Abstract
Most of the inland open-waters, especially lakes, have been the victim of enhanced man-induced perturbations and are in a critical phase of ecological transition. Lakes function as long-term sinks for many pollutants including Potentially Toxic Elements (PTE), so the problems would further be accentuated due to environmental degradation and impending climate change scenario. Hence, a study was designed to evaluate the distribution of PTEs in water, sediments and fish (Pethia conchonius and Cyprinus carpio) of Sumendu (Mirik) Lake situated in the Eastern Himalayan part of India. It plays a significant role in providing means of livelihood to many sectors. Thirty-three sediment and water specimens were acquired from 11 sampling points to characterise the PTEs (Fe, Mn, Zn, Cu, Cr, Co, Cd, Ni, Pb, and As) concentrations levels using ICP-MS. Spatial distributional trends and pollution quality indexes were assessed for sediment samples. The degree of PTE exposure in the water and fishes were found to be within acceptable limits. The geostatistical prediction map showed that the concentration range of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn in sampling points were 2.61–10.1 µg g−1, 0.02–0.48 µg g−1, 4.85–17.5 µg g−1, 32.2–84.7 µg g−1, 24.2–47.1 µg g−1, 13,007–61029 µg g−1, 128.3–629.9 µg g−1, 18.9–49.8 µg g−1, 3.80–20.8 µg g−1, 95.0–282.4 µg g−1, respectively. Moreover, the sediment samples were found to be low to moderately polluted according to several pollution assessment indices viz. Degree of Contamination, Contamination Factor, Ecological Risk Index, etc. The findings of this research will aid in understanding potential contamination sources and significant pollution hotspots that could be utilised to develop contamination control strategies and targeted management tactics for Himalayan fresh water lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inland open water resources (wetlands, lakes, reservoirs, rivers, etc.) area are significant source of drinking water, irrigation, agriculture, and aquaculture operations, and they play a significant part in urban ecosystem services. Lakes among them play a momentous role in the global water cycle as they offer essential ecosystem services to both humans and wildlife [12] and they constitute only 1% of the total surface area [71]. Lakes have supported human survival and development for millions of years, providing crucial and valued ecosystem services like water resources (for consumption, commerce, and farming), water management, aquaculture, biodiversity, hydroelectric, mobility, entertainment, and beauty [17, 71]. However, the acceleration of civilization, agriculture, urbanisation, and demographic expansion over the past few decades has resulted in more nutrient intake, higher primary production, deteriorated quality of the water, and decreased species richness in about 40% of all lakes globally [17]. Lakes are relatively confined water systems with longer retention times, making them potential long-term sinks for pollutants like pesticides, heavy metals and microplastics among other freshwater resources [53]. Understanding and controlling lakes’ vulnerability to anthropogenic eutrophication has long been the main objective of limnological study [71]. The Sustainable Development Goals (SDGs) of the United Nations (Goal #6) place a high priority on lakes as a supply of water, yet the SDGs’ success is seriously threatened by lake eutrophication [60]. Lakes are sensitive to environmental changes because of their intimate connection to the environment, and their current geochemistry reflects both natural and anthropogenic aspects of the entire catchment area [57].
It was reported that a major environmental disaster has arisen due to the contamination of inland water resources with potentially hazardous elements [24]. The PTEs contamination might significantly alter the microbial community composition changing their functionality in various biotic and abiotic compartments of aquatic systems resulting in harmful effects on the survivability of higher vertebrates like fish. The public’s attention has recently been called to various studies on PTE accretion in the hydrosphere because of their soaring lethality, extensive persistence, bioaccumulation, and biomagnification behaviour [25, 51, 58]. The input from the discharge of industrial and urban sewage or atmospheric deposition in the watershed affects the levels of PTEs in sediments of fluvial and estuarine settings [52]. When PTE concentrations rise above a particular level, they may have a negative influence on living things along the food chain [43]. Even while few trace metals are inherently crucial for aquatic life, PTE concentrations beyond certain limits are dangerous for living creatures [39]. The majority of PTEs available in scenery are also common and have a propensity for bioaccumulation, making them an intriguing topic. PTEs including heavy metals in the environment can move to surface deposits by adhering to suspended particles, becoming complex, and sedimenting [27]. As a result, sediments serve as the ecosystem’s source, destination, and container for heavy metals [44]. Under certain circumstances, PTEs adsorbed on sediments could leak into the water nearby, harming aquatic life [72]. PTEs continuously bioaccumulate in aquatic life, where they are non-degradable [44]. They are significant in ecotoxicology because metals can migrate hierarchically down the food chain and biomagnify over time, which can have toxic impacts on human society [68]. Due to their lethality, endurance, mutagenic nature, and irrevocable properties, PTEs had been categorised as priory pollutants [4]. They could build up in soil due to both natural and man-made processes. The nature of the source materials and weathering processes have a big impact on how heavy metals naturally accumulate. Urban and peri-urban soil characteristics have changed from their original states as a result of human-induced activities (agricultural, manufacturing, urban outflow, waste disposal, and atmospheric deposits). To protect the biodiversity of freshwater ecosystems and the benefits they give, the quality of ground to surface waters must be promoted [23]. Understanding the geographical patterns of HMs in water and sediment is, crucial for risk assessment and environmental protection of the freshwater ecosystem.
The Himalayan lakes are of particular interest to environmentalists since they have been subjected to biotic strain for many thousands of years and are still degrading quickly as a result of this stress. In the current study, the primary threat sources are looked at, along with the pertinent threat causes. The water, sediment, and fish tissue of Mirik lake in the Darjeeling Hills, Himalaya, were examined for the first time in a preliminary assessment of PTE contamination and pollution. The purpose of the current investigation was (i) to determine the amounts of PTEs (Ni, Cr, Zn, Mn, Pb, Cu, As, Cd, Co, and Hg) in the water, sediment, and fish species (ii) using number of sediment quality criteria and pollution indexes (both single and integrated) which may be employed to measure PTE enrichment and pollution levels in order to evaluate the pollution quality metrics (iii) Source sharing out and geographic distribution of PTE dispersion in Mirik lake sediment.
