Abstract
Traditional steels and alloys face challenges in resisting high-temperature oxidation and corrosion, especially as boilers are operated at elevated temperatures. This issue has become increasingly serious. Consequently, the surface modification of materials becomes crucial to protect against various forms of degradation and enhance component's operational performance while minimising costs. In recent years, numerous researchers have explored methods to reduce the hot corrosion of boiler steels, with a particular focus on surface coatings. Surface modification techniques such as thermal spray coating, electroplating, and other metallurgical approaches are commonly employed. The use of composite coatings on steel has grown in popularity as a possible way to enhance the material's mechanical, metallurgical, erosion, and heat-resistant qualities. This review paper has reported an in-depth and critical analysis of the existing literature, focusing on various thermal spray coatings and their applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
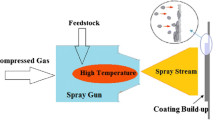
The rapid rise in the demand for electrical power is attributed to the advancements in mechanisation and automation. Demand from society is increasing because it is used to operate industrial equipment as well as for domestic purposes. In India, the substantial demand for electric power is met through diverse sources such as solar, hydro, gas, bio, and thermal power plants [1,2,3,4]. However, a significant portion of electricity production is accomplished through a coal-fired thermal power plant, constituting approximately 75% of the total power generation, according to the Ministry of Coal. An air heater, boiler, pulverising mill, coal handling facilities, ash disposal, stack emissions, and boilers are all impacted by the properties of coal, also referred to as coal quality. The efficiency, dependability, and operational availability of the boiler and emissions control equipment may be impacted by the diverse properties and heat content that coal displays [5,6,7,8,9,10]. When boiler tubes and superheaters are used at high temperatures, it has been shown that the use of low-grade coal causes a considerable amount of erosion and corrosion. Usually, low-ranked coal has a significant amount of ash and hard quartz, which is the cause of the tube surface corrosion [11,12,13]. Hot corrosion occurs on the boiler's metallic surface as a result of the formation of salt coatings including vanadium pentoxide, sodium chloride, and sodium sulphate due to coal fire. The presence of hot corrosion and erosion poses significant challenges during boiler operation, contributing to increased maintenance costs, breakdown expenses, and damage to the tubes [1, 2], as illustrated in Fig. 1. Addressing hot corrosion and erosion on the boiler tube surface can be encountered through the application of an appropriate surface engineering method.
Hot Corrosion
Impurities found in low-grade coal, including Sodium (Na), Vanadate (V), and Sulphur (S), give rise to low-melting-point compounds. These compounds, in turn, expedite oxidation on the metallic surface, particularly under elevated temperatures ranging from 600 to 900 °C [14,15,16,17,18].
Some researchers have investigated the chemical processes involved in salt formation during fuel combustion and their findings suggest that the Sulphur content in coal and fuel oils, upon burning, produces sulphur dioxide, which gradually oxidises into sulphur trioxide. Concurrently, Sodium chloride reacts with sulphur oxides (SO3) and water vapours at combustion temperatures, resulting in the formation of sodium sulphate [3]. This sodium sulphate can then deposit on metal surfaces and persist in a liquid state at higher temperatures. The trace of vanadium contained in fuel will generate vanadium pentoxide when it is burned, and then react with sodium sulphate (Na2SO4) to produce highly corrosive sodium vanadate. At higher temperatures, the deposited sodium sulphate is in a liquid state and will seriously corrode metal surfaces and alloys [19,20,21,22].
Hot corrosion on superalloys typically involves two major stages: the Initiation stage and the Propagation stage. In the Initiation stage, the process typically begins with the rupture of the protective oxide layer on the surface. Subsequently, the oxide layer undergoes a self-repair mechanism to restore its integrity. If the salt is present on the surface, which is already covered with a protective oxide, then initially there’s no reaction [23,24,25,26]. In the broken protective oxide layer, there must be some accelerated oxidation, and this can occur in four manners. (i) The mechanical interruption of the oxide, i.e. for example by thermal cycling, and by placing the oxide surface in tension (ii) Sulphur scattering occurs within the oxide, leading to the formation of chromium-rich sulphide structures inside the metal. This is followed by the subsequent advancement of the external oxide layer or its rearrangement, especially if the oxide layer is mechanically removed. (iii) Disintegration of the defensive oxide from the salts. (iv). During the combustion, a reduced area of the environment may be shaped because of inadequate consumption of fuel [27,28,29,30,31]. This kind of decreasing air may harm the surface oxide layer, particularly within the sight of impurities like Na2SO4. Suppose the repair of the oxide is not at all possible, then propagation of phases leads to rapid degradation of the alloy. As soon as coating penetration occurs, the spread stage prompts catastrophic corrosion. Various components influence the time taken to corrosion moves from the initiation stage to the propagation stage. Some of the factors like alloy composition, fabrication condition, gas deposition and its velocity, salt deposition, rate of salt deposition, temperature, specimen geometry, and erosion play a major role in identifying the sort of reaction product that is shaped inside the propagation stage of hot corrosion [32,33,34,35]. The propagation of corrosion was studied in laboratory-level experiments and the standard test cycle structure aims to simulate the cyclic nature of thermal and environmental stresses that materials might encounter in practical applications, especially in high-temperature and corrosive environments. Each test cycle consisted of 1 h of heating at a specified temperature followed by cooling at the ambient temperature for 20 min [36,37,38,39,40].
Thermal Spray Process
The use of basic raw materials is typically limited to specific applications due to inherent properties. Certain application areas require characteristics that are challenging to achieve with basic materials alone. This has led to an increased reliance on alloys, superalloys, composites, and coatings to enhance the performance of materials, allowing them to withstand the working environment economically and efficiently [1, 41,42,43]. Protective coating has gained the attention of researchers due to its increased hardness. Surface Engineering deals with the modifying surface structure, chemistry, and properties of substrate material to reach good performance and durability by either mechanical, metallurgical, physical, or chemical means or by producing a thick or thin layer of coating. Surface engineering involves altering the surface properties to innovate to scale down the degradation over time by making a robust surface. Some applications require materials with sufficient erosion and corrosion resistance. Enhancing the performance of components in such applications may involve considering a composite structure, which combines a base material with a protective coating layer having a distinct structure. This approach serves as an alternative for achieving a combination of material properties [4, 44,45,46,47]. Coatings play a crucial role in preventing the degradation and corrosion of materials by forming a protective layer that shields the surface. The primary coating techniques include Chemical Vapour Deposition (CVD), Physical Vapour Deposition (PVD), and thermal spray methods. Among these, flame spraying with powder, plasma, and HVOF spraying is gaining increasing importance. The coating can help to enhance the functionality and performance of the equipment or product. It helps to create surface corrosion resistance and wear resistance which enables it to withstand high temperatures. Thermal spraying evolved when early experiments in which a stream of high-pressure gas could cause liquid droplets to break into fine particles. The area of interest was to produce powders rather than constructing coatings. The scientist named Dr. Max Ulrick School of Zurich observed the possibility that the molten particles impinging upon themselves could cause the coating on the substrate. His work, and that of his collaborators, resulted in the establishment of the Thermal spray process.
The coating layer formed, depending on its microstructure, can be used in various industries including Electronic, Automotive, Aeronautic, and Thermal to provide.
-
Wear resistance, abrasion, and erosion.
-
Thermal barrier coating to protect structures
-
Protection against resistance to higher temperature oxidation, erosion, and corrosion
-
Building composite structures of metals and ceramics
The principle of the thermal spray process involves heating feedstock materials, (Wire or Powder) and then speeding it up to a high speed, and then allowing the molecules to hit the workpiece surface. The molecules thus deposited will deform and solidify onto the workpiece surface. Millions of molecules are deposited on top of each other to build the coating (Fig. 2).
Mechanical or metallurgical bonding is used to adhere these particles to the substrate. There are different coating deposition methods available and the selection of the right one relies on the operational requirements for coating, whether the coating material is suitable for the planned method, the requirement of coating adhesion, and the availability of facility and coating cost [48,49,50,51,52,53,54,55,56,57,58,59,60].
Choosing a particular method involves selecting specific process parameters. Several parameters are integral to the thermal spray process, such as Spray Material, Spraying Distance, Spraying Angle, Gas Flow Rates, Carrier Gas Flow, Powder Feed Rate, Electric Current, Plasma Gas Power, Cooling System, and Spray Pattern Control. The optimisation of these parameters is essential to attain the targeted coating properties, encompassing thickness, adhesion, hardness, and porosity. The specific values for these parameters will be contingent on both the chosen thermal spray method and the material designated for spraying.
Types of Thermal Spray Coating Processes
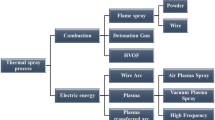
Several types of advanced thermal spraying techniques are being used in industries. They are as follows (Ref. Fig. 3):
Powder or Wire Flame Spray
In 1912, Swiss Engineer MU Schoop invented the spraying process, which was initially used for spraying metals with low melting points, such as tin and lead. It was then expanded to include the spraying of refractory metals and ceramics. It’s a low-energy thermal spraying method. The coating material used for deposition is in wire form, and the spraying cost is minimal compared to other methods (Fig. 4).
