Abstract
Zoledronic acid (ZOL) is one of the most potent nitrogen containing bisphosphonates (N-BP) which are used for cancer-induced skeletal disease by inhibiting osteoclast-mediated bone resorption. It acts by inhibiting the farnesyldiphosphate synthase which is one of several key enzymes in the mevalonate pathway consequently leading to osteoclast apoptosis. From recent studies, direct anti-cancer effects of bisphosphonates, particularly ZOL, was demonstrated in breast cancers. The synergic anti-cancer effects of ZOL treated with other anti-cancer agents in breast cancer cells were also reported. However, pharmacokinetic properties of ZOL have limited its cytotoxic activities because almost 60 % of injected ZOL are disposed into bone and 40 % of the dose cleared rapidly from blood, so that not enough dose of ZOL delivered to the cancer cells. For this reason, we introduced transferrin-conjugated long-circulating liposomes as targeted delivery system for enhancing the anti-cancer effect of ZOL. Transferrin receptors have been explored as a target to deliver therapeutics into cancer cells due to their increased expression on malignant cells. Liposomes were prepared by a reverse-phase evaporation vesicle method with aqueous ZOL solution. Transferrins were attached to the neutral liposomes via free thiols (–SH) to the PDP-end of the pyridylthiopropionoylamino-PEG-distearoyl-phosphatidyethanolamine (PDP-PEG-DSPE). Liposomes were characterized by measuring size distribution and zeta potential. The mean diameter of liposomes was 190 nm, and the encapsulation efficiency of ZOL was about 10 %, which was determined by HPLC analysis. Based on the 50 % inhibitory concentration (IC50) of ZOL against breast cancer cell MDA-MB-231, increased anti-cancer effect of transferrin-conjugated liposomal ZOL was evaluated compared to free ZOL and non-conjugated liposomal ZOL. In conclusion, transferrin-conjugated liposome can enhance the anti-cancer effect of ZOL and it is suggested that ZOL can be used as an anti-cancer agent with this formulation in breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen containing bisphosphonates (N-BPs) are used for osteoclast-mediated bone loss caused by osteoporosis, metastatic breast cancer, and Paget’s disease. N-BPs induce apoptosis of osteoclast, thereby showing anti-resorptive effects in bone (Drake et al. 2008). Recently, direct anti-cancer effects of N-BPs, especially zoledronic acid (ZOL), have been demonstrated in many researches. In these researches, ZOL exerts not only its direct anti-cancer effects such as inhibition of cell growth and induction of apoptosis, but its indirect effects such as inhibition of invasive and angiogenic properties in various cancer cells including breast cancer (Yamada et al. 2009; Aft 2011; Winter et al. 2008; Holen and Coleman 2010; Caraglia et al. 2010), prostate cancer (Clyburn et al. 2010; Lee et al. 2001), melanoma (Forsea et al. 2004), pancreatic cancer (Tassone et al. 2003), and other solid tumors. In various breast cancer cell lines, ZOL induced the apoptosis and inhibited cell growth in a time- and dose-dependent manner, with a 50 % inhibitory concentration (IC50) in the range of 10–100 μM (Senaratne and Colston 2002; Senaratne et al. 2000). The synergic anti-cancer effects of ZOL treated with other anti-cancer agents such as paclitaxel (Jagdev et al. 2001), doxorubicin (Ottewell et al. 2012), letrozole (Neville-Webbe et al. 2010) in breast cancer cells (Zhao et al. 2012) were also reported. Moreover, there are some clinical evidences of direct anti-cancer effects of ZOL added to neoadjuvant chemotherapy in breast cancer (Coleman et al. 2010). It is already known that adjuvant use of bisphosphonates reduces the rates of recurrence and death in patients with early-stage breast cancer (Coleman et al. 2011).
The ZOL exerts the anti-cancer effect on cancer cells through inhibition of farnesyl pyrophosphate synthase which is the one of key enzymes in mevalonate pathway. Accordingly, ZOL suppresses prenylation of small GTPases including Ras, Rho and Rac that regulate the proliferation, invasive properties and proangiogenic activity of human cancer cells (Goffinet et al. 2006; Luckman et al. 1998).