Materials and Methods
Area of Research
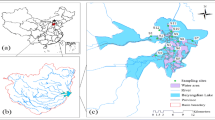
The Sumendu Lake, popularly known as Mirik lake, a nest in the Lesser Himalaya’s. It also plays a significant role in providing means of livelihood to many sectors [21]. Mirik lake is a created wetland that is surrounded by a large natural drainage network and is located in a valley surrounded by hill ranges. It stretches about 1.25 km in length, 110 ha in area with maximum depth of 8 m. It is located at 26o53.163′–26°53.620′N latitude and 88o11.190′–88o10.943′E longitude at an altitude of 1767 m above msl, under Kurseong subdivision of Darjeeling district. The lake collects sewage from populated areas through a number of inlets because it is located in a valley surrounded by hill ridges with a substantial natural drainage network. The river Mechi receives spillover water from the lake through a single outfall point that is located on the lake’s western shore. The catchments region is made up of residential areas, undeveloped land, and business districts including hotels, restaurants, and other stores.
Figure 1 Provides a thorough description of the study site.
Sample Collection and Storage
A total of 33 sediments and water samples were taken from 11 sampling points in July 2022. High-density polyethylene bottles were used to collect water samples. For PTEs analysis, the obtained water samples were promptly preserved by addition of HNO3-acid. Surface sediment samples were collected using the Peterson grab. Three sediment samples were chosen at random from each sampling location. The samples were transported to the laboratory for treatment after being placed in sterile plastic bags. From the Mirik lake, Pethia conchonius and Cyprinus carpio were collected in three replicates each. All the fishes were stored in the icebox until they were processed for PTEs.
Preparation of Samples for Chemical Analysis
After adding 1.0 mL of pure HNO3 (Trace Metal TM Acids, Thermo Fisher Scientific) to the 100 ml of water samples, they were digested on a hot plate until nearly dry followed by addition of MiliQ water creating a final volume of 50 ml. In order to investigate general parameters and trace metal analyses, using a 2 mm sieve, ground-up samples of air-dried sediment were examined. Organic carbon concentration was assessed by wet digestion [22, 55]. A pH metre was used to measure pH, and the sediment to water ratio was 1:2.5 [40]. Shade-dried sediment (1 g) was digested with Triacid (HNO3:HClO4:H2SO4 ratio of 10:4:1) in a hot plate system (130–200 °C) (Trace Metal TM Acids, Thermo Fisher Scientific). The digested samples were diluted to a level of 50 ml using Milli Q water, then screened using Whatman paper 42 after cooling to room temperature. After that, an ICP-MS was used to check the samples for PTE content (NexION 1000, PerkinElmer, USA). The Mein hart nebulizer and cyclonic spray chamber were utilised in conjunction with the ICP-MS system (deflector voltage: 20 V, ICP RF Power: 1600 W, CeO/Ce = 0.018, Ce++/Ce+ = 0.028, nebulizer gas flow rates: 1.5 L/min, auxiliary gas flow rates: 2 L/min, plasma argon flow rates: 15 L/min). In Milli Q water, the solutions were made. For the examination of fish samples, 1 g of fish flesh (on a weight-per-weight basis) was digested with Triacid (HNO3:HClO4:H2SO4 ratio of 10:4:1) (Trace Metal TM Acids, Thermo Fisher Scientific) in a hot plate system using the same procedures as for the analysis of sediment samples.
Assessment of Metal Contamination
The pollution levels in this research were determined using a variety of Indexes. (I) Indexes with a single element and (II) Indexes with multiple elements are the two categories into which these indices fall [22, 31].
Indexes with a Single Element
These metrics quantify the pollution brought on by a single PTE.
Contamination Factor (CF)
An indicator called CF is used to rate the quality of sediment. CF is a helpful method for monitoring environmental contamination over time that is brought on by a single PTE [9]. The gradual pollution of a certain element in sediments, can be examined using CF values [1]. Each element’s CF is calculated by dividing the element PTE concentration that was recorded at specific sampling sites by the background element PTE concentration [15]. Some scientists use the typical Earth’s crustal shale values found in clay in sedimentary rocks, as described by [31], as background concentration data for the investigated sites are not accessible. However, statistical techniques were used to determine the baseline concentration or background values because the concentration range is much different from what was seen in this research region [31]. The statistical methods selected include identifying the critical statistical parameters in a “clean” data set after first eliminating outlier values (expected to be anthropogenically undisturbed). Median plus twice the absolute median deviation (2DMA) was used to generate the background value [31, 46]. Sediment is considered to be contaminated by human-induced activity when CF > 1 and uncontaminated when CF ≤ 1 [3].
Potential Contamination Index (C p)
The Potential Contamination Index is another tool for measuring element contamination in sediment (Cp). Although the CF and Cp indexes only take into account one aspect when evaluating pollution, the Cp index also evaluates the PTE’s divergence from the background value across all sampling locations. CF simply takes into account background value and aids in determining how contamination varies from background value over a particular sampling site.
Cp = (Metal) sample maximum/(Metal) Background.
When.
Cp < 1: low degree of contamination;
1 ≤ Cp < : 3: contamination of moderate degree;
Cp > 3: contamination of greater degree [8].
Indexes with Multiple Element
Potential Ecological Risk Index (RI)
The Potential Ecological Risk Index is exploited to determine the amount of ecological damage caused by PTEs in sediment [59]. It is possible to use the risk index to represent how PTEs affect the ecology of the environment [41]. According to [15], the potential ecological risk index is calculated as Eir = Tir CF, where Eir stands for potential ecological hazard, Tir for toxic response factor, and CF for contamination factor. The Tir illustrates the possible risk of heavy metal pollution by emphasising the toxicity of some heavy metals and the susceptibility of the ecosystem to contamination [7]. Cd, Cr, Cu, Ni, Pb, Co, Mn, and Zn each have hazardous reaction factors of 30, 2, 5, 5, 2, 1, and 1, respectively, as per Hakanson’s (1980) standardised toxic response factor (Kumar et al., 2020).
