Abstract
Phytoextraction is a cost-effective technique to remediate contaminated soil. The efficiency of the phytoextraction process is limited by the slow growth, small biomass production of hyper-accumulator plants, and lower phytoavailability of contaminants in soil. The study is focused on comparing the efficiency of the three reported accumulator plants for phytoextraction of zinc (Zn) and copper (Cu) from contaminated soil and their effect on the bioavailability/toxicity of the elements after harvest. In a pot experiment, sunflower, marigold, and spinach were grown in Zn and Cu-contaminated soil. After harvest, the effect of phytoextraction on the distribution of Zn and Cu in various soil-solid phases was studied through a fractionation study as an indicator of bioavailability. The efficiency of phytoextraction was compared in terms of the metal uptake ability of the plants. The highest biomass yield of accumulator plants was obtained with marigold (30.1 g pot−1), followed by sunflower (16.3 g pot−1) and spinach (7.75 g pot−1). The concentrations of Zn and Cu in the three plants ranged from 58.0 to 222 mg kg−1 and 6.33 to 13.3 mg kg−1, respectively. In both the cases of Zn and Cu, sunflower was found superior to the other two plants in terms of phytoextraction of the metals from the contaminated soil. A fractionation study showed that in sunflower and marigold-grown soil, the carbonate bound fraction of Zn enriched water-soluble and exchangeable fraction of Zn, while in spinach-grown soil, the dissolved carbonate bound fraction of Zn enriched the organically bound fraction. Thus, it can be inferred that sunflowers and marigolds increased the bioavailability and toxicity of Zn and Cu more than that of spinach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace element contamination is a matter of concern across the globe including India. This type of contamination not only degrades the quality of soil, water, and food crops but also impairs human health by getting into the food chain. Trace metals like zinc (Zn), Copper (Cu), and manganese (Mn) are micronutrients i.e. they have essential functions for usual plant growth at low concentrations [35] but they act as toxic elements for the growth of plants and food chain when become bioavailable at an elevated concentration [24,25,26, 34]. For example, Zn at its higher concentration impedes reproduction and impairs the growth of the embryo causing various types of anaemia [46]. Long-term exposure to Cu at high levels can irritate the nose, eyes, and mouth as well as headaches, dizziness, stomach aches, and acute gastrointestinal effects including vomiting and diarrhoea [45]. The average total concentration of Zn in soil ranges from 50 to 80 mg kg−1 [26], whereas, the maximum permissible limit of Zn in the soil is 300 mg kg−1 [14, 18]. The concentration of Cu in soil generally varies from 5 to 30 mg kg−1 [6], but, when the concentration of this trace element exceeds 60–125 mg kg−1, it becomes toxic even for tolerant plants.
Phytoextraction has been gaining the worldwide attention of researchers as an environmentally friendly and potentially cost-effective technique to remove toxic metals from soil [47]. The technique makes use of hyper-accumulator plant species, which remove the toxic metals from soil or water through their roots and translocate to their above-ground portion i.e. shoot [16, 17, 28]. Hyper-accumulator plants have an exceptionally high capacity to accumulate trace elements in their aerial parts. However, the efficiency of the phytoextraction process is limited by their slow growth and small biomass production of hyper-accumulator plants, restricted root contact with heavy metals in soil, and lower phytoavailability of heavy metals in soil [2, 13, 33]. The successful outcome of phytoextraction is often associated with the recurring weather and climatic conditions greatly [3]. Therefore, exploration of hyperaccumulator plants that can be cultivated in agriculture fields, and well adapted to the local condition, and have high biomass production capacity is a more practical and feasible solution in remediating metal-contaminated soil. The ability of sunflower (Helianthus annuus L.), French marigold (Tagetes patula L.) and spinach (Spinacea oleracea L.) to accumulate heavy metals has been reported in several literatures [9, 50, 51]. These plants are well suited to local climatic conditions, cultivated in agricultural fields, and have high biomass. Sunflowers and marigolds are comparatively high biomass-producing plants. Possibility of phytoremediation of metal contaminated (namely Cr, Mn, Fe, Cu, Zn, and Pb) soil by using sunflower (Helianthus annuus L.), marigold (Tagetes patula L.) and cock’s comb (Celocia cristata L.) was evaluated [9]. Average Cu accumulation in sunflower was the highest (32 mg kg−1 DW) followed by marigold (22 mg kg−1 DW), and cock’s comb (16 mg kg−1 DW). Zinc accumulation in the root of sunflower was the highest (469 ± 24.4 mg kg−1 DW), followed by cock’s comb (437 ± 48.2 mg kg−1 DW). A significant amount of accumulation of Zn in the leaf of the plants was also found with the highest concentration in cock’s comb (365 ± 38.9 mg kg−1 DW), followed by sunflower (355 ± 45.3 mg kg−1 DW). Phytoremediation by use of these two plants (sunflower and marigold) has advantages as these plants are especially used for floriculture, which are economically important, non-edible, ornamental species and are aesthetically pleasing [9, 29]. A significant reduction in Zn and Cu to the tune of 17.4 and 8.76%, respectively, was observed in the contaminated soil by growing spinach as an accumulator plant [44]. The ability to take multiple cuttings from spinach allows for the periodic removal of metals, thereby facilitating the substantial extraction of metals over time.
Total concentration of metals in soil is a poor indicator of their bioavailability and toxicity, as a metal associated with different solid phases of soil vary in their bioavailability to a great extent [54]. Therefore, the determination of metals in their various chemical forms present in the soil is of utmost importance to judge their degree of contamination/toxicity [54]. Several sequential extraction procedures were developed aiming to reveal the different forms of association of metals in soil and their level of bioavailability [8, 10, 37, 39, 53]. Moreover, DTPA extractable metal content is a more appropriate chemical extraction procedure to determine the trace element availability to the plant than the determination of total metal content in the plant [30, 31, 56]. In past studies, the chemical fraction of Zn and other metals was impacted by land application of sewage sludge [12, 41], wastewater [15, 23], compost [1, 58], nanoparticles [22], and chemical fertