matthews I, Fredriksen P, Bjørnstad PG, Thaulow E, Gronn M. Reduced pulmonary function in children with the Fontan circulation affects their exercise capacity. Cardiol Young. 2006;16(3):261–7.
Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125(18):2232–42.
Ali L, Pingitore A, Piaggi P, Brucini F, Passera M, Marotta M, et al. Respiratory training late after Fontan intervention: impact on cardiorespiratory performance. Pediatr Cardiol. 2018;39(4):695–704.
Hedlund ER, Ljungberg H, Söderström L, Lundell B, Sjöberg G. Impaired lung function in children and adolescents with Fontan circulation may improve after endurance training. Cardiol Young. 2018;28(9):1115–22.
Marino B, Lipkin P, Newburger J, Peacock G, Gerdes M, Gaynor J, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–72.
Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. 2015;4(12):e002302.
Luyckx K, Rassart J, Goossens E, Apers S, Oris L, Moons P. Development and persistence of depressive symptoms in adolescents with CHD. Cardiol Young. 2015;26(6):1115–22.
Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, et al. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137(2):158–64.
DeMaso DR, Calderon J, Taylor GA, Holland JE, Stopp C, White MT, et al. Psychiatric disorders in adolescents with single ventricle congenital heart disease. Pediatrics. 2017;139(3):e20162241.
McCrindle BW, Williams RV, Mitchell PD, Hsu DT, Paridon SM, Atz AM, et al. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113(8):1123–9.
Gurvitz M, Valente A, Broberg C, Cook S, Stout K, Kay J, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61(21):2180–4.
Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol. 2008;125(1):62–5.
Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, et al. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues. Circulation. 2011;123(13):1454–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology
Rights and permissions
About this article
Cite this article
Simmons, M.A., Elder, R.W. Modern Day Care of Patients With Single Ventricle Heart Disease: Late Complications of Fontan Palliation. Curr Pediatr Rep 7, 53–61 (2019). https://doi.org/10.1007/s40124-019-00192-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-019-00192-7