RI is the collective term for the possible ecological risk that all of the metals evaluated pose. When: An Eir score of 40 indicates a low risk to the environment; an Eir score of 80 indicates a moderate risk to the environment; an Eir score of 80 plus 160 plus 320 equals a severe ecological risk; and an Eir score of 320 equals a very high ecological hazard [15]. A RI score of 150 indicates a low potential ecological risk; a RI value of 300 indicates a moderate ecological risk; a RI value of 600 indicates a considerable ecological risk; and a RI value of 600 indicates an exceptionally high ecological risk [43].
Comparison of Environmental Risk Assessment to Sediment Quality Standards (SQGs)
The PTEs environmental risk (Cd, Co, Cr, Cu, Fe, Ni, Pb, Zn, Mn, As) were calculated by comparing their concentrations to sediment quality guidelines [30] such as the Lowest Effect Level (LEL), Threshold Effects Level (TEL), Severe Effect Level (SEL), and Probable Effects Level (PEL) in order to ascertain whether the detected level of concentration will have adverse biological effects [38, 70]. Below the LEL threshold, sediments are regarded as clean to mildly contaminated, but no effects on the majority of organisms that live in sediment are anticipated [37]. PEL stands for the concentration at which unfavourable consequences are expected to occur frequently [47]. PEL offers aquatic organisms a reduced level of defence [49]. The lowest attention level (TEL) is the level at which detrimental consequences are least likely to occur [47]. As a result, it is believed to provide aquatic organisms with a higher level of defence [49]. Over the SEL threshold, sediments are considered to be significantly contaminated, and adverse effects on the majority of species that live in sediment are anticipated [37].
Data Analysis
The natural or anthropogenic origins of PTEs were determined using correlation analysis [16, 64]; multivariate statistical analysis of Principal Component Analysis (PCA); and Cluster Analysis (CA). The PCA and CA, as well as all other statistical analyses, were carried out using SPSS version 16.0. In order to create the graphs, Origin 2021 was used (Academic). A useful method for finding patterns, classifying them, modelling them, and locating contamination sources is PCA-based factor analysis [36]. Using cluster analysis, several geochemical groupings were found, allowing samples with similar metal concentrations to be categorised. The samples are grouped by CA according to how similar they are. The amalgamation rule was Ward’s method, and the metric for cluster analysis was the squared Euclidean distance. Dendrogram representation from cluster analysis showed the similarities between the variables [35, 45].
The spatial autocorrelation and distribution of PTEs in the surface sediment were investigated using the analytical interpolation method Inverse Distance Weighted (IDW) in ArcGIS 10.2.2. The method was also used to investigate the ecological risk value of several PTEs in the vicinity of Mirik lake. IDW makes the supposition that the rate of correlation and similarity increases in direct proportion to the separation between nearby locations. The value of the cell will resemble the value of the sample point more closely the closer a point is to the prediction cell. There is more weight when the measured location is close to the centre of the prediction cell. The analysis was conducted using ArcGIS 10.2.2. We created a polygon that closely resembled Mirik lake and used it to interpolate data. The entire root mean square error was kept around 1 to accomplish effective data interpolation (0.001–0.002).
Results and Discussion
Distribution of PTEs (μg L−1) in the Water of Mirik Lake
The levels of PTEs-Cd, Cr, Pb, Ni, Co, Fe, Mn, Cu, Zn, and As in the water from Mirik lake was compared with various drinking water quality standards (supplementary Table 1) [54, 61]. Concentration of all potentially toxic elements we found within the permissible limit. According to the findings, PTE absolute concentrations varied in the following order: Fe > Zn > Mn > Cr > Ni > Cu > Pb > As > Cd > Co. Fe concentration varied from 25.3 to 59.0 μg L−1 and Mn concentration varied from 2.0 to 6.9 μg L−1. As was found in the water and it varied from 0.03 to 0.35 μg L−1with mean ± Sd was 0.17 ± 0.11 µgL−1. According to the current investigation, the concentration of As in drinking water was within the acceptable limits of 0.05 mg L−1 and 0.01 mg L−1 [61]. The element As is categorised as the first priority toxic element by the WHO and US EPA. It is the most common drinking water pollutant on a global basis [19]. To explain how environmental influences are causing As in water to behave differently, several theories have been put forth. As(V) and As(III) are the two As species that predominate in aquatic environments, and their presence changes depending on the pH and redox circumstances [5]. Fe-oxide minerals cause strong arsenate adsorption in the middle-acidic pH range and desorption has been seen at pH 8 [34]; these processes are related to As mobility [20]. Highest As was recorded at outlet point followed by S9, S6, S4, S7 and inlet. Location and the degree of anthropogenic water contamination is found to be maximum in inlet station which has highest concentration of most of PTEs examined except Mn and As. The inlet station is located near the tourist assemble point where disposal of plastics, garbage and sewage wastes from different tourist vehicles, hotels and residential areas takes place [10]. Travelers congregate here to see the fishes that congregate primarily in this location as the fishes consume different foods tossed by the people [33]. Additionally, run-off from tea fields and resorts transporting pesticides and fertilisers, human excreta from kaccha latrines and bathing, etc., are all dumped into the Mirik lake [21].
Distribution of PTEs (μg g−1) in the Sediment of Mirik Lake
The spatial variability of PTEs in sediment is shown in supplementary Figure 1. Fe > Mn > Zn > Cr > Ni > Cu > Pb > Co > As > Cd were the potentially toxic elements concentrations in the sediment that were investigated. Fe concentration ranged between 12,980–61032 µg g−1 with an overall average of 37,823 µg g−1. The station Outlet had the highest concentration of Fe (61,032 g g−1), which may have been caused by the quick deposition of waste from nearby bathing and washing activities. Also, outlet station of Mirik lake is connected to the Mechi River through weirs where through intermixing deposited nutrients are substantially concentrated near the outlet of the lake by which it gets discharged in the Mechi river [21].
The control of desorption and absorption processes by river hydrodynamics may also be used to explain the highly changeable pollution levels [19]. This is related to the lake sediments carried by waterways combine with river water, which has a big impact on how much pollution gets deposited [48]. The physical characteristics and chemical environment of the sediment are altered by hydrodynamic circumstances, which in turn affects the concentration of heavy metals [66]. The mobility of heavy metals and their behaviour in sediments is affected by the hydrological and depositional conditions of the river. The kind of sediments, which are composed primarily of clay particles, likewise considerably affects the distribution of iron [11, 32]. The lowest Fe values (12,980 g g−1 and 19,155 g g−1) were observed close to sampling sites S1 and S4. Zn concentration ranged between 95.0 and 282.4 µg g−1 with an overall average of 141.4 µg g−1. Station S9 has the greatest concentration of Zn (282.4 µg g−1). S9 is the location where residential waste and industrial effluents, particularly from Mirik bazaar, are released. At this location, people bathe and wash their garments. Mn concentration ranged between 128.0 and 630.3 µg g−1 with an average concentration of 418.3 µg g−1. Greater levels of Mn concentration were found near to stations s6 and outlet point. Cr concentration ranged between 32.1 and 84.7 µg g−1 with an average concentration of 64.4 µg g−1. The greatest quantity of chromium was found near outlet point (84.7 µg g−1). After prolonged exposure, the element Cr, which is mutagenic, extremely poisonous, and carcinogenic, has the ability to travel and change in surface sediments [19]. Ni concentration ranged between 18.9 and 49.8 µg g−1 with an average concentration of 36.1 µg g−1. Highest concentration of Ni was found near station outlet point (49.8 µg g−1). Cu concentration ranged between 24.2 and 47.1 µg g−1 with an average concentration of 32.8 µg g−1. Highest concentration of Cu was found at station S9 (47.1 µg g−1). The concentration of Pb ranged between 3.79 and 20.8 µg g−1 with an average concentration of 6.0 µg g−1. Highest concentration of Pb was found in station outlet point (20.8 µg g−1). Co concentration ranged between 4.84 and 17.5 µg g−1 with an average concentration of 13.3 µg g−1. Highest concentration of Co was found in station S6 (17.5 µg g−1). The As concentration ranged between 2.61 and 10.1 µg g−1 with an average concentration of 6.69 µg g−1. Highest concentration of the As was found in station inlet point (10.1 µg g−1). Cd concentration ranged between 0.02 and 0.48 µg g−1 with an overall average of 0.23 µg g−1. Highest concentration of Cd was found in station S9 (0.48 µg g−1).
PTEs in the Fish Tissue
Fish species Pethia conchonius and Cyprinus carpio were studied for their PTE levels from different stations of Mirik lake are presented in Table 1 and compared with WHO and FAO prescribed limit for human consumption. These fish species were chosen because they were often consumed by the locals and could be used to assess PTE bioaccumulation. Cd, Co, Cr, Cu, Fe, Ni, Pb, Zn, Mn and As concentration were found to be 0.17 µg g−1, 0.03 µg g−1, 0.75 µg g−1, 1.31 µg g−1, 106.4 µg g−1, 0.15 µg g−1, 0.23 µg g−1, 95.2 µg g−1, 0.63 µg g−1 and 0.05 µg g−1 for Pethia conchonius. Similarly, in Cyprinus carpio PTE concentration were found to be 0.23 µg g−1, 0.07 µg g−1, 0.95 µg g−1, 1.61 µg g−1, 123.2 µg g−1, 0.17 µg g−1, 0.28 µg g−1, 110.2 µg g−1, 0.76 µg g−1 and 0.09 µg g−1 for Cd, Co, Cr, Cu, Fe, Ni, Pb, Zn, Mn and As, respectively. The analysis of fish samples revealed that the distribution of PTEs were in the order of Zn > Fe > Mn > Pb > Cr > Cu Ni > As > Cd > Co. PTE concentrations in fish tissue were found to be within ranges suitable for human consumption prescribed by FAO 1983; World Health Organization 1989 are presented in Table 1. Different fish species residing in the same body of water but with varying levels of water contamination may be the cause of the difference. The heavy metals found in the sediment are due to sediment water interface, which is the primary habitat of Cyprinus carpio. Enhanced mineralization processes take place at the interface, especially in the oxic zone. It may be due to organic matter suspended in the water, which initiates oxidation reactions at the sediment- water interface. Second, eating habits and biological metabolisms of creatures affected how much metal was present in them. When dietary consumption of these two metals exceeds the levels that could possibly endanger human health, Zn and Cu, common, essential minerals with many health advantages, may become toxic [67]. The presence of potentially harmful substances in the fish sample may be the result of anthropogenic activities such as residential trash, neighbouring chemical factories, picnic and tourist garbage, sewage effluents, illegal waste disposal, and untreated industrial discharges. In order to safeguard consumers, it is necessary to monitor the PTEs content in fish tissue to verify compliance with food safety regulations [67].
Risk Assessment
The CF is used to estimate the PTE sources and the degree of anthropogenic influence [19]. According to the current investigation, average CF values for all potentially harmful elements differed in the following order: Zn > Cu > Ni > Cr > Fe > Co > Cd > As > Mn > Pb (Fig. 2.). The calculated CF value for Zn exceeded 1 in stations 8, 9 and inlet point. Overall average Zn conc. was found to be near unity (0.95). CF value for Cu exceeded 1 in stations S9, outlet and inlet and average CF was found to be 0.90. CF value for Fe exceeded 1 in stations S5, outlet and inlet. So, 27% of the sites for Zn, Cu and Fe indicated moderate contamination indicating an anthropogenic input for these PTEs [2]. Diesel fuel, shoe rubber and rubber from tyre dust are significant potential sources of zinc contamination [62]. Zn and Cu has a good level of remobilization from water to sediments, which is one of its characteristics [28]. It can precipitate with sulphides in an anoxic environment under extreme circumstances (floods), which cause resuspension [19]. CF value for Ni exceeded 1 in stations S9 and outlet and near to unity in station inlet (0.96). For Cr, CF value was found to be 1 in station outlet and near to 1 in Stations inlet and S9. CF value for As was found to be near 1 (0.98) in Station inlet. In stations S9 and inlet CF value for Cd exceeded 1. The CF value was less than 1, which indicates no contamination, in the remaining PTEs. However, there is a considerable likelihood that the stations with CF values close to 1 will be increasingly polluted in the future due to anthropogenic reasons.