The Powder or Wire Flame Spray process offers cost-effectiveness, versatility, and applicability to large surfaces. However, it also has limitations, including the potential for lower coating density, bond strength, and temperature resistance. The selection of this process depends on the specific requirements of the application, the desired properties of the coated material, and considerations related to cost and production efficiency.
Detonation Gun Spray
A mixture of fuel and oxygen (O2) is ignited in a long barrel using a spark plug in this process. The detonation pressure wave arises from the explosion of the mixture, which heats up and propels the powder particles with a high velocity towards the surface of the substrate. It deposits a variety of materials and ceramic coatings onto workpieces at supersonic speeds using the controlled explosion of oxygen and acetylene (Fig. 5). Detonation Gun Spray offers advantages such as high particle velocities, low oxidation, and versatility in material choices and it has limitations, including challenges in achieving very thick coatings, equipment complexity, and cost, and restrictions on the size of coated parts.
High-Velocity Oxy Fuel (HVOF)
The procedure, shown in Fig. 6, involves converting the powdered or wired raw materials into a liquid condition and propelling them quickly onto a surface by the use of fuel gas combinations and oxygen. Various types of fuel gases are employed in this process, including propylene, propane, hydrogen, methane, acetylene, ethylene, crylene, SPRAL-29 kerosene, MAPP gas, LPG, and others [5]. The process starts by burning the chosen fuel gas in the presence of oxygen. This combustion generates a high-velocity, high-temperature gas stream. A carrier gas is used to transport the powdered coating material from a feeder unit to the HVOF gun. At the gun, the transported powder is ignited by the high-temperature gas stream, causing it to rapidly accelerate towards the substrate. During the process, high-speed powder particles strike the substrate's surface, stick to it, and build up to form a coating. This procedure results in a thick covering with very little porosity. The HVOF process is renowned for its capability to generate top-tier coatings characterised by exceptional properties such as high bond strength, minimal porosity, and enhanced resistance to wear and corrosion. This technique finds extensive use across diverse industries for applications related to surface protection and improvement.
HVOF process is a widely used and effective coating technique with several advantages, particularly in producing high-density and high-adhesion coatings. However, it also has limitations, including equipment costs, substrate size constraints, and potential thermal stress issues.
Plasma Spray Coating
A surface engineering technique called plasma spray coating involves depositing material onto a substrate—usually in the form of wire or powder. The first step in the procedure involves using a plasma torch. The plasma jet produced by this torch has a very high temperature. An ionised gas with a temperature of at least 10,000 Kelvin (K) is called plasma. The substance to be deposited is introduced into the powerful plasma jet in the form of wire or powdered particles. The injected material melts quickly due to the plasma jet's high temperature. It is crucial to remember that the plasma torch's temperatures are far greater than the material's melting point. After melting, the substance is shot towards the substrate that has to be coated at very high speeds. Melted droplets of the material emerge from the plasma torch. The molten droplets quickly cool and solidify when they come into contact with the substrate. As a result, a coating develops on the substrate. The substance that is deposited sticks to the substrate and solidifies rapidly. A coating thickness that generally ranges from approximately 50–500 µm may be achieved by control of the plasma spray process, contingent upon the particular application and needs. In many different sectors, plasma spray coating provides a flexible method for applying functional and protective coatings on surfaces. It is renowned for its capacity to produce coatings with advantageous characteristics including thermal insulation, wear resistance, and corrosion resistance. Because of the process's versatility, a large range of materials may be deposited, making it appropriate for a variety of applications.
Plasma Spray Coating offers advantages such as material versatility, high coating density, and controlled coating thickness, and its limitations, include high equipment costs, challenges in achieving very thick coatings, and potential issues related to substrate overheating.
Effect of Thermal Spray Coating on Corrosion
Thermal spray coatings are widely employed to protect substrates from severe erosion and high-temperature corrosion. Among these methods, the HVOF technique has become a preferred choice in critical applications due to its ability to produce a uniform coating with improved mechanical strength properties and superior resistance to oxidation and corrosion. The survey results demonstrate the technique's reliability, as it consistently delivers coatings with uniform thickness and high hardness when applied to metal surfaces.
Thermal spray technology represents a dynamic and evolving field of research with applications in metallurgical improvement. Thermal spray coating provides effective solutions to address challenges associated with wear, erosion, and oxidation in high-temperature environments, making it a valuable area of study and innovation in the field of materials science and engineering. One of the primary applications of thermal spray coatings is in environments with elevated temperatures, where these coatings are employed to improve the resistance against wear, erosion, and oxidation. The selection of coating materials in thermal spray coating is a critical consideration. Carbides, oxides, and ceramics are promising materials for coatings designed to protect against erosion, oxidation, and wear. Solid lubricant coatings have demonstrated enhanced wear resistance, particularly at higher temperatures. In recent years, numerous studies have been published exploring a variety of coating materials aimed at developing resistance to erosion, wear, and oxidation in thermal spray coatings.
Nagabhushana et al. [6], used an atmospheric plasma spray technique to coat Super Ni 76 with NiCrBSi, fly ash, Chromium Oxide (Cr2O3), Molybdenum (Mo), and Titanium dioxide (TiO2) composite coating. SEM (Scanning Electron Microscopy) was employed to assess the surface morphology and cross-section of the coating. This was done to ascertain the characteristics and nature of the deposited coatings. The highest microhardness 708HV0.02 was observed for composite coating NiCrBSi/fly-ash combination while the lowest 620HV0.02 was observed for NiCrBSi/fly-ash/Mo coating. In all the cases composite coating had higher microhardness than the substrate material. According to the study from Amrinder Mehta et al. [7] the thermal barrier coating methods have mainly been used for providing thermal and corrosive insulation for diesel, marine, and steam turbine engines at high-temperature environmental conditions. The lamellar microstructure created by the quick solidification of impinging liquid droplets and cohesiveness among splats distinguishes thermal spray coatings.
High-temperature corrosion of two superalloys, Inconel 617 and Inconel 738, was examined in a research by Wadi et al. [8]. This study was conducted for a maximum of 100 h and included three different temperature ranges: 700, 800, and 900 °C. The corrosion conditions were intentionally accelerated on the specimen surfaces by introducing sodium chloride (NaCl) and sodium sulphate (Na2SO4) into the experimental setup. Both Inconel 617 and Inconel 738 demonstrated remarkable resistance to oxidation, particularly at elevated temperatures of 800 and 900 °C. The main oxides that formed on the surface of the alloys during the corrosion process were identified as chromium oxide (Cr2O3) and nickel–chromium oxide (NiCr2O4). These oxide layers are known for their protective qualities and can act as barriers against further corrosion. Moreover, the study highlights the importance of understanding how corrosion rates can vary with temperature, which is vital for materials used in applications subject to high-temperature conditions.
Cesanek et al.'s research [9] explores how heated corrosion processes impact coatings made of Cr3C2-25%NiCr, Hastelloy C-276, and Stellite-6. These coatings were created at 750 °C in a high-temperature environment using the High-Pressure High-Velocity Oxygen Fuel (HP/HVOF) techniques. On the specimen surfaces, a combination of 60% V2O5 and 40% Na2SO4 was applied to speed up the corrosion conditions. He tested each specimen cyclically for 50 cycles, with a 20-min cooling interval in between. He then compared the corrosion resistance of three coatings: Cr3C2-25%NiCr, Stellite-6, and Hastelloy C-276, at various temperatures. Hastelloy C-276 showed the best corrosion resistance among these coatings. The exceptional ability of Hastelloy C-276 to withstand corrosion was ascribed to the development of a shielding oxide layer on its exterior. This oxide layer consisted of significant phases, including chromium oxide (Cr2O3), nickel–chromium oxide (NiCr2O4), and nickel-molybdenum oxide (NiMoO4). These oxides act as barriers, shielding the underlying material from further corrosion.
The investigation carried out by Vasudev et al. [10] had the objective of comparing the hot corrosion behaviour between Inconel-718 coatings, deposited using the HVOF method, and bare cast iron. The investigation involved subjecting both materials to 50 hot corrosion cycles, each followed by a 20-min cooling period. The research sought to understand the protective capabilities of Inconel-718 against hot corrosion at a high temperature of 900 °C. The study compared the response of Inconel-718 coatings with that of bare cast iron when exposed to a hot corrosion environment. The analysis revealed that Inconel-718 outperformed cast iron in terms of hot corrosion resistance at the elevated temperature of 900 °C. The enhanced corrosion resistance of Inconel-718 was attributed to the formation of stable oxide phases within the coating. These phases included NiCr2O4, Cr2O3, aluminum oxide (Al2O3), and TiO2. The presence of these oxides acted as protective layers, guarding the underlying material against further corrosion. The study suggested that the Inconel-718 coating effectively protected the substrate from the damaging effects of hot corrosion. This protective role is crucial for applications where materials are subjected to aggressive high-temperature environments.
Delaunay et al.'s investigation [11] concentrated on the superalloy Inconel-718's high-temperature oxidation behaviour. The goal of the research was to comprehend the behaviour of this alloy at high temperatures (900 °C) during oxidation. The author proposed that Inconel-718 exhibits resistance to oxidation when exposed to high temperatures, specifically at 900 °C. Oxidation resistance is a critical property for materials used in applications subjected to elevated temperatures. According to the research, the development of oxide layers including minor alloying elements, such as titanium oxide (TiO2) and aluminium oxide (Al2O3), is responsible for Inconel-718's resistance to oxidation. These oxide layers act as protective barriers, shielding the underlying material from further oxidation. Additionally, the study focused on the intermetallic phase Ni3-Nb forming on a quasi-continuous scale at the interface of the alloy and oxide layer. Such intermetallic phases can contribute to the overall oxidation resistance of the material.
In order to better understand how two nickel-based superalloys behaved at high temperature conditions namely, 900 °C Mudgal et al. [12] undertook research. The study's main objectives were to learn more about these metals' resistance to corrosion and the makeup of the oxide scales that are produced when corrosion occurs. The research contrasted a cobalt-based superalloy's high-temperature corrosion resistance with that of two nickel-based superalloys. It was discovered that at 900 °C, the nickel-based alloys outperformed the cobalt-based alloys in terms of resistance to corrosion. The superior corrosion resistance of the nickel-based alloys was attributed to the development of stable oxide layers containing key components such as nickel chromite (NiCr2O4) and chromium oxide (Cr2O3). These oxide layers acted as protective barriers, preventing further corrosion of the alloys. In contrast, the cobalt-based alloy exhibited the formation of a thick oxide scale that contained components including cobalt oxide (CoO), nickel tungstate (NiWO4), nickel chromite (NiCr2O4), and cobalt chromite (CoCr2O4). This oxide scale was less effective at protecting the alloy, which contributed to its lower corrosion resistance. Based on the study's findings, the authors recommended avoiding the use of bare cobalt-based alloys in certain circumstances, particularly when exposed to high-temperature corrosion environments.
The study conducted by Javed et al. [13] focused on the development of a coating using the HVOF method and compared it with the Atmospheric Plasma Spraying (APS) coating as well as bare Monel K500 substrates. The objective was to assess the performance of these coatings for marine applications, particularly in a corrosive environment. The coating samples were subjected to Neutral Salt Spray testing for 1553 h, which simulated a harsh marine environment with exposure to saltwater mist. This testing is designed to assess the corrosion resistance of materials and coatings in such conditions. The test results revealed that the HVOF-coated samples exhibited excellent corrosion resistance. The HVOF-coated samples did not exhibit any signs of corrosion attack, indicating that the coating was successful in protecting the substrate against corrosion. During testing, however, the uncoated Monel K500 substrate displayed a localised corrosion attack. Pits measuring as much as 0.6 mm in breadth and 25 μm in depth were found on the exposed substrate. This underscores the vulnerability of unprotected materials to corrosion in marine environments. These findings highlight the importance of coatings in enhancing the longevity and performance of materials in marine applications.
The HVOF approach was used in the work by Singh et al. [14] to create a composite coating for a boiler tube. Evaluating the coatings' resistance to heat corrosion was the main goal. The research compared two types of coatings, namely NiCrAlY-SiC and NiCrAlY-B4C, and examined their composition and performance under hot corrosion conditions. These coatings were specifically designed to enhance the hot corrosion resistance of the tube's surface. Hot corrosion is a critical concern in boiler applications where materials are exposed to high temperatures and corrosive environments. The NiCrAlY-SiC coatings were found to contain layers consisting of Cr2O3, SiO2, Al2O3, and NiO, along with spinals phases. On the other hand, the NiCrAlY-B4C coatings had layers containing Boron trioxide (B2O3), alumino-boron carbide (Al3BC), Al2O3, Cr2O3, and spinal phases. The composition of these coatings is significant as it determines their protective properties. The study's results indicated that the NiCrAlY-SiC coatings outperformed the NiCrAlY-B4C coatings in terms of hot corrosion resistance. This suggests that the NiCrAlY-SiC coating provided superior protection against hot corrosion in the boiler tube environment.
The study conducted by Patil et al. [15] focused on the development of a composite coating on T22 boiler steel using the HVOF spray method. The research aimed to investigate the microstructure and mechanical properties of the as-deposited coatings, with a particular focus on aspects such as porosity, microhardness, coating thickness, and coating density. The study provided insights into the microstructure and mechanical properties of the coating, including porosity, microhardness, thickness, and density. The microhardness values exhibited a decrease from the coating to the bare alloy. This suggests that the coating had different hardness properties compared to the underlying T22 boiler steel.
At severe temperatures, Praveen et al. [16] examined the oxidation and erosion resistance of WC–Co reinforced with SS304 stainless steel substrate and HVOF-coated NiCrSiB reinforced with Al2O3. Micro-structured Al2O3 supplemented NiCrSiB fared better than other coatings in terms of oxidation resistance. The study indicates that the NiCrSiB/WC–Co coating has enhanced erosion resistance.
In Sun et al.'s study [17], an HVOF spraying approach was used to provide an amorphous Fe-based coating on 8090 Al-Li alloy. The primary aim of the study was to investigate the influence of this amorphous coating on the corrosion resistance of the 8090 Al-Li alloy. The evaluation included a comparison of the 8090 alloy's corrosion resistance between its coated and naked forms. Corrosion resistance is a crucial factor for materials used in various applications, as it determines their ability to withstand degradation when exposed to corrosive environments. The study yielded several important findings, one of which was that the coated 8090 alloy had seven times more corrosion resistance than the naked 8090 alloy. This indicates a substantial improvement in the alloy's ability to resist corrosion when it is protected by the Fe-based amorphous coating.
In a work on Cr3C2–NiCr cermet coatings, Zhang et al. [18] used HVOF with shroud plasma spraying to deposit the coating. Both coatings underwent an erosion test that replicated the multi-angle, high-speed erosion environment brought on by tiny particles on turbine blades. Comparing the HVOF coating to the SPS coating, the findings showed that the former had more density and fewer flaws.
The study conducted by Kumari et al. [19] focused on investigating the hot corrosion behaviour of a composite coating composed of 93% tungsten carbide (WC) and 7% chromium carbide (Cr3C2) with a nickel (Ni) binder. This composite coating was applied using the HVOF method on ASME SA213-T91 steel material. The research aimed to assess how well this coating performed under conditions of hot corrosion, particularly focusing on weight gain and the formation of protective oxide scales. According to the study's findings, the composite-coated material showed no weight increase in tests including cyclic hot corrosion. Additionally, the study noticed that protective oxide scales were forming on the coated material's surface. These results show that the ASME SA213-T91 steel was effectively protected against heat corrosion by the composite covering. According to Vasudev et al. [20], the coatings' microhardness showed an increasing trend as the Al2O3 concentration increased, whereas their density, porosity, and surface roughness showed a decreasing trend. Out of all the composite coatings, the one with the greatest fracture toughness rating was the one with 20 weight percent alumina reinforcement.
Ribu et al. [21] have developed WC-10Co composite coating on 35CrMo steel and the results showed 35% higher wear resistance compared to uncoated steel. In order to enhance surface qualities for corrosion-related applications, Patil et al. [22] have effectively coated the SA213-T22 bare alloy with a high entropy alloy NiCrMoFeCoAl. The research involved a comprehensive characterisation of the deposited coatings concerning their microstructure and mechanical properties. The evaluation encompassed parameters such as porosity, microhardness, coating thicknesses, and coating density. Interestingly, the study revealed a decline in microhardness values when transitioning from the coating to the bare alloy.
Using the HVOF spraying technique, coatings' mechanical characteristics and wear behaviours were the main subject of a research by Khuengpukheiw et al. [23]. After applying coatings to AISI 1095 steel, the researchers looked at the performance of WC–Co/NiSiCrFeB, NiSiCrFeB, and WC–Co. Through frequent contact with relatively soft sliding steel components, these experiments sought to determine how well these coatings might tolerate cumulative wear. NiSiCrFeB coating wear rates were shown to be rather high by the investigation. For applications requiring moderately hard counterwork materials, including medium to high carbon steels, WC-Co/NiSiCrFeB and WC-Co coatings were thus thought to be more appropriate than NiSiCrFeB coatings.
Verma et al. [24] used the HVOF spray technique to investigate the corrosion behaviour of steel that was not coated vs steel that was coated with IN 625. The study aimed to assess the protective properties of the IN 625 coating at varying temperatures and examine the phases of oxide scale present on the coated samples. Because of corrosion, the oxide scale on the uncoated steel peeled off and spalled. Conversely, the IN 625 coating displayed notable corrosion resistance. This suggests that the coating effectively protected the steel substrate from corrosion-related damage. The study noted that the IN 625 coating provided better protection against corrosion at 700 °C compared to 900 °C. This temperature-dependent behaviour is essential for understanding the coating's performance under different operating conditions. The study distinguished between oxides and spinels of nickel and chromium as the main components in the oxide scale of coated samples. Comprehending the oxide scale's composition is crucial for evaluating the coating's ability to form protective layers. These findings have practical implications for potential applications of HVOF spray coatings on boiler tubes operating between 700 and 900 °C. Coatings like IN 625 can provide improved protection against corrosion in high-temperature environments, crucial for boiler applications.