Even with these potentials of ZOL as an anti-cancer agent, the pharmacokinetic properties of ZOL have made difficult to evaluate its anti-cancer effects in vivo. After i.v. administration, approximately 60 % of the dose were rapidly accumulated within bone matrix, hydroxyapatite, and about 40 % of the dose were eliminated from plasma by kidney within 24 h (Chen et al. 2002; De Luca et al. 2011; Asikoglu and Durak 2009; Weiss et al. 2008). Because of these properties, very low concentrations of ZOL reaches into non-skeleton tissues including tumor tissues, so that ZOL can’t exert enough anti-cancer effects. And administration of high ZOL concentrations has the possible risks of side effects such as osteonecrosis of the jaws (ONJ) (Haidar et al. 2009; Mehrotra and Ruggiero 2006). From this consideration, we introduced one of the most studied delivery system, liposome, for enhancing the anti-cancer effects of ZOL and overcoming its non-favorable pharmacokinetic properties.
There are some researches about other bisphosphonates encapsulated in liposomes, including alendronate (N-BPs) and clodronate (BPs) targeting monocytes and macrophages (Epstein-Barash et al. 2010; Zeisberger et al. 2006). However, in these studies, liposome vehicles enhanced the half-life of drugs in plasma but were captured in reticulo-endothelial system (RES) randomly. It is suggested that conventional liposomal formulation is not enough to deliver the ZOL specifically into the tumor tissues.
“Stealth liposomes” (PEGylated liposomes) are the liposomes modified with hydrophilic polymer, polyethyleneglycol (PEG), on their surface. They have long-circulating property and increased the half-life in the plasma compared with conventional liposomes (non-PEGylated liposomes). The mechanism of long-circulating effect is the evasion of RES by reducing opsonization affected by hydrophilicity and steric hindrance effect of PEG (Epstein-Barash et al. 2010; Maruyama 2011). Thereby, stealth liposomes can be accumulated passively in solid tumor tissues much more than normal tissues by enhanced permeability and retention (EPR) effect (Matsumura and Maeda 1986; Maeda 2001). This phenomenon explains the leakage of nano-particles to tumor tissues through leaky vasculature (100–780 nm pore size) and retain in that tissues due to the lack of lymphatic drainage system when the particle size is in range of 90–200 nm (Liu et al. 1992).
On the other hand, enough concentration of ZOL for anti-cancer effects is higher than other conventional anti-cancer agents, for example, IC50 range of ZOL was μM range compared with the nM range of other anti-cancer agents like paclitaxel, letrozole (Jagdev et al. 2001; Neville-Webbe et al. 2010). Thus, active targeting strategy is needed for delivery of ZOL into the tumor tissues more selectively and efficiently, in addition to passive targeting by PEGylation.
Transferrin receptor (TfR, CD71) is a type II transmembrane glycoprotein (180 kDa) significantly over-expressed in various malignant cells (Faulk et al. 1980; Shindelman et al. 1981) that require large amount of iron for proliferation. Besides, cancer cells with high metastatic properties show higher expression of TfR (Inoue et al. 1993). In malignant breast cells, TfR expression was detected up to 4–5 times higher than normal breast cells (Walker and Day 1986). And transferrin (Tf) is a monomeric glycoprotein (76–81 kDa) transporting iron into cells via TfR by receptor-mediated endocytosis. In this regard, there have been many studies on targeted delivery of therapeutics into various cancer cells using Tf-modified delivery systems (Hong et al. 2010; Ishida et al. 2001; Li et al. 2009). So, we conjugated Tf to the distal end of the PEG chains of the PEGylated liposomes for targeting the tumors.