The potential contamination index (Cp) was found to be in sequence of Cd > Zn > Cu > Ni > Mn > As > Pb > Fe > Co > Cr (Table 2). Cp value for Cd and Zn were found to moderately polluted (2.97and 2.75, respectively). For Cu, Ni and Mn, Cp was found to be 1.94, 1.66 and 1.50 indicating low pollution. For rest of the potentially toxic elements Cp was found to be less than 1.5 indicating extremely low level of pollution. Although for As, Cp was found to be 1.47 but proper monitoring is required to stop it further contaminating.
Figure 3a shows that the potential ERIS presented by every PTE in the Mirik lake sediments. ERIS for Cd, Co, Cr, Cu, Ni, Pb, Zn, Mn and As ranged from 1.76–41.83, 0.51 -1.87, 0.76–2.00, 3.32–6.46, 2.15–5.68, 0.81–4.46, 0.63–1.89, 0.19–0.94 and 2.53–9.87 with mean values of 20.04, 1.41, 1.52, 4.49, 4.12, 2.85, 0.95, 0.62 and 6.49, respectively. The potential ecological risk indices showed that the PTEs' pollution intensity declines in the order of: Cd > As > Cu > Ni > P > B > Cr > Co > Z > Mn. Similar findings were found for the levels of Cd pollution using the Hakanson potential ecological risk indicator and other examined indices. Recent research suggests a substantial correlation between tourism and cadmium contamination, which impacts the entire world [42, 66]. The pattern helps to explain why cadmium is found in high tourism areas. Land contamination caused by settlement sedimentation, commercial and residential trash outflow, construction activity and municipal trash have been linked to anthropogenic PTE concentrations in the Mirik lake [21]. Through the Ecological Risk Index, a prediction map was created to examine the spatial pattern of the overall potential ecological risk (ERI) (Fig. 3b) which ranged from 14.65 to 72.78. 37% of the stations were found to be little risk of pollution (ERI < 40); while, 63% of stations were found to be moderate risk of pollution (ERI < 80). The figure clearly depicts a high risk towards inlet and stations S9, and Species richness may be greatly impacted by this, especially for macroinvertebrates, planktonic communities, and littoral vegetation [21]. ERI values, in notably, highlight the ecological risk associated with certain biological species’ sensitivity to PTEs [4]. The highest Cd, Cu, and Zn levels are seen in the navy to blue coloured zones, which also exhibit very high to high ecological risk (72.79). The areas were mostly those where wastewater from the Mirik market is released. At this location, people also bathe and wash their clothes [10].
Comparing PTE Pollution Danger to Sediment with SQGs
Assessing PTEs’ concentrations in SQGs allowed for the determination of their pollution status (Supplementary Table 2). For all sampling locations, the present amount of Cd in sediments was lower than the LEL, PEL, TEL, SEL, and TET, indicating that sediments are regarded as clean to slightly contaminated and that no effects on the majority of species that live in sediment are anticipated [37]. Cr, Cu, and Ni contents were higher than LEL in all sample locations but lower than SEL and TET, indicating that sediments were moderately polluted. The Pb content in sediment was lower than the LEL, PEL, TEL, SEL, and TET from all monitoring stations, ranging from 3.79 to 20.82 g g−1. It shows that sediments were not thought to be polluted by Pb. The current investigation found that, in the majority of the sampling stations, the As concentration in sediments above the TEL in accordance with SQGs. Additionally, it was shown that the physicochemical characteristics of sediment affect how much pollution is present at any one time. Sediment biogeochemical activities and environmental physicochemical variables have shown to be significantly impacted by sediment contamination [56].