The research conducted by Hanumanthlal et al. [25] focused on the behaviour of high-temperature hot corrosion in plasma-sprayed coatings. The study aimed to assess the impact of different coating compositions, particularly the inclusion of fly ash cenosphere powder, on the resistance to hot corrosion. The research involved the development of coatings with a specific composition consisting of Fe17Cr2Ni0.18C and fly ash cenosphere powder. Different weight ratios were maintained, including 0, 5, 10, and 15% of cenosphere powder. The coatings were applied to T22 boiler steel material. Boiler steels are exposed to high-temperature and potentially corrosive environments, making them a suitable test substrate for assessing the coatings' performance. In a liquid salt environment consisting of 60% V2O5 and 60% Na2SO4, both the coated and naked steel specimens underwent a hot corrosion test. At a maximum temperature of 600 °C, there were 17 cycles in the test, each lasting 51 h. In hot corrosion testing, the extreme temperatures that materials may encounter in industrial applications are simulated. When compared to the uncoated steels, the FeCrNiC/cenosphere-coated steels demonstrated a higher level of resistance to heat corrosion, according to the study's results. This indicates that even under the severe hot corrosion test circumstances, the coatings successfully prevented the T22 boiler steel from deteriorating. When compared to the uncoated steels, the coated steels' corrosion behaviour obeyed the parabolic rate law, and their parabolic rate constant values were less. Slower corrosion rates are shown by lower rate constants, which also show how efficient the coatings are in protecting. Because some compounds, such as mullite, alumina, and an oxide layer of silicon that forms as protection at high temperatures, are stable at high temperatures, the coated steels showed enhanced resistance to hot corrosion.
The research conducted by Verma et al. [26] aimed to investigate the impact of copper (Cu) on the high-temperature wear behaviour of a high-entropy alloy composed of Co, Cr, Fe, Ni, and varying amounts of copper (CoCrFeNiCux). The alloy was produced using the arc melting method. The study explored how the addition of copper affected the wear rate of the alloy at different temperatures. The research findings indicated that the inclusion of copper in the CoCrFeNi alloy led to a reduction in the wear rate. This reduction in wear rate was observed at both room temperature and higher temperatures. The wear rate reduction is a desirable characteristic as it signifies improved resistance to wear and frictional forces. An interesting aspect of the study was the formation of a copper oxide (Cu oxide) layer on the alloy's surface. This oxide layer was found to contribute to the increased wear resistance of the CoCrFeNiCu alloy, particularly at elevated temperatures. The Cu oxide layer appeared to play a role in reducing wear, especially at 600 °C.
The research conducted by Venkata et al. [27] focused on investigating the high-temperature tribology behaviour of a Ti6Al4V-TiC composite coating. This coating was produced using the cold-spray technique and was evaluated for wear resistance when subjected to different temperatures. The study also involved using a WC-Co sphere as a counter face in tribological testing. The primary focus of the study was to assess the wear resistance of the composite coating at various temperatures. Wear resistance is a crucial factor in applications where materials are exposed to abrasive or erosive conditions. The research findings indicated that the Ti6Al4V-TiC composite coating demonstrated superior wear resistance at all temperatures tested. This suggests that the composite coating effectively protected the substrate from wear, even under high-temperature conditions. The research also determined that the Ti6Al4V coatings' primary wear mechanism was abrasive wear. Nevertheless, the wear on the composite coating was reduced by the existence of a tribolayer, a layer created at the interface as a result of wear and friction. This implies that the formation of the tribolayer improved the wear resistance of the composite coating. The generation of oxide layers, such as WO3, CoWO4, and TiO2 compounds, over 200 °C was crucial for enhancing the Ti6Al4V-TiC composite coating's wear resistance. These oxide layers possess the capacity to function as barriers that avert wear and corrosion.
The effects of the aluminium (Al) content on the high-temperature oxidation and wear resistance of an AlxCoCrFeMnNi alloy, a high-entropy alloy, were studied by Fuxing et al. [28]. The research sought to determine how changes in aluminium concentration influenced the characteristics of the alloy, which was produced via plasma arc cladding. Examining how Aluminium (Al) affects the behaviour of the alloy, specifically with regard to hardness, wear resistance, and oxidation resistance, was the main goal of the study. Material characteristics may be greatly impacted by the addition of Al. According to the study results, the AlxCoCrFeMnNi alloy's hardness and wear resistance improved when the Al content was raised. This indicates that Al was a major factor in improving the alloy's resistance to wear and friction. The research found that metal atom diffusion significantly influenced the behaviour of the alloy at a high temperature of 900 °C in the environment. One important discovery was that the alloy formed a finer and denser oxide coating, mostly made up of Cr2O3 and Al2O3, as the amount of Al increased. The diffusion of oxygen and base elements was hindered by this oxide coating. Thus, it enhanced the alloy's ability to withstand oxidation at elevated temperatures.
The study by Adnan et al. [29] included the manufacture of α-Al2O3 nanoparticles using a straightforward sol–gel method. In order to stop high-temperature corrosion in boiler tubes, both with and without fuel ash, these artificial nanoparticles were used as a coating layer. NiCrAlY alloy was combined with them. The thermal plasma process was used to apply the coating. Austenitic stainless-steel tube corrosion rates were determined at different temperatures using the weight loss technique. Using XRD, SEM, and EDS examination, the generated α-Al2O3 nanoparticles and the surface morphology of the austenitic stainless-steel tubes were characterised. The findings reveal that the degree of corrosion increases with rising temperatures both in the presence and absence of the coating. The maximum coating performance was 82% in the absence of fuel ash and 88% in its presence.
Effect of Heat Treatment on Thermal Spray Coating
Even with sophisticated coating techniques, it has been discovered that getting completely flawless coatings is difficult. Modern coating methods may be used, however during coating processing; a few small flaws will inevitably show up [30,31,32]. There are a few voids at the splat borders after the coating procedure. The susceptibility of the substrate to corrosion is increased by these flaws [33]. The heat treatment method modifies the remaining stresses in surfaces coated with HVOF. After cooling, the secondary stress field that was created is where residual stresses are directed by elevated-temperature heat treatment. A secondary stress field exists as a result of the substrates and coatings differing coefficients of thermal expansion. Heat treatment may boost the adhesive strength because the coating and substrate diffuse more readily [34, 35]. Chatha et al. [36] looked at the impact of post-heat treatment on T91 steel coated with Cr3C2–NiCr using the HVOF spray method. While the coating's hot corrosion resistance has improved, the post-heat treatment procedure has also led to an improvement in hot corrosion resistance. By causing oxides to develop on splat boundaries quickly, the heat treatment speeds up the process of filling pores. By using this method, heated corrosion may be effectively prevented from spreading.
Lee and Min et al. [37] verified the characteristics and microstructure of the coated surface using the heat treatment method. An increase in annealing temperature from 550 to 750 °C and 900 °C results in an improvement in the surface's corrosion resistance. A decrease in porosity, a rise in grain coarsening and other related changes occurred with the raising of the annealing temperature. The most effective method for reducing porosity in HVOF-sprayed coatings has been identified as heat treatment.
Shabanlo et al.'s [38] research showed that porosity is greatly reduced by heat treatment at 1045 °C. Defects of all kinds, including microstructural faults and unmelted particles, are encountered throughout the spraying process. Wear resistance and hot corrosion behaviour have both improved. Additionally, re-melting of particles has been found to enhance adhesion between the coating and substrate. Thermal spray plays a vital role in contemporary engineering applications, and there is a growing recommendation for the adoption of techniques like additive manufacturing. Moreover, the utilisation of superalloys and reinforcements is advised in industrial applications where thermo-mechanical strength is necessary, contributing to the prevention and reduction of hot corrosion and erosion.
Durga Prasad et al. [39] studies reported the development of a protective coating consisting of CoMoCrSi–Cr2C3 applied through the HVOF spraying method on grade 15 titanium alloy. After the initial spraying, the coatings undergo a microwave heating step. This additional heat treatment was applied to induce specific microstructural changes, optimise coating properties and to enhance adhesion. This combination aims to enhance the high-temperature sliding wear resistance of the material, making it suitable for applications where durability and resistance to wear at elevated temperatures are critical, such as in aerospace or industrial settings. The specific composition and application method are chosen to provide optimal properties for withstanding the intended wear conditions. The post-heat treatment has led to the inter-diffusion of elements. This suggests that atoms from the coating and substrate have migrated across the interface, creating a region where the composition of elements is mixed. Inter-diffusion is a common phenomenon during heat treatment and can contribute to the formation of various phases. As a result of the recrystallisation and elements migration, the microstructures of the annealed coatings have become more compact as compared to sprayed coating [36,37,38,39].
Guo et al. [40] study investigates the microstructures, mechanical properties, and tribological behaviours of HVOF sprayed CoMoCrSi coatings across a broad temperature range. A key focus is on the comparison between as-sprayed coatings and those subjected to an 800 °C annealed treatment. The annealed coatings exhibit well-crystallised structures, improved microstructures, and enhanced properties. Notably, the surface microhardness and adhesive strength increased by 13.3 and 12.5%, respectively, after annealing. A compact oxide layer forms on the annealed coating surface, comprising Cr2O3, Co3O4, MoO3, and CoMoO4, contributing to increased microhardness and excellent tribological behaviours. The annealed coatings demonstrate lower friction coefficients and wear rates than their as-sprayed counterparts across a wide temperature range. Consequently, the 800 °C annealed treatment proves effective in significantly improving the microstructures and properties of HVOF sprayed CoMoCrSi coatings, positioning them as promising candidates for abrasion-resistant materials in diverse temperature environments.