In this study, we described the procedures of preparing Tf-conjugated PEGylated liposomes encapsulating ZOL and evaluated their characteristics such as particle size, zeta potential, and drug encapsulation efficiency. After optimizing properties of delivery system, anti-cancer effect of ZOL with this formulation compared to free ZOL was determined in malignant breast cancer cell MDA-MB-231.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), cholesterol (Chol), human transferrin, 4-(p-maleimidophenyl) butyric acid N-hydroxysuccinimide ester (SMPB), dithiothreitol (DTT), tetrabutylammonium bromide (TBA), 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), chloroform, Sephadex G-75 were purchased from Sigma Chemical Co. (St. Louis Mo, USA). 1,2-Distearoyl-sn-glycero-3-phosphoenthanolamine-N-[methoxy-(polyethylene glycol)-2000] (DSPE-mPEG2000), 1,2-distearoyl-sn-glycero-3-phosphoenthanolamine-N-[PDP-(polyethylene glycol)-2000] ammonium salt (DSPE-PEG2000-PDP) were purchased from the Avanti Polar Lipids (Alabaster, AL, USA). Diethyl ether, methanol (HPLC grade), acetonitrile (HPLC grade), dimethylsulfoxide (DMSO) were purchased from Duksan Co., LTD (Ansan, Korea). Zoledronic acid (Zometa™, Novartis, Switzerland) was purchased from Taeyoung Pharm. Co., LTD (Seoul, Korea). MDA-MB-231, human breast cancer cell, was obtained from the Korea Cell Line Bank (KCLB, Seoul, Korea). RPMI 1640 medium (RPMI 1640), fetal bovine Serum (FBS), trypsin–EDTA, penicillin/streptomycin and Dulbecco’s phosphate buffered saline (DPBS) were purchased from WelGENE Inc. (Daegu, Korea). BSA protein assay agent was purchased from Bio-Rad (BIO-RAD Lab., CA, USA). Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from BD Pharmingen (San Diego, CA). All other reagents were of analytical grade and used without further purification.
Preparation of PEGylated liposome
PEGylated liposomes were prepared by the reverse-phase evaporation (REV) method (Szoka and Papahadjopoulos 1978). The lipid mixture composed of DPPC:Chol:DSPE-mPEG2000:DSPE-PEG2000-PDP (6.7:3.3:0.1:0.06 molar ratio) were dissolved in 1 ml of chloroform. The organic solvent was removed by a rotary evaporator (Laborota 4000, Heidolph, Italy) under nitrogen gas flow. The dry lipid film was resuspended in 1.5–2 ml of fresh diethyl ether then 1 ml of ZOL solution was added. The emulsion was sonicated in a bath type sonicator (Branson 2200, USA) for 3 min in cold water. And the resulting one-phase semi-solid gel suspension was evaporated for removing organic solvent. Unencapsulated ZOL was removed by passing the liposome suspension through Sephadex G-75 column. The liposome suspension was downsized by extrusion through 0.2 μm cellulose membrane filter (Whatman Int. Ltd., England) 10 times using Lipex™ extrusion device (Northern Lipids Inc., Vancouver, Canada).
Conjugation of transferrin to PEGylated liposome
The liposome containing 0.6 mol% DSPE-mPEG-PDP was incubated with DTT (25 mM in final solution) for 30 min at room temperature (20–25 °C), then separated from unreacted DTT through Sephadex G-75 column. The transferrin (Tf) dissolved in DPBS (0.2 mM) was reacted with SMPB solution (25 mM) dissolved in dimethylformamide to give a 1:20 molar ratio of Tf:SMPB at room temperature for 1 h, and MPB-Tf was separated from unreacted SMPB through Sephadex G-75 column. The thiolated liposome, reduced by DTT, and MPB-Tf were mixed and incubated overnight at room temperature. After incubation, unbound MPB-Tf was removed using Sephadex G-75 column. In this gel chromatography, 500 μl of final liposome was collected in clean plastic tube and each sample was measured absorbance at 280 nm wavelength which is λmax of proteins.
Size distribution and zeta potential analysis
The mean diameter and zeta potential of liposomes were measured using dynamic laser-light scattering system (NICOMP 380ZLS, Inc., Santa Babara, CA, USA). Each sample was diluted in DPBS and analyzed scattering angle was 90°. All measurements were performed at room temperature.