Identification of the Sources of PTEs
Understanding the distribution of potentially harmful substances can be achieved by analysing their origins. The relationship and source of the possibly harmful materials were examined using principal components analysis (PCA), Pearson’s correlation analysis, and cluster analysis. To locate possible sources of PTEs, the statistical technique known as PCA has been well-researched and proven to work [63, 65]. Instead of a variance–covariance matrix, a correlation matrix was used to calculate PCA, giving each variable an equal weight. The PCA results for the PTE levels in the surface sediment samples are presented in supplementary Table 4. The PCA revealed four main components with Eigen values greater than one based on Varimax rotation that cumulatively explained 88.12% of their total variance in Mirik lake sediment. The first principal component (PC1) explained 55.5% of the total variation (eigenvalue = 2.88) (Fig. 4), and was strongly correlated with Co, Cr, Ni, Fe, Pb, Mn and As (loading value 0.785–0.956) implying a common source [58]. Perhaps due to external anthropogenic release. This theory is further corroborated by Pearson’s correlation analysis (supplementary Table 3), which reveals that all heavy metals have a substantial positive connection with one another (r > 0.70, p 0.01). Co–Ni (r = 0.70), Co–Fe (r = 0.78, Co–Pb (r = 0.73, Co–Mn (r = 0.88, Co–As (r = 0.84), Co–Cr (r = 0.85, Co–Ni (r = 0.70), Co–Fe (r = 0.78, Co–Pb), Co–Mn (r = 0.78), Co–As (r = 0.84). According to Pearson’s correlation coefficient (r), r value between 0.5 and 0.7 is regarded as having a moderate correlation; while, r > 0.7 is viewed as having a high correlation [45]. A strong correlation coefficient between two metals can imply that they will behave similarly in a given environment [29]. There is a loading for Cd, Cu, Zn, and Silt in PC2, which explained 12.8% of the total variance. These metals are seen in the same PCA quadrant with varying loadings, pointing to one or more sources of pollution [19]. It is possible to explain their close association and related origin by the strong organo-metallic complexes that organic complexes like humic and fulvic acids create with Cu and Cd [70]. Also silts play a significant role in incorporating the cations on their surface and raising the level of PTE contents [50]. Even by correlation analysis and coefficients of variation, moderate loading may suggest the presence of a mixed source that is less geogenic and more linked to anthropogenic activities. Pearson’s correlation analysis shows Cd–Cu (r = 0.77), Cd–Zn (r 0.81) and EC–Cd (r = 0.71). PC3 which described 11.4% of the total variance, has a strong loading for OC, sand and clay. PC4 which described 8.4% of the total variance and Eigen value 1.12 has strong loading for pH. The strong correlation and availability of metals is governed by the sediment’s texture and organic content though its powerful complexing capacity with metallic pollutants. PTEs from the ecosystem can be absorbed by OC in sediment [18] and is considered to be a significant sink or carrier for heavy metals providing an excellent record of the impacts of industry and anthropogenic activity [69]. Strong correlation indicating that PTEs originated from the same places, are interdependent, behaved similarly while being transported, and were relocated together [43]. Weak correlations between PTEs demonstrate that metals are controlled by a variety of geochemical interactions and support rather than by a single cause [46]. PTEs, however, also show intricate interactions among themselves, with alterations in correlations relating to anthropogenic as well as biological, chemical, and physical processes [43]. Complex inter elementary correlations point to a mixed source of origin, including both anthropogenic and lithogenic sources [14, 26]. PTE’s natural sources include weathering of metal-rich rocks and continental dust migration [6, 7]
Hierarchical Cluster Analysis (HCA) is an effective method for locating or grouping the correlations between the parameters that have been studied, or for putting sampling sites into groups based on their similarities [13] To create a heat map that represents the severity of each PTE for each sampling site, HCA was used to create dendrograms linked by coloured mosaics (Fig. 5). The whole dataset of 11 sample sites (right of the map) and PTE concentrations (bottom of the map) was created into a 2-D HCA heat map, as shown in Fig. 5. Sediment samples were clustered into 3 groups, S9, Inlet, outlet and S5 as Group 1; S8, S6, S3, S7 and S2 as Group 2 and S4, S1 as Group 3. As shown, three clusters that were clearly distinguished and coincided with three groups differentiated by the concentrations of PTEs according to results from 2-D HCA heat map and PCA identified by HCA. Similar geochemical and human factors may have an impact on the concentration of stations along the Mirik lake and the dispersion of PTEs in sediments. Therefore, using their commonality, the sampling sites could be totally clustered using HCA.
Conclusions
Potential toxic elements distribution, ecological risk, and source localization in the sediments, organisms, and surface water of Mirik lake was studied. Due to wastewater and other rubbish disposal, PTE concentrations (Mn, Ni, Pb, Cu, Fe, Cr, Cd, Co, and Zn) were higher especially at the inlet side of the wetland according to the spatial distribution maps. Ecological risk assessment indicated that Cd and As were the most polluted PTEs in sediments and need greater concern. The findings of the CA and PCA indicated that there was a substantial positive association between the concentrations of PTEs and were mostly influenced by the texture of the sediments in the research area. PTE concentrations in fish tissue were found to be within ranges suitable for human consumption. In general, the study’s findings offer preliminary data on the level of PTE exposure in sediments, water, and fish as well as the pollution status, which may be useful in the creation of acceptable environmental safety measures.
Availability of Data and Material
All data generated or analysed during this study are included in this manuscript.
References
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ Nanotechnol Monit Manag 5:27–35
Arisekar U, Shakila RJ, Shalini R et al (2022) Distribution and ecological risk assessment of heavy metals using geochemical normalization factors in the aquatic sediments. Chemosphere 294:133708
Barik SK, Muduli PR, Mohanty B et al (2018) Spatial distribution and potential biological risk of some metals in relation to granulometric content in core sediments from Chilika Lake, India. Environ Sci Pollut Res 25:572–587