The researcher Zhang [41] investigates the impact of laser heat (LH) treatment on the microstructure, microhardness, and wear performance of HVOF-sprayed WC–CrC–Ni coatings. Laser scanning velocity is explored as a crucial parameter during LH treatment. The results reveal substantial changes in the microstructure after LH treatment, with WC and Cr3C2 identified as primary phases in both HVOF- and LH-treated coatings. Notably, a compact interface forms between the coating and substrate after laser heating. Decreasing laser scanning velocity reduces porosity and thickness in LH-treated coatings, leading to a gradual increase in microhardness. In wear tests, optimal laser heating conditions (600W power, 300 mm/min scanning velocity) significantly enhance friction and wear resistance. This improvement is attributed to reduced porosity, increased hardness, and the formation of oxide tribofilms on the coating. Overall, LH treatment proves effective in modifying the microstructure and mechanical properties of conventional coatings, enhancing wear performance.
The literature highlights the development of various composites and high-entropy alloys to address the challenges posed by elevated-temperature wear, erosion, and corrosion environments. Among these, composite coatings have gained significant attention, and several composite coatings with their respective applications have been reported. Table 1 presents information on composite coatings and their applications. Table 2 provides a list of composite coating combinations along with the bonding strength of the developed coatings. Additionally, Table 3 summarises the development of high entropy alloys for thermal spray applications, while Fig. 7 showcases the reporting of basic steel material designed for corrosion resistance applications.
Hardness of High-temperature steels [42]
Despite the use of steels and coatings, various composites and alloys have been developed using different technologies for applications in high-temperature corrosion and wear environments. A comparative study reveals that the hardness of ceramic composites and high-entropy alloys (as shown in Fig. 8) is higher than that of steels and coatings.
Hardness of High-temperature alloys [42]
Electrochemical Corrosion Analysis
The use of nickel-based coating powder (NiCrMoNb), or Inconel 625, coated on carbon steel and stainless steel by HVOF thermal spray technique was studied by Boudi et al. [43]. In specifically, the research sought to ascertain the coatings' tensile bond strength in a corrosive media both before to and during electrochemical testing. A solution bath containing 0.1N H2SO4 and 0.05N NaCl was used for the static electrochemical test. After three weeks of testing in the corrosive medium, the main conclusion to be drawn from the data is that the tensile bond strength dramatically declines. This implies that the corrosive environment has a negative impact on the coated materials' ability to bind. The work conducted by Guilemany et al. [44] investigates the corrosion resistance of heat-sprayed HVOF coatings, with a particular emphasis on cermet coatings that have different amounts of CrC-NiCr layers. The goal of this research is to comprehend how coating thickness affects how steel that is coated behaves as it corrodes. Higher porosity and residual strains in cermet coatings, such CrC-NiCr, are known to affect corrosion resistance, even though thicker metallic coatings typically provide superior corrosion protection. Electrochemical tests in a 3.4% NaCl solution are also used in the study. These experiments include Open-Circuit Potential (EOC), Polarisation Resistance (R), Cyclic Voltammetry (CV), and Electrochemical Impedance Measurements (EIS). Optical microscope and SEM are used in structural characterisation together with EDS research. Research results show that the behaviour of the substrate largely determines the corrosion resistance of the overall system, and that the anticorrosive qualities are not greatly improved by increasing the number of deposited layers. According to the research, tension produced during the spray deposition process is a major factor in how coated steel responds to corrosion processes. In general, the study offers valuable perspectives on the intricate interactions among coating thickness, composition, porosity, residual stresses, and substrate effect on corrosion resistance in thermally sprayed ceramic coatings. [45] Liang et al. A Cr3C2–NiCr interlayer and a top DLC film were combined to form a duplex coating by the use of HVOF spraying and closed-field unbalanced magnetron sputtering (CFUBMS). In comparison with single DLC and Cr3C2–NiCr coatings, the electrochemical behaviour of the Cr3C2–NiCr/DLC duplex coatings in a 3.5 wt% NaCl solution was examined. Without changing their microstructure, the cermet interlayer made the DLC films' surfaces rougher. Dynamic potential polarisation tests revealed that the duplex coatings had excellent surface integrity even after localised damage. Excellent corrosion resistance was achieved by the cermet interlayer preventing penetrating fractures and hence preventing substrate corrosion. Vijaya Lakshmi's work [46] looks at the wear and corrosion behaviours of ODS FeAl powder coatings sprayed by detonation on T91 substrate by electrochemical means. In 3.5 weight percent NaCl (sea water), 2N H2SO4 (acid), and 3.5 weight percent Na2SO4 (neutral salt), the electrochemical corrosion resistance of these coatings is compared with T91 substrate and detonation-sprayed Cr3C2–25NiCr coatings. To find out how quickly the coatings corroded, potentiodynamic polarisation (PDP) experiments were performed. When compared to T91 substrate, ODS FeAl coatings perform better in terms of corrosion resistance in 3.5 weight percent Na2SO4, while Cr3C2–25NiCr coatings continuously outperform it in all tested aqueous environments. According to the corrosion processes, Cr3C2–25NiCr coatings have localised and severe corrosion, while FeAl coatings have a greater rate of corrosion, which is offset by uniform corrosion. Solid particle erosion wear experiments, on the other hand, reveal that ODS FeAl coatings perform better than Cr3C2–25NiCr coatings, with improvements in wear resistance of 4–5 times at ambient temperature and 1.5–2.5 times at 400 °C. Additionally, ODS FeAl coatings perform slightly better at both temperatures than T91 substrate. The Impedance/Gain-phase analyser SI 1260 and the electrochemical interface SI 1287 from SOLARTRON, UK were used to evaluate the electrochemical corrosion behaviour of coated samples. According to the findings, ODS FeAl coatings have the potential to be innovative thermal spray feedstock materials that provide a techno-economic advantage over the widely used Cr3C2–25NiCr coatings in the industry.
Discussions
The investigation of thermal spraying techniques aims to identify the most suitable and effective surface engineering technology. These techniques are commonly employed for applying coatings that enhance wear, erosion, and corrosion resistance in various industrial applications. The findings of such studies often reveal coatings with outstanding properties, including exceptional corrosion and oxidation resistance, a high melting point, impressive hardness, strength, and excellent wear resistance at elevated temperatures. Notably, it has been noted that the HVOF method yields thick coatings with lower porosity and inclusion levels, which makes it a viable option for several applications [61,62,63,64,65].
Coating materials come in various forms, including powder, wire, and rod, and their selection is crucially dependent on specific applications and operating conditions, such as temperature and exposure to corrosive substances. Modern industrial turbines often operate at temperatures exceeding 900 °C. This extreme heat puts significant stress on materials, necessitating the use of high-temperature-resistant coatings. Turbine components are expected to have a long operational life, typically ranging from 25,000 to 50,000 h [47, 66,67,68,69,70,71,72]. This longevity requirement poses challenges in terms of material durability and performance. Monolithic materials, which are uniform and homogeneous, may not possess the necessary qualities to withstand the harsh service conditions experienced by turbine components over their operational lifespan. These conditions include high temperatures, wear, erosion, and oxidation. Given the demanding operational environment, the selection of appropriate coating materials is of utmost importance. Coatings must exhibit high-temperature stability and resistance to wear, erosion, and oxidation [73,74,75,76,77,78]. These characteristics are essential for protecting turbine components and ensuring their extended lifespan and reliability.
Hardness, resistance to wear and erosion, and chemical inertness are just a few of the remarkable qualities that set WC-Co-based cermet coatings apart. The wear resistance of engineering components may now be increased with the use of these coatings [49, 79,80,81,82]. In order to further improve its resistance to wear and corrosion, WC is often mixed with other metals, including nickel (Ni) or cobalt (Co), to make composite powder materials. The performance of these composites is enhanced under demanding conditions. The matrix ingredient in WC-Co coatings is often cobalt (Co). The hardness and general qualities of the coating may be greatly influenced by the selection of the matrix material. Interestingly, the hardness of WC-Co coatings tends to decrease as the cobalt (Co) content increases [50, 83,84,85]. This relationship between Co content and hardness is an important consideration when designing cermet coatings [50]. WC-Co coatings may exhibit a relatively high friction coefficient when in contact with opposing surfaces. This characteristic can be problematic in some applications, as it can lead to increased wear on the contacting surfaces. To mitigate the challenges posed by high friction coefficients, it is possible to incorporate solid lubricants into WC-Co coatings. These lubricants can reduce friction and provide better protection to the surfaces in contact with the coatings [86,87,88].