Encapsulation efficiency of zoledronic acid into liposomes
The concentration of ZOL was calculated by high performance liquid chromatography (HPLC). The chromatographic separation was achieved on a CAPCELLPAK C18 UG120 column (150 mm × 4.6 mm, I.D. 5 μm) using a mobile phase composed of 5 mM phosphate buffered saline, methanol, and acetonitrile in ratio of 97:2:1 vol% (pH 7.4). And 7 mM tetrabutylammonium (TBA) bromide was added as a ion-pairing reagent for retention of ZOL in a reverse-phase column (Rao et al. 2005; Jiang et al. 2004). The flow rate of mobile phase was 1 ml/min and the injection volume was 15 μl. Separated ZOL was detected at 215 nm wavelength by Waters 486 tunable absorbance detector (Waters, MA, USA). The chromatograms were analyzed by Millennium 32 program (Waters). ZOL encapsulated in the liposome samples was extracted using non-ionic surfactant Triton X-100 which solubilizes the lipid membrane into aqueous phase (Goni et al. 1986; Chern et al. 2006). Briefly, 5 μl of Triton X-100 added into the 100 μl of prepared liposomes (5 % of liposomal sample in volume ratio). The mixture was mixed for 1 h by vortexing, and centrifuged for 20 min at 14,000 rpm. Finally, lipids in the sample fell down and hydrophilic materials including ZOL were contained in supernatant, so that aqueous supernatant was analyzed by HPLC. The amount of ZOL encapsulated in liposome sample was determined from standard curve of ZOL. And the encapsulation efficiency of ZOL (%) was calculated using the following formula;
Encapsulation efficiency of ZOL (%) = Amount of ZOL in the supernatant from liposome/Initial loading amount of ZOL in preparing process × 100
Protein assay
The amount of Tf attached to the liposomes was calculated by Bradford Protein assay (Bradford 1976). The standard solution was prepared by dissolving Tf with DPBS, and diluted in the concentration between 0 and 1 mg/ml, which were used in preparing for the standard curve. The 1:80 dilution of Tf-conjugated liposome was used as a sample. Non-conjugated liposome was used as a control group. To each 800 μl sample, 200 μl of 1:5 dilution Bradford reagent (Bio-RAD Protein assay reagent, BIO-RAD Lab., CA, USA) was added. The mixture was incubated at room temperature for 15 min. Absorbance was measured at 595 nm using a UV/VIS spectrophotometer.
Lipid assay
The concentration of phospholipids was measured by the Bartlett’s phosphorus assay (Bartlett 1959). The standard solution for phosphorus assay was KH2PO4 solution. Standard solution between 0.1 and 0.4 μM of phosphorus were prepared by adding 50–200 μl of the stock solution which contains 1 mg/ml phosphorus (2.195 g KH2PO4 in 500 ml DPBS) in clean borosilicate glass tubes. Sample phospholipids were extracted from liposome by the Bligh and Dyer (1959) method. To each tube of standard and sample, 400 μl of 10 N sulfuric acid was added and the tube were heated in a heating block at 165 °C for 30 min. After 30 min, the tubes were cooled down, and 30 μl of 30 % hydrogen peroxide was added to each tube, which were then heated again at 165 °C for 30 min. After further cooling, 4.6 ml of 22 % (w/w) ammonium molybdate solution was added to each tube, followed by 0.2 ml of Fiske–Subba Row reagent with immediate mixing. These tubes were heated in boiling water for 10 min, and after cooling, the absorbance was measured at 830 nm wavelength using a spectrophotometer.
Number of transferrin attached to a liposome
In order to calculate the number of Tf attached to each liposome, some assumptions were made;
-
1.
the thickness of lipid bilayer of each liposome was 40 Å
-
2.
the area occupied by each phospholipid was 70 Å
-
3.
the size of liposome was 200 nm in diameter
Using these assumptions, the number of liposomes of 200 nm diameter per 1 μM lipid was 1.8 × 1012. And the molecular weight of Tf is 76–81 kDa, which means each Tf weight is 1.26 × 10−19–1.35 × 10−19 g. With these informations, we could calculate the actual number of Tf on a liposome from the result of Bradford protein assay and Bertlett’s phosphorus assay.
Cell culture
MDA-MB-231 human breast cancer cells were purchased from the Korea Cell Line Bank (KCLB, Seoul, Korea) and maintained in RPMI 1640 medium with 10 % fetal bovine serum (FBS), 25 mM HEPES, l-glutamine and 100 unit/ml penicillin/streptomycin at 37 °C in a 5 % CO2 incubator (Sanyo, Electric Co. Ltd., Japan). Cells were grown in 25 or 75 cm2 polystyrene tissue culture flask (Nunc, Denmark). The cells were washed with serum free medium, trypsinized and collected from culture flask. The trypsinized cells were centrifuged and cell pellet was resuspended with fresh medium containing FBS, then seeded in culture flask or dishes. The culture medium was replaced every other day with fresh one.