Bhuiyan MAH, Karmaker SC, Bodrud-Doza M et al (2021) Enrichment, sources and ecological risk mapping of heavy metals in agricultural soils of dhaka district employing SOM, PMF and GIS methods. Chemosphere 263:128339
Bondu R, Cloutier V, Rosa E, Roy M (2020) An exploratory data analysis approach for assessing the sources and distribution of naturally occurring contaminants (F, Ba, Mn, As) in groundwater from southern Quebec (Canada). Appl Geochem 114:104500
Chen R, Chen H, Song L et al (2019) Characterization and source apportionment of heavy metals in the sediments of Lake Tai (China) and its surrounding soils. Sci Total Environ 694:133819
Chen Y, Liu M, Zhang X et al (2021) Metals in Yellow River estuary sediments during the 2018 water-sediment regulation scheme period. Mar Pollut Bull 166:112177
Dauvalter V, Rognerud S (2001) Heavy metal pollution in sediments of the Pasvik River drainage. Chemosphere 42:9–18
Díaz-de Alba M, Galindo-Riano MD, Casanueva-Marenco MJ et al (2011) Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (South of Spain) using chemometric tools. J Hazard Mater 190:177–187
Dutta S (2019) Water pollution at Mirik lake in Darjeeling, West Bengal: a local socioeconomic impact analysis. Int J Adv Res (Indore) 7:915–925. https://doi.org/10.21474/IJAR01/9115
El-Amier YA, Elnaggar AA, El-Alfy MA (2017) Evaluation and mapping spatial distribution of bottom sediment heavy metal contamination in Burullus Lake, Egypt. Egypt J Basic Appl Sci 4:55–66
Feng Y, Zhang H, Tao S et al (2022) Decadal lake volume changes (2003–2020) and driving forces at a global scale. Remote Sens (Basel). https://doi.org/10.3390/rs14041032
Ghosh AR, Mondal S, Kole D (2018) Environmental impact assessment: a case study on East Kolkata Wetlands. In: Wastewater management through aquaculture. Springer, pp 285–303
Guan Q, Zhao R, Pan N et al (2019) Source apportionment of heavy metals in farmland soil of Wuwei, China: comparison of three receptor models. J Clean Prod 237:117792
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Han H, Ma Q, Li C et al (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9:1123–1130
Ho LT, Goethals PLM (2019) Opportunities and challenges for the sustainability of lakes and reservoirs in relation to the Sustainable Development Goals (SDGs). Water (Basel) 11:1462
Huang Z, Liu C, Zhao X et al (2020) Risk assessment of heavy metals in the surface sediment at the drinking water source of the Xiangjiang River in South China. Environ Sci Eur 32:1–9
Iordache AM, Nechita C, Zgavarogea R et al (2022) Accumulation and ecotoxicological risk assessment of heavy metals in surface sediments of the Olt River, Romania. Sci Rep. https://doi.org/10.1038/s41598-022-04865-0
Karimian N, Johnston SG, Burton ED (2018) Antimony and arsenic partitioning during Fe2+-induced transformation of jarosite under acidic conditions. Chemosphere 195:515–523
Khamaru L, Chakraborty J, Samanta S et al (2022) Assessment and monitoring of urbanisation on Himalayan lacustrine environment-a case study in Mirik municipality area. GeoJournal 87:703–722
Khan S, Naushad M, Lima EC et al (2021) Global soil pollution by toxic elements: current status and future perspectives on the risk assessment and remediation strategies–A review. J Hazard Mater 417:126039
Koudenoukpo ZC, Odountan OH, Guo C et al (2022) Understanding the patterns and processes underlying water quality and pollution risk in West–Africa River using self-organizing maps and multivariate analyses. Environ Sci Pollut Res 1–20
Kumar V, Parihar RD, Sharma A et al (2019) Global evaluation of heavy metal content in surface water bodies: a meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 236:124364
Li X, Li Y (2021) 2.5 Persistent organic pollutants and heavy metal pollution. Oceanography of the Yellow Sea and East China Sea 153
Likuku AS, Mmolawa KB, Gaboutloeloe GK (2013) Assessment of heavy metal enrichment and degree of contamination around the copper-nickel mine in the Selebi Phikwe Region, Eastern Botswana
Liu B, Wang J, Xu M et al (2019) Spatial distribution, source apportionment and ecological risk assessment of heavy metals in the sediments of Haizhou Bay national ocean park, China. Mar Pollut Bull 149:110651
Liu Q, Jia Z, Liu G et al (2023) Assessment of heavy metals remobilization and release risks at the sediment-water interface in estuarine environment. Mar Pollut Bull 187:114517
Llorca M, Ábalos M, Vega-Herrera A et al (2020) Adsorption and desorption behaviour of polychlorinated biphenyls onto microplastics’ surfaces in water/sediment systems. Toxics 8:59
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Martínez-Guijarro R, Paches M, Romero I, Aguado D (2019) Enrichment and contamination level of trace metals in the Mediterranean marine sediments of Spain. Sci Total Environ 693:133566
Masoud AA (2014) Groundwater quality assessment of the shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. J Afr Earth Sci 95:123–137
Mondal D, Pal J, Ghosh TK, Biswas AK (2012) Abiotic characteristics of Mirik Lake water in the hills of Darjeeling, West Bengal, India. Adv Appl Sci Res 3:1335–1345
Norra S, Berner ZA, Agarwala P et al (2005) Impact of irrigation with As rich groundwater on soil and crops: a geochemical case study in West Bengal Delta Plain, India. Appl Geochem 20:1890–1906
Pal M, Maity R, Ratnam JV et al (2020) Long-lead prediction of ENSO modoki index using machine learning algorithms. Sci Rep 10:1–13
Pardo R, Vega M, Debán L et al (2008) Modelling of chemical fractionation patterns of metals in soils by two-way and three-way principal component analysis. Anal Chim Acta 606:26–36
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario
Rajeev M, Sushmitha TJ, Aravindraja C et al (2021) Exploring the impacts of heavy metals on spatial variations of sediment-associated bacterial communities. Ecotoxicol Environ Saf 209:111808
Rinklebe J, Antoniadis V, Shaheen SM et al (2019) Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ Int 126:76–88
Sajjadi S-A, Mohammadzadeh A, Tran HN et al (2018) Efficient mercury removal from wastewater by pistachio wood wastes-derived activated carbon prepared by chemical activation using a novel activating agent. J Environ Manag 223:1001–1009
Saleh SMK, Amer AT, Al-Alawi A (2018) Potential ecological risk of heavy metals in surface sediments from the Aden coast, Southern Yemen. J Environ Sci Toxicol Food Technol 12:42–55
Sarimn AS, Mohamed CAR (2012) Elements content in otolith as pollution indicator for cultured sea bass (Lates calcarifer) of Malaysia. J Environ Prot (Irvine, Calif) 3:1689–1703
Sarkar SK, Mondal P, Biswas JK et al (2017) Trace elements in surface sediments of the Hooghly (Ganges) estuary: distribution and contamination risk assessment. Environ Geochem Health 39:1245–1258
Shetaia SA, Khatita AMA, Abdelhafez NA et al (2022) Human-induced sediment degradation of Burullus lagoon, Nile Delta, Egypt: heavy metals pollution status and potential ecological risk. Mar Pollut Bull 178:113566
Shil S, Singh UK (2019) Health risk assessment and spatial variations of dissolved heavy metals and metalloids in a tropical river basin system. Ecol Indic 106:105455
Singh H, Pandey R, Singh SK, Shukla DN (2017) Assessment of heavy metal contamination in the sediment of the River Ghaghara, a major tributary of the River Ganga in Northern India. Appl Water Sci 7:4133–4149
Smith SL, MacDonald DD, Keenleyside KA et al (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638
Srilert C, Van TP (2022) Spatial and temporal variabilities of surface water and sediment pollution at the main tidal-influenced river in Ca Mau Peninsular, Vietnamese Mekong Delta. J Hydrol Reg Stud 41:101082
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Suresh G, Sutharsan P, Ramasamy V, Venkatachalapathy R (2012) Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol Environ Saf 84:117–124
Tang KHD (2021) Interactions of microplastics with persistent organic pollutants and the ecotoxicological effects: a review. Trop Aquat Soil Pollut 1:24–34
Trifuoggi M, Ferrara L, Toscanesi M et al (2022) Spatial distribution of trace elements in surface sediments of Hooghly (Ganges) river estuary in West Bengal, India. Environ Sci Pollut Res 29:6929–6942
Tsering T, Sillanpää M, Viitala M, Reinikainen S-P (2022) Variation of microplastics in the shore sediment of high-altitude lakes of the Indian Himalaya using different pretreatment methods. Sci Total Environ 849:157870
USEPA (2006) Science Policy Council Handbook: Peer Review, 3rd ed.; Office of Science Policy, Office of Research and Development, 3RD EDITIO. Office of Science Policy, Office of Research and Development, Washington, DC, USA
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wan W, Zhang Y, Cheng G et al (2020) Dredging mitigates cyanobacterial bloom in eutrophic Lake Nanhu: shifts in associations between the bacterioplankton community and sediment biogeochemistry. Environ Res 188:109799
Wang Q, Li Y, Liu L et al (2023) Human impact on current environmental state in Chinese lakes. J Environ Sci 126:297–307
Wang X, Zhang C, Li S et al (2021) Ecological risk, health risk assessment, and pollution source analysis of Xinli Lake wetland based on triangular fuzzy number. Environ Sci Pollut Res 28:22334–22347
Wieczorek J, Baran A, Urbański K et al (2018) Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ Geochem Health 40:2325–2342
Woolway RI, Kraemer BM, Lenters JD et al (2020) Global lake responses to climate change. Nat Rev Earth Environ 1:388–403
World Health Organization (2008) Guidelines for drinking-water quality, second add Bis (2012) Drinking water specification IS: 10500: 2012
Worthing M, Bosworth L, Papandrea M et al (2020) HHpXRF study of recent zinc and lead pollution on lava stepping stones from Pompeii: Tourist footfall, tyre dust and leaded petrol. Archaeometry 62:1042–1066
Xia F, Zhang C, Qu L et al (2020) A comprehensive analysis and source apportionment of metals in riverine sediments of a rural-urban watershed. J Hazard Mater 381:121230
Xiao Y, Yin H, Zhang Y et al (2021) A dual-stage attention-based Conv-LSTM network for spatio-temporal correlation and multivariate time series prediction. Int J Intell Syst 36:2036–2057
Yalcin MG, Tumuklu A, Sonmez M, Erdag DS (2010) Application of multivariate statistical approach to identify heavy metal sources in bottom soil of the Seyhan River (Adana), Turkey. Environ Monit Assess 164:311–322
Yao Q, Chen L, Mao L et al (2022) Co-effects of hydrological conditions and industrial activities on the distribution of heavy metal pollution in Taipu River, China. Int J Environ Res Public Health 19:10116
Yap CK, Al-Mutairi KA (2022) Copper and zinc levels in commercial marine fish from Setiu, East Coast of Peninsular Malaysia. Toxics 10:52
Yin K, Wang Q, Lv M, Chen L (2019) Microorganism remediation strategies towards heavy metals. Chem Eng J 360:1553–1563
Zhang H, Jiang Y, Ding M, Xie Z (2017) Level, source identification, and risk analysis of heavy metal in surface sediments from river-lake ecosystems in the Poyang Lake, China. Environ Sci Pollut Res 24:21902–21916
Zhang Y, Li H, Yin J, Zhu L (2021) Risk assessment for sediment associated heavy metals using sediment quality guidelines modified by sediment properties. Environ Pollut 275:115844
Zhou J, Leavitt PR, Zhang Y, Qin B (2022) Anthropogenic eutrophication of shallow lakes: Is it occasional? Water Res 221:118728
Zhuang W, Zhou F (2021) Distribution, source and pollution assessment of heavy metals in the surface sediments of the Yangtze River Estuary and its adjacent East China Sea. Mar Pollut Bull 164:112002
Acknowledgements
The ICAR-Central Inland Fisheries Research Institute, Barrackpore provided the researchers with a variety of infrastructure facilities, for which the researchers are grateful (ICP-MS). Authors duly acknowledge the In-charge Chemistry Laboratory, Fishery Resource Assessment and Informatics Division of the institute for providing the facility for the digestion of samples. The researchers also like to thank Mr. Asim Jana and Mr. Jayanta Pramanik for their assistance with sample collecting.
Funding
ICAR-Central Inland Fisheries Research Institute.
Author information
Authors and Affiliations
Contributions
T. Banerjee contributed to conceptualization, data analysis, writing, and original draft preparation; V. Kumar contributed to sample collection, review and editing; D.J. Sarkar contributed to review and editing; S. Roy contributed to editing; C. Jana contributed to map preparation; B. K. Behera contributed to conceptualization, supervision; B. K. Das contributed to overall supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
The experiment was conducted on dead fish, collected from fishermen however, the present research was conducted as per the guideline of ICAR—Central Inland Fisheries Research Institute, Barrackpore, Kolkata.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors. No experiment was conducted with live fish samples in this study. Only caught fish by local fishermen were used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, T., Kumar, V., Sarkar, D.J. et al. Potentially Toxic Elements: Distribution, Ecological Risk Assessment and Sources Identification in a Himalayan Lake in India. Agric Res 13, 529–541 (2024). https://doi.org/10.1007/s40003-024-00720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-024-00720-y