High-temperature applications often use NiCrAlY alloys, which exhibit exceptional resistance to corrosion and oxidation when the ratios of Al and Cr particles are optimised [51]. However, since NiCrAlY coatings are less hard than carbides, ceramics, and oxides, they are not used as the primary coating in the industry. Meanwhile, hard phases such as WC, Al2O3, Cr3C2, and Cr2O3 in the Ni or Co matrix may be reinforced to greatly increase the wear resistance of these coatings [52]. Cr3C2–NiCr coatings find frequent applications in high-temperature scenarios demanding erosion, wear, and corrosion resistance. The incorporation of carbide particles within a NiCr matrix imparts exceptional hardness and strength, particularly effective up to temperatures of 900 °C. Moreover, the thermal expansion coefficient of Cr3C2 (10.3 × 10–6 °C-1) is lower than that of iron (11.4 × 10–6 °C-1) and nickel (12.8 × 10–6 °C-1), the base alloys for high-temperature applications. In a high-temperature environment, selecting such a composition may greatly minimise thermal mismatch [53,54,55]. Furthermore, oxide ceramics, which include titania, alumina, zirconia, chromia, silica, and yttria, are widely used as coating materials to improve resistance to corrosion, fretting, erosion, wear, and cavitation. Applications needing a mix of wear and corrosion resistance are especially well-suited for these ceramics [56]. For mass-scale application, material costs need to be carefully taken into account.
Recent investigations have included the integration of nanomaterials, including silicon carbide, titanium dioxide, and alumina, with nanocrystalline metal matrix coatings to create nanocomposite coatings. The incorporation of these nanoparticles has shown to improve resistance to corrosion. For instance, it has been shown that adding SiC nanoparticles to Ni, Ni-W, or Ni-Co alloys increases their corrosion resistance. SiC nanoparticles act as inert physical barriers, preventing corrosive flaws from starting and spreading. Additionally, they have the ability to change the nickel layer's microstructure [89,90,91].
Future Scope and Conclusions
As a result of exposure to high temperatures and oxidising environments including molten salts, alloys undergo rapid deterioration known as "hot corrosion." When non-protective oxides at the salt/metal interface become more soluble, the corrosive attack speeds up and eventually saturates the whole molten salt layer with porous metal oxides. This process continues along the salt/gas contact. One of the best ways to reduce corrosion and increase the lifespan of components that are exposed to high temperatures is to use anti-corrosion coatings. Using protective oxide layers like Cr2O3, SiO2, and Al2O3, these coatings fend against corrosion. Thermal spray coating is the most widely used method for surface modification, according to literature reviews.
-
Power plants run at high capacity due to the increasing demand for electricity from various applications. The abundant and cost-effective supply of low-rank coal makes coal-based thermal power plants a major contributor to India's electricity production.
-
To protect boiler materials from hot corrosion, the use of composite coatings is a widespread strategy. HVOF and Plasma Spray demonstrate exceptional qualities such as bond strength, hardness, porosity, wear resistance, and corrosion resistance, outperforming plasma spray, detonation gun spray, flame spray, and various other thermal spray coatings.
-
The hot corrosion on the boiler tube surface is intensified by the elevated temperature of low-grade coal, leading to rapid oxidation facilitated by the presence of salt layers.
-
To prolong the lifespan of a coating, additional heat treatment is administered to the developed coatings and these further enhance the corrosion, and wear resistance due to its improved microstructure and bonding strength.
-
In this specific domain, nano-structured powder materials and rare earth materials have demonstrated potential applications. However, it's important to note that there is still much work to be done in terms of research and development. Researchers need to continue their efforts in exploring various combinations of coatings and materials to further advance and optimise their applications in this field.
Data Availability
Not applicable.
References
J. Parkash, H.S. Saggu, H. Vasudev, A short review on the performance high-velocity oxy-fuel coatings in boiler steel applications. Mater. Today: Proc. (2022). https://doi.org/10.1016/j.matpr.2021.09.014
H. Nalbandian, Expert systems and coal quality in power generation (Aug 2011)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Studies on the properties of high-velocity oxy- fuel thermal spray coatings for higher temperature applications. Mater. Sci. (2005). https://doi.org/10.1007/s11003-006-0047-z
A. Rani, N. Bala, C.M. Gupta et al., Combating hot corrosion of boiler tubes with plasma spraying coatings: a review. Int. J. Res. Mech. Eng. Technol. 3(2), 31–35 (2003)
H. Saini, D. Kumar, V.N. Shukla, Hot corrosion behaviour of nanostructured cermet-based coatings deposited by different thermal spray techniques: a review. Mater.: Today Proc. (2017). https://doi.org/10.1016/j.matpr.2017.01.055
N. Nagabhushana, Microstructure and microhardness of plasma sprayed NiCrBSi/fly ash composite coatings. Int. J. Innovative Res. Sci. Eng. Technol. 7(7), (2018)
A. Mehta (2022) A Book by CRC and Chapter “Performance status of Different advanced thermal barrier coatings against failure Mechanisms in Gas Turbine”.
G.A. El-Awadi, S. Abdel-Samad, E.S. Elshazly, Hot corrosion behavior of Ni-based Inconel 617 and Inconel 738 superalloys. Appl. Surf. Sci. 378, 224–230 (2016)
Z.C. Esanek, S. Houdkova, F. Lukac, High-temperature corrosion behavior of selected thermally sprayed coatings in corrosive aggressive environment. Mater. Res. Express (2018). https://doi.org/10.1088/2053-1591/aae956
H. Vasudev, L. Thakur, A. Bansal, H. Singh, S. Zafar, High temperature oxidation and erosion behaviour of HVOF sprayed bi-layer Alloy-718/NiCrAlY coating. Surf. Coat. Technol. 362, 366–380 (2019)
F. Delaunay, C. Berthier, M. Lenglet, J. Lameille, SEM-EDS and XPS studies of the high-temperature oxidation behaviour of Inconel 718. Mikrochim. Acta 132, 337–343 (2000)
D. Mudgal, S. Singh, S. Prakash, Hot corrosion behavior of some superalloys in a simulated incinerator environment at 900C. J. Mater. Eng. Perform. 23(1), 238–249 (2014)
M.A. Javed, A.S.M. Ang, C.M. Bhadra, R. Piola, W.C. Neil, C.C. Berndt, M. Leigh, H. Howse, S.A. Wade, Corrosion and mechanical performance of HVOF WC-based coatings with alloyed nickel binder for use in marine hydraulic applications. Surf. Coat. Technol. 418, 127239 (2021). https://doi.org/10.1016/j.surfcoat.2021.127239
G. Singh, N. Bala, V. Chawla, Y.K. Singla, Hot corrosion behavior of HVOF-sprayed carbide based composite coatings for boiler steel in Na2SO4–60 % V2O5 environment at 900 °C under cyclic conditions. Corro. Sci. 190, 109666 (2021)
V. G. Patil, B. Somasundaram, S. Kandaiah, Sachinkumar, Microstructure characteristics and properties of NiCrMoFeCoAl HVOF coating for T22 boiler tube steel. Mater. Today: Proc. 54(2), 468–471 (2022). https://doi.org/10.1016/j.matpr.2021.10.346
A.S. Praveen, A. Arjunan, High-temperature oxidation and erosion of HVOF sprayed NiCrSiB/Al2O3 and NiCrSiB/WCCo coatings. Appl. Surf. Sci. Adv. 7, 100191 (2022). https://doi.org/10.1016/j.apsadv.2021.100191
Y.J. Sun, R. Yang, L. Xie, Y.B. Li, S.L. Wang, H.X. Li, W.R. Wang, J.S. Zhang, Interfacial bonding and corrosion behaviors of HVOF-sprayed Fe-based amorphous coating on 8090 Al-Li alloy. Surf. Coat. Technol. 436, 128316 (2022). https://doi.org/10.1016/j.surfcoat.2022.128316
X. Zhang, Comparison on multi-angle erosion behavior and mechanism of Cr3C2-NiCr coatings sprayed by SPS and HVOF. Surf. Coat. Technol. (2020). https://doi.org/10.1016/j.surfcoat.2020.126366
J. Nalini Kumari, Development of CNT mixed HVOF coating for water tube boiler material to improve the degradations. Surf. Coati. Technol. (2022). https://doi.org/10.1016/j.matpr.2021.10.177