Inhibition of proliferation in MDA-MB-231 cells
For cell density optimization, MDA-MB-231 cells were plated at various cell densities in 96-well plates. The proper cell density which allowed the cells to grow at the same rate for 3 days was determined by MTT assay. Growth inhibition of the cells by zoledronic acid was also evaluated using the MTT assay. MDA-MB-231 cells (3000 cells/well) were plated in 96-well plate and incubated at 37 °C, 5 % CO2 condition. After 24 h, medium was suctioned out and the cells were treated with DPBS (control) and various formulations of ZOL, such as in free drug, conventional liposomes (Conv-Lipo), and PEGylated liposomes (PEG-Lipo). And the toxicity of liposome itself was evaluated by empty liposomes which is the formulation not encapsulating ZOL. After 48 h incubation, the treatments were suctioned out and MTT solution (1 mg/ml in serum free medium) was added to each well and incubated for additional 4 h in a CO2 incubator. The MTT containing medium was aspirated off and 100 μl of DMSO was added to dissolve the formed crystal formazan. The absorbance was measured at 570 nm wavelength using an ELISA reader (EL800, BIO-TEK, USA). The relative cell viability of drug treated cells was presented in percentage of control (DPBS).
Apoptosis assay by FACS
The apoptosis of MDA-MB-231 breast cancer cells was analyzed by FACS which detects the externalized phosphatidylserin (PS) by fluorescein isothiocyanate (FITC) conjugated annexin-V and integrity of cellular membrane by propidium iodide (PI). MDA-MB-231 cells (1 × 105 cells/well) were plated in 6-well plates and incubated at 37 °C, 5 % CO2 condition. After 24 h, medium was removed and the cells were treated with DPBS, empty liposomes, free form of ZOL, conventional liposomal ZOL, PEGylated liposomal ZOL, and Tf-conjugated PEGylated liposomal ZOL with fresh serum-free medium for 24 h. Then, the cells were washed with DPBS, and trypsinized and collected. After centrifugation, the cell pellets were resuspended to the concentration of 1 × 106/ml in 1× binding buffer and stained with 5 μl of FITC annexin-V and 5 μl of PI using FITC annexin-V apoptosis detection kit (BD Pharmingen™, San Diego, CA). After incubation of cells in the dark at room temperature for 15 min, 100 μl of the cells were diluted with 400 μl of 1× binding buffer according to the manufacturer’s instructions, and measured the fluorescenc intensity by dual color FACS analysis using FACSCanto II (BD Biosciences, San Joes, CA). Viable cells with intact cellular membranes excluded PI, while membranes of dead or damaged cells were permeable to PI. For this reason, we can consider both the FITC annexin-V and PI negative cells as viable. On the other hand, FITC annexin-V positive and PI negative cells were considered as early apoptotic; and FITC annexin-V positive and PI positive cells were considered as late apoptotic or necrotic ones.
Statistical analysis
The results were expressed as a mean ± standard deviation (SD).
Results
Conjugation of transferrin to the PEGylated liposome
The final transferrin-conjugated liposomal zoledronic acid (Tf-Lipo–ZOL) collected from gel chromatography was measured using the absorbance at 280 nm (Fig. 1). The collection fraction 9 and 10 showed the highest absorbance and were used for the following experiments. The collection fraction 25 was estimated as an unbound Tf.
Size distribution and zeta potential analysis
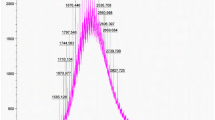
The mean diameter of Tf-Lipo–ZOL was 189.5 ± 69.9 nm (Fig. 2a). And the average zeta potential of the liposomes was −0.04 mV (Fig. 2b). There was no significant changes of size (between 150 and 250 nm) and aggregation or precipitation of liposomal ZOL during the storage at 4 °C environment up to a month (data not shown).
Encapsulation efficiency of zoledronic acid into liposomes
The encapsulation efficiency of ZOL was calculated by HPLC analysis. In this HPLC condition, retention time of ZOL was 2.46 min (Fig. 3). The encapsulation efficiency of ZOL extracted from liposomes was determined from the calibration curve of standard free ZOL solution with the initial loading amount of ZOL. The encapsulation efficiency of ZOL encapsulated in the Tf-Lipo was about 9.16 %. On the other hands, the encapsulation efficiency of conventional liposomal ZOL (Conv-Lipo–ZOL) and PEGylated liposomal ZOL (PEG-Lipo–ZOL) was about 15 % (Table 1). It is considered that reduced encapsulation efficiency of ZOL in Tf-Lipo is due to the dilution during several separation processes of unreacted materials in the Tf-conjugation procedure.