H. Vasudev, Effect of addition of Al2O3 on the high-temperature solid particle erosion behaviour of HVOF sprayed Inconel-718 coatings. Mater. Today Commun. 30, 103017 (2022). https://doi.org/10.1016/j.mtcomm.2021.103017
D.C. Ribu, Experimental investigation of erosion-corrosion performance and slurry erosion mechanism of HVOF sprayed WC-10Co coatings using design of experiment approach. J. Mater. Res. Technol. (2022). https://doi.org/10.1016/j.jmrt.2022.01.134
V.G. Patil, Microstructure characteristics and properties of NiCrMoFeCoAl HVOF coating for T22 boiler tube steel. Mater. Today: Proc. (2022). https://doi.org/10.1016/j.matpr.2021.10.346
R. Khuengpukheiw, Wear behaviors of HVOF-sprayed NiSiCrFeB, WC-Co/NiSiCrFeB, and WC-Co coatings evaluated using a pin-on-disc tester with C45 steel pins. Wear (2021). https://doi.org/10.1016/j.wear.2021.203699
R. Verma, “Comparative high-temperature oxidation studies of HVOF IN 625 coating on T22 boiler steel at 900℃ and 700. Mater. Today: Proc. (2021). https://doi.org/10.1016/j.matpr.2020.08.756
S. Hanumanthlal, Microstructural characterization and hot corrosion behavior of plasma-sprayed Fe17Cr2Ni0.18C/Fly ash cenosphere-based composite coating. SAE Int. J. Mater. Manf. (2021). https://doi.org/10.4271/05-14-03-0017
A. Verma, P. Tarate, A.C. Abhyankar, M.R. Mohape, D.S. Gowtam, V.P. Deshmukh, T. Shanmugasundaram, High-temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 161, 28–31 (2019)
V.N.V. Munagala, T.B. Torgerson, T.W. Scharf, R.R. Chromik, High-temperature friction and wear behavior of cold-sprayed Ti6Al4V and Ti6Al4V-TiC composite coatings. Wear 426–427, 357–369 (2019)
F. Ye, Z. Jiao, S. Yan, L. Guo, L. Feng, Y. Jianxing, Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high-temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 174, 109178 (2020)
A.A. Mohammed, Z.T. Khodair, A.A. Khadom, Preparation, characterization, and application of Al2O3 nanoparticles for the protection of boiler steel tubes from high-temperature corrosion. Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.07.172
S. Stewart, R. Ahmed, T. Itsukaichi, Surf. Coat. Technol. 190, 171 (2005)
U. Dragos, M. Gabriela, B. Waltraut, C. Ioan, Solid State Sci. 7, 459 (2005)
G. Sreedhar, M.D. MasroorAlam, V.S. Raja, Surf. Coat. Technol. 204, 291 (2009)
S. Kamal, R. Jayaganthan, S. Prakash, Surf. Coat. Technol. 203, 1004 (2009)
F. Ghadami, M.H. Sohi, S. Ghadami, Effect of bond coat and post-heat treatment on the adhesion of air plasma sprayed WC-Co coatings. Surf. Coat. Technol. 261, 289–294 (2015)
F. Ghadami, S. Ghadami, H. Abdollah-Pour, Structural and oxidation behavior of atmospheric heat-treated plasma sprayed WC-Co coatings. Vacuum 94, 64–68 (2013)
S.S. Chatha, H.S. Sidhu, B.S. Sidhu, The effects of post-treatment on the hot corrosion behavior of the HVOF-sprayed Cr3C2–NiCr coating. Surf. Coat. Technol. (2012). https://doi.org/10.1016/j.surfcoat.2012.04.026
C.H. Lee, K.O. Min, Effects of heat treatment on the microstructure and properties of HVOF-sprayed Ni-Cr-W-Mo-B alloy coatings. Surf. Coat. Technol. 132(1), 49–57 (2000). https://doi.org/10.1007/s11666-015-0365-5
M. Shabanlo, R. Amini Najafabadi, A. Meysami, Evaluation and comparison the effect of heat treatment on mechanical properties of NiCrBSi thermally sprayed coatings. Anti-Corros. Methods Mater. 65(1), 34–37 (2018). https://doi.org/10.1108/ACMM-02-2017-1756
C. Durga Prasad, M.R. Sharnappa Joladarashi, M.S. Ramesh, B.H. Srinath, Channabasappa., Effect of microwave heating on microstructure and elevated temperature adhesive wear behavior of HVOF deposited CoMoCrSi-Cr3C2 composite coating. Surf. Coat. Technol. 374, 291–304 (2019). https://doi.org/10.1016/j.surfcoat.2019.05.056
H. Guo, Microstructures, mechanical properties and tribological behaviors of HVOF-sprayed CoMoCrSi alloy coatings before and after 800 °C-annealed treatment. J. Alloy. Compd. 909, 164662 (2022)
S.-H. Zhang, Investigation on microstructure, surface properties and anti-wear performance of HVOF sprayed WC–CrC–Ni coatings modified by laser heat treatment. Mater. Sci. Eng. B 162, 127–134 (2009)
V.M. Gopinath, S. Arulvel, A review on the steels, alloys/high entropy alloys, composites, and coatings used in high-temperature wear applications. Mater. Today: Proc. 43, 817–823 (2021)
A.A. Boudi, M.S. Hashmi, B.S. Yilbas, HVOF coating of Inconel 625 onto stainless and carbon steel surfaces: corrosion and bond testing. J. Mater. Process. Technol. 30(155), 2051–2055 (2004)
J.M. Guilemany, Effects of thickness coating on the electrochemical behavior of thermal spray Cr C –NiCr coatings. Surf. Coat. Technol. 153, 107–113 (2002)
S. Liang, Effect of cermet interlayer on the electrochemical behavior of Cr3C2- NiCr/DLC duplex coating. Surf. Coat. Technol. 469, 129813 (2023)
D. Vijaya Lakshmi, Electrochemical corrosion and solid particle erosion response of Y2O3 dispersed FeAl coatings deposited by detonation spray. Intermetallics 155, 107844 (2023)
S.D. Choudhury, U.C. Bhakta, Superalloys in NTPC gas turbine and their properties. R&D J. 2–1, 41–46 (1996)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Characterizations and hot corrosion resistance of Cr3C2-NiCr coating on Ni-base superalloys in an aggressive environment. J. Therm. Spray Technol. 15(4), 811–816 (2006)
M. Jafari, M.H. Enayati, M. Salehi, S.M. Nahvi, C.G. Park, Microstructural and mechanical characterizations of a novel HVOF-sprayed WC-Co coating deposited from electroless Ni–P coated WC-12Co powders. Mater. Sci. Eng. A 578, 46–53 (2013)
R.J.K. Wood, Tribology of thermal sprayed WC-Co coatings. Int. J. Refract Metal Hard Mater. 28(1), 82–94 (2010)
D. Seo, K. Ogawa, Y. Suzuki, K. Ichimura, T. Shoji, S. Murata, Comparative study on oxidation behavior of selected MCrAlY coatings by elemental concentration profile analysis. Appl. Surf. Sci. 255(5), 2581–2590 (2008)
B. Cai, Y.-F. Tan, H.E. Long, T. Hua, X.-L. Wang, Tribological behavior and mechanisms of graphite/CaF2/TiC/Ni-base alloy composite coatings. Trans. Nonferrous Metals Soc. China 23(2), 392–399 (2013)
J.M. Guilemany, J.M. Miguel, S. Vizcaıno, C. Lorenzana, J. Delgado, J. Sanchez, Role of heat treatments in the improvement of the sliding wear properties of Cr3C2–NiCr coatings. Surf. Coat. Technol. 157(2–3), 207–213 (2002)
M. Manjunatha, R.S. Kulkarni, M. Krishna, Investigation of HVOF thermal sprayed Cr3C2-NiCr cermet carbide coatings on erosive performance of AISI 316 molybdenum steel. Proc. Mater. Sci. 5, 622–629 (2014)
G.-J. Yang, C.-J. Li, S.-J. Zhang, C.-X. Li, High temperature erosion of HVOF sprayed Cr 3 C 2-NiCr coating and mild steel for boiler tubes. J. Therm. Spray Technol. 17(5–6), 782–787 (2008)
M.A. Zavareh, A.A. Sarhan, D. Mohammed, B.B. Razak, W.J. Basirun, The tribological and electrochemical behavior of HVOF-sprayed Cr3C2–NiCr ceramic coating on carbon steel. Ceram. Int. 41(4), 5387–5396 (2015)
K. Goyal, H. Singh, R. Bhatia, Behaviour of carbon nanotubes-Cr2O3 thermal barrier coatings in actual boiler. Surf. Eng. 36(2), 124–134 (2020)
V. Singh, K. Goyal, R. Goyal, Improving the hot corrosion resistance of boiler tube steels by detonation gun sprayed coatings in actual boiler of thermal power plant. Anti-Corros. Methods Mater. 66(4), 394–402 (2019)
S. Dehgahi, R. Amini, M. Alizadeh, Corrosion, passivation and wear behaviors of electrodeposited Ni-Al2O3-SiC nano-composite coatings. Surf. Coat. Technol. 304, 502–511 (2016)
M.R. Vaezi, S.K. Sadrnezhaad, L. Nikzad, Electro deposition of Ni-SiC nanocomposite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A 315(1–3), 176–182 (2008)
C. Cai, X.B. Zhu, G.Q. Zheng, Y.N. Yuan, X.Q. Huang, F.H. Cao, J.F. Yang, B. Zhang, Electro deposition and characterization of nano-structured Ni-SiC composite films. Surf. Coat. Technol. 205, 3448–3454 (2011)
C. Durga Prasad, S. Kollur, C.R. Aprameya, T.V. Chandramouli, T. Jagadeesha, B.N. Prashanth, Investigations on tribological and microstructure characteristics of WC-12Co/FeNiCrMo composite coating by HVOF process. JOM The J. Min. Metals Mater. Soc. (TMS) (2023). https://doi.org/10.1007/s11837-023-06242-2
C. Durga Prasad, S. Kollur, M. Nusrathulla, G. Satheesh Babu, M.B. Hanamantraygouda, B.N. Prashanth, N. Nagabhushana, Characterisation and wear behaviour of SiC reinforced FeNiCrMo composite coating by HVOF process. Trans. IMF (2023). https://doi.org/10.1080/00202967.2023.2246259
H. Sharanabasava, M. Raviprakash, C. Durga Prasad, M.R. Ramesh, M.V. Phanibhushana, H. Vasudev, S. Kumar, Microstructure, mechanical and wear properties of SiC and Mo reinforced NiCr microwave cladding. Adv. Mate. Process. Technol. (2023). https://doi.org/10.1080/2374068X.2023.2257937
G. Madhu Sudana Reddy, C. Durga Prasad, S. Kollur, A. Lakshmikanthan, R. Suresh, C.R. Aprameya, Investigation of high temperature erosion behaviour of NiCrAlY/TiO2 plasma coatings on titanium substrate. JOM The J. Min. Metals Mater. Soc. (TMS) (2023). https://doi.org/10.1007/s11837-023-05894-4
G. Madhusudana Reddy, C. Durga Prasad, P. Patil, N. Kakur, M.R. Ramesh, High temperature erosion performance of NiCrAlY/Cr2O3/YSZ plasma spray coatings. Trans. IMF (2023). https://doi.org/10.1080/00202967.2023.2208899
H. Sharanabasva, C. Durga Prasad, M.R. Ramesh, Characterization and wear behavior of NiCrMoSi microwave cladding. J. Mate. Eng. Perform. (2023). https://doi.org/10.1007/s11665-023-07998-z
G. Madhusudana Reddy, C. Durga Prasad, P. Patil, N. Kakur, M.R. Ramesh, Investigation of plasma sprayed NiCrAlY/Cr2O3/YSZ coatings on erosion performance of MDN 420 steel substrate at elevated temperatures. Int. J. Surf. Sci. Eng. 17(3), 180–194 (2023). https://doi.org/10.1504/IJSURFSE.2023.10054266
H. Sharanabasva, C. Durga Prasad, M.R. Ramesh, Effect of Mo and SiC reinforced NiCr microwave cladding on microstructure, mechanical and wear properties. J. Inst. Eng. (India): Series D (2023). https://doi.org/10.1007/s40033-022-00445-8
H.S. Nithin, K.M. Nishchitha, D.G. Pradeep, C. Durga Prasad, M. Mathapati, Comparative analysis of CoCrAlY coatings at high temperature oxidation behavior using different reinforcement composition profiles. Weld. World 67, 585–592 (2023). https://doi.org/10.1007/s40194-022-01405-2
G. Madhusudana Reddy, C. Durga Prasad, G. Shetty, M.R. Ramesh, T. Nageswara Rao, P. Patil, Investigation of thermally sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ cermet composite coatings on Titanium alloys. Eng. Res. Express, IOP (2022). https://doi.org/10.1088/2631-8695/ac7946
G. Madhusudana Reddy, C. Durga Prasad, P. Patil, N. Kakur, M.R. Ramesh, Elevated temperature erosion performance of plasma sprayed NiCrAlY/TiO2 coating on MDN 420 steel substrate. Surf. Topogr.: Metrol. Prop. IOP 10, 025010 (2022). https://doi.org/10.1088/2051-672X/ac6a6e
G. Madhusudana Reddy, C. Durga Prasad, G. Shetty, M.R. Ramesh, T. Nageswara Rao, P. Patil, High temperature oxidation behavior of plasma sprayed NiCrAlY/TiO2 & NiCrAlY /Cr2O3/YSZ coatings on titanium alloy. Weld. World (2022). https://doi.org/10.1007/s40194-022-01268-7
T. Naik, M. Mathapathi, C. Durga Prasad, H.S. Nithin, M.R. Ramesh, Effect of laser post treatment on microstructural and sliding wear behavior of HVOF sprayed NiCrC and NiCrSi coatings. Surf. Rev. Lett. 29(1), 225000 (2022). https://doi.org/10.1142/S0218625X2250007X
G. Madhusudana Reddy, C. Durga Prasad, G. Shetty, M.R. Ramesh, T. Nageswara Rao, P. Patil, High temperature oxidation studies of plasma sprayed NiCrAlY/TiO2 & NiCrAlY /Cr2O3/YSZ cermet composite coatings on MDN-420 special steel alloy. Metallogr. Microstruct. Anal. 10, 642–651 (2021). https://doi.org/10.1007/s13632-021-00784-0
C. Durga Prasad, S. Lingappa, S. Joladarashi, M.R. Ramesh, B. Sachin, Characterization and sliding wear behavior of CoMoCrSi+Flyash composite cladding processed by microwave irradiation. Mater. Today Proc. 46, 2387–2391 (2021). https://doi.org/10.1016/j.matpr.2021.01.156
G. Madhu, K.M. Mrityunjaya Swamy, D.A. Kumar, C. Durga Prasad, U. Harish, Evaluation of hot corrosion behavior of HVOF thermally sprayed Cr3C2 -35NiCr coating on SS 304 boiler tube steel. Am. Inst. Phys. 2316, 030014 (2021). https://doi.org/10.1063/5.0038279
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, M.S. Srinath, Microstructure and tribological resistance of flame sprayed CoMoCrSi/WC-CrC-Ni and CoMoCrSi/WC-12Co composite coatings remelted by microwave hybrid heating. J. Bio Tribo-Corros. 6, 124 (2020). https://doi.org/10.1007/s40735-020-00421-3
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, Comparative investigation of HVOF and flame sprayed CoMoCrSi coating. Am. Inst. Phys. 2247, 050004 (2020). https://doi.org/10.1063/5.0003883
M. Mathapati, K. Amate, C. Durga Prasad, M.L. Jayavardhana, T. Hemanth Raju, A review on fly ash utilization. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2021.09.106
C. Durga Prasad, A. Jerri, M.R. Ramesh, Characterization and sliding wear behavior of iron based metallic coating deposited by HVOF process on low carbon steel substrate. J. Bio Tribo-Corros. 6, 69 (2020). https://doi.org/10.1007/s40735-020-00366-7
M.S. Reddy, C. Durga Prasad, P. Patil, M.R. Ramesh, N. Rao, Hot corrosion behavior of plasma sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ cermets coatings on alloy steel. Surf. Interfaces 22, 100810 (2021). https://doi.org/10.1016/j.surfin.2020.100810
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, M.S. Srinath, B.H. Channabasappa, Comparison of high temperature wear behavior of microwave assisted HVOF sprayed CoMoCrSi-WC-CrC-Ni/WC-12Co composite coatings. SILICON 12, 3027–3045 (2020). https://doi.org/10.1007/s12633-020-00398-1
R. Dinesh, S. Rohan Raykar, T.L. Rakesh, M.G. Prajwal, M. Shashank Lingappa, C. Durga Prasad, Feasibility study on MoCoCrSi/ WC-Co cladding developed on austenitic stainless steel using microwave hybrid heating. J. Mines Metals Fuels (2021). https://doi.org/10.18311/jmmf/2021/30113
K.G. Girisha, R. Rakesh, C. Durga Prasad, K.V. Sreenivas Rao, Development of corrosion resistance coating for AISI 410 grade steel. Appl. Mech. Mater. 813–814, 135–139 (2015). https://doi.org/10.4028/www.scientific.net/AMM.813-814.135
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, M.S. Srinath, B.H. Channabasappa, Development and sliding wear behavior of Co-Mo-Cr-Si cladding through microwave heating. SILICON 11, 2975–2986 (2019). https://doi.org/10.1007/s12633-019-0084-5
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, M.S. Srinath, B.H. Channabasappa, Microstructure and tribological behavior of flame sprayed and microwave fused CoMoCrSi/CoMoCrSi-Cr3C2 coatings. Mater. Res. Express, IOP 6, 026512 (2019). https://doi.org/10.1088/2053-1591/aaebd9
K.G. Girisha, K.V. Sreenivas Rao, C. Durga Prasad, Slurry erosion resistance of martenistic stainless steel with plasma sprayed Al2O3–40%TiO2 Coatings. Mater. Today Proc. 5, 7388–7393 (2018). https://doi.org/10.1016/j.matpr.2017.11.409
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, M.S. Srinath, B.H. Channabasappa, Influence of microwave hybrid heating on the sliding wear behaviour of HVOF sprayed CoMoCrSi coating. Mater. Res. Express IOP 5, 086519 (2018). https://doi.org/10.1088/2053-1591/aad44e
C. Durga Prasad, S. Joladarashi, M.R. Ramesh, A. Sarkar, High temperature gradient cobalt based clad developed using microwave hybrid heating. Am. Inst. Phys. 1943, 020111 (2018). https://doi.org/10.1063/1.5029687
K.G. Girisha, C. Durga Prasad, K.C. Anil, K.V. Sreenivas Rao, Dry sliding wear behaviour of Al2O3 coatings for AISI 410 grade stainless steel. Appl. Mech. Mater. 766–767, 585–589 (2015). https://doi.org/10.4028/www.scientific.net/AMM.766-767.585
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors state that none of their personal or financial relationships might have impacted the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakkannavar, V., Yogesha, K.B., Prasad, C.D. et al. A Review on Tribological and Corrosion Behaviour of Thermal Spray Coatings. J. Inst. Eng. India Ser. D (2024). https://doi.org/10.1007/s40033-024-00636-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40033-024-00636-5