Number of transferrin attached to a liposome
The number of Tf attached to each liposome was optimized by changing the concentration of Tf solution (data not shown). Finally, 0.2 mM Tf solution and 0.06 molar ratio of DSPE-PEG2000-PDP: total lipids were used for proper number of Tf attachment. According to the Bradford protein assay, 2.063 × 10−4 g Tf was conjugated to 1 ml of Tf-Lipo. And the 1 ml of Tf-Lipo contained 4.878 × 10−5 mol phosphorus, which was determined by the Bartlett’s phosphorus assay. Therefore, the actual number of Tf molecules attached to each liposome is about 17~19.
Inhibition of proliferation in MDA-MB-231 cells
For the in vitro experiments, the seeding density of MDA-MB-231 cells should be determined. The optimum cell density was between 2,500 and 5,000 cells in a 96-well flat-bottomed microassay plate, so that we chose 3,000 cells for the future study (data not shown). The concentration of treated ZOL was determined by inhibitory concentration assay using free form of ZOL in MDA-MB-231 cells (Fig. 4). The IC50 of free ZOL was about 75 μM. The anti-proliferative effect of ZOL was enhanced with the liposomal formulation compared with free form of ZOL (Fig. 5).
Effect of transferrin-conjugated liposomal zoledronic acid on apoptosis
As shown in Fig. 6, most of the cells treated with empty liposome were not positively labeled with FITC annexin-V, indicating a low fraction of apoptotic cells. When the cells were treated with transferrin-conjugated liposomal zoledronic acid (Tf-Lipo–ZOL), the apoptotic population was enhanced (60.3 %) 2-times more than free ZOL (33.7 %) and 1.5-times more than non-conjugated liposomal ZOL (about 43 %).
FACS analysis of apoptosis induced by various formulation of ZOL; control (DPBS), empty liposome (Lipo-Empty), free zoledronic acid (Free ZOL), conventional liposomal zoledronic acid (Conv-Lipo-ZOL), PEGylated liposomal zoledronic acid (PEG-Lipo-ZOL), transferrin-conjugated liposomal zoledronic acid (T f -Lipo-ZOL). The spots appeared in lower left quadrant (FITC−, PI−) represent viable cells, in lower right quadrant (FITC+, PI−) represent early-apoptotic cells, and upper right quadrant (FITC−, PI+) represent necrotic cells
Discussion
The most potent nitrogen containing bisphosphonate, zoledronic acid, has been used for osteoclast-mediated bone disease and used for combination therapies with anti-cancer agents in adjuvant and neoadjuvant cancer therapy (Fox 2010; Coleman et al. 2010). Beside the adjuvant anti-cancer effect, many studies have demonstrated the direct anti-cancer effects of ZOL in vitro and the molecular pathway for anti-cancer effect is similar to that observed in osteoclasts (Winter et al. 2008). Through this mechanism, ZOL inhibited proliferation and induced apoptosis in the various cancer cells (Gnant 2011; Clyburn et al. 2010; Tassone et al. 2003). But the poor pharmacokinetic property of rapid elimination from the plasma and accumulation into the bone limited the study and evaluation of anti-cancer effect of ZOL in vivo (Chen et al. 2002). In this regard, we introduced liposomal delivery system for reducing bone affinity and delivering of ZOL selectively to the tumor tissues.
In this study, we prepared liposomal ZOL and evaluated its characteristics and the in vitro anti cancer effect on the breast cancer cells. The mean diameter of Tf-Lipo–ZOL was about 190 nm, which is optimum for being accumulated in tumor tissues by EPR effect (Maeda 2001). And with the size, neutral zeta potential (−0.03 mV) is favorable to evade from the RES. In the study of clodronate encapsulated in liposomes, neutral liposomes were less detected to macrophages or monocytes (Epstein-Barash et al. 2010). The transferrins attached to liposomes are also key strategy for targeting and internalizing in cancer cells. The targeting efficiency is dependent on targeting moiety content of the liposome. Less number of Tf per liposome can lead to lower targeting effect and too high concentration of proteins can result in aggregation of liposome in blood stream. The actual number of transferrin attached to a liposome was calculated as 17–19, and this number seems to be optimal as a drug targeting moiety.
From the results of in vitro anti-cancer effect, liposomal formulation can be evaluated as a safe and less toxic delivery system. The empty liposome showed less than 10 % cytotoxic effects (4.22 % of anti-proliferation effect and 8.9 % of apoptosis inducing). On the other hand, anti-cancer effects of the liposomal ZOL were higher than free ZOL but not significant, especially in non-conjugated liposomal ZOL. Therefore it is considered that active targeting is essential for evaluating the anti-cancer effect in vivo study.
From these results, we suggest that the potential use of ZOL as an anti-cancer agents can be materialized with the targeted delivery systems such as liposomes. Moreover, with the additional properties of ZOL, such as tolerable side effects and well known anti-resorptive activity, ZOL encapsulated in targeted liposomes can be a promising therapeutic for malignant breast cancer and other metastatic cancer patients by dual effects of enhanced anti-cancer effects in tumor sites and anti-resorptive effect in bone.
References
Aft R (2011) Bisphosphonates in breast cancer: antitumor effects. Clin Adv Hematol Oncol 9(4):292–299
Asikoglu M, Durak FG (2009) The rabbit biodistribution of a therapeutic dose of zoledronic acid labeled with Tc-99m. Appl Radiat Isotopes 67(9):1616–1621
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234(3):466–468
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caraglia M, Marra M, Naviglio S, Botti G, Addeo R, Abbruzzese A (2010) Zoledronic acid: an unending tale for an antiresorptive agent. Expert Opin Pharmacother 11(1):141–154
Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A (2002) Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 42(11):1228–1236
Chern CS, Chiu HC, Yang YS (2006) Interactions between nonionic Triton X surfactants and cholesterol-containing phosphatidylcholine liposomes. J Colloid Interface Sci 302(1):335–340
Clyburn RD, Reid P, Evans CA, Lefley DV, Holen I (2010) Increased anti-tumour effects of doxorubicin and zoledronic acid in prostate cancer cells in vitro: supporting the benefits of combination therapy. Cancer Chemother Pharmacol 65(5):969–978
Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, Gil M, Ritchie D, Passos-Coelho JL, Wheatley D, Burkinshaw R, Marshall SJ, Thorpe H (2010) The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer 102(7):1099–1105
Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W, Bell R (2011) Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 365(15):1396–1405
De Luca A, Lamura L, Gallo M, Daniele G, D’Alessio A, Giordano P, Maiello MR, Pergameno M, Perrone F, Normanno N (2011) Pharmacokinetic evaluation of zoledronic acid. Expert Opin Drug Metab Toxicol 7(7):911–918
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83(9):1032–1045
Epstein-Barash H, Gutman D, Markovsky E, Mishan-Eisenberg G, Koroukhov N, Szebeni J, Golomb G (2010) Physicochemical parameters affecting liposomal bisphosphonates bioactivity for restenosis therapy: internalization, cell inhibition, activation of cytokines and complement, and mechanism of cell death. J Control Release 146(2):182–195
Faulk WP, Hsi BL, Stevens PJ (1980) Transferrin and transferrin receptors in carcinoma of the breast. Lancet 2(8191):390–392
Forsea AM, Muller C, Riebeling C, Orfanos CE, Geilen CC (2004) Nitrogen-containing bisphosphonates inhibit cell cycle progression in human melanoma cells. Br J Cancer 91(4):803–810
Fox KR (2010) Adding zoledronic acid to endocrine therapy in the adjuvant treatment of hormone-sensitive breast cancer in premenopausal women: a new care standard or a provocative idea? Curr Oncol Rep 12(1):1–3
Gnant M (2011) Anticancer activity of bisphosphonates in breast cancer. Anticancer Agents Med Chem 12:114–122
Goffinet M, Thoulouzan M, Pradines A, Lajoie-Mazenc I, Weinbaum C, Faye JC, Seronie-Vivien S (2006) Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer 6:60
Goni FM, Urbaneja MA, Arrondo JL, Alonso A, Durrani AA, Chapman D (1986) The interaction of phosphatidylcholine bilayers with Triton X-100. Eur J Biochem 160(3):659–665
Haidar A, Jonler M, Folkmar TB, Lund L (2009) Bisphosphonate (zoledronic acid)-induced osteonecrosis of the jaw. Scand J Urol Nephrol 43(6):442–444
Holen I, Coleman RE (2010) Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res 12(6):214
Hong M, Zhu S, Jiang Y, Tang G, Sun C, Fang C, Shi B, Pei Y (2010) Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles. J Control Release 141(1):22–29
Inoue T, Cavanaugh PG, Steck PA, Brunner N, Nicolson GL (1993) Differences in transferrin response and numbers of transferrin receptors in rat and human mammary carcinoma lines of different metastatic potentials. J Cell Physiol 156(1):212–217
Ishida O, Maruyama K, Tanahashi H, Iwatsuru M, Sasaki K, Eriguchi M, Yanagie H (2001) Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm Res 18(7):1042–1048
Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI (2001) The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer 84(8):1126–1134
Jiang Y, Zhang XQ, Xu ZR (2004) Analysis of zoledronic acid and its related substances by ion-pair RP-LC. Chromatographia 60(7–8):405–409
Lee MV, Fong EM, Singer FR, Guenette RS (2001) Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res 61(6):2602–2608
Li X, Ding L, Xu Y, Wang Y, Ping Q (2009) Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm 373(1–2):116–123
Liu D, Mori A, Huang L (1992) Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta 1104(1):95–101
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13(4):581–589
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41:189–207
Maruyama K (2011) Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev 63(3):161–169
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46(12 Pt 1):6387–6392
Mehrotra B, Ruggiero S (2006) Bisphosphonate complications including osteonecrosis of the jaw. Hematol Am Soc Hematol Educ Program 356–360:515
Neville-Webbe HL, Coleman RE, Holen I (2010) Combined effects of the bisphosphonate, zoledronic acid and the aromatase inhibitor letrozole on breast cancer cells in vitro: evidence of synergistic interaction. Br J Cancer 102(6):1010–1017
Ottewell PD, Brown HK, Jones M, Rogers TL, Cross SS, Brown NJ, Coleman RE, Holen I (2012) Combination therapy inhibits development and progression of mammary tumours in immunocompetent mice. Breast Cancer Res Treat 133:523–536
Rao BM, Srinivasu MK, Rani ChP, kumar SS, Kumar PR, Chandrasekhar KB, Veerender M (2005) A validated stability indicating ion-pair RP-LC method for zoledronic acid. J Pharm Biomed Anal 39(3–4):781–790
Senaratne SG, Colston KW (2002) Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res 4(1):18–23
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW (2000) Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 82(8):1459–1468
Shindelman JE, Ortmeyer AE, Sussman HH (1981) Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int J Cancer 27(3):329–334
Szoka F Jr, Papahadjopoulos D (1978) Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA 75(9):4194–4198
Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D’Alessandro A, Galea E, Goel A, Abbruzzese A, Boland CR, Venuta S (2003) Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer 88(12):1971–1978
Walker RA, Day SJ (1986) Transferrin receptor expression in non-malignant and malignant human breast tissue. J Pathol 148(3):217–224
Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H (2008) Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 36(10):2043–2049
Winter MC, Holen I, Coleman RE (2008) Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 34(5):453–475
Yamada J, Tsuno NH, Kitayama J, Tsuchiya T, Yoneyama S, Asakage M, Okaji Y, Shuno Y, Nishikawa T, Tanaka J, Takahashi K, Nagawa H (2009) Anti-angiogenic property of zoledronic acid by inhibition of endothelial progenitor cell differentiation. J Surg Res 151(1):115–120
Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA (2006) Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 95(3):272–281
Zhao M, Tominaga Y, Ohuchida K, Mizumoto K, Cui L, Kozono S, Fujita H, Maeyama R, Toma H, Tanaka M (2012) Significance of combination therapy of zoledronic acid and gemcitabine on pancreatic cancer. Cancer Sci 103:58–66
Conflict of interest
All authors (M Choi, DH Shin and JS Kim) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, M., Shin, D.H. & Kim, JS. Repositioning of zoledronic acid for breast cancer using transferrin-conjugated liposome. Journal of Pharmaceutical Investigation 43, 461–469 (2013). https://doi.org/10.1007/s40005-013-0091-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-013-0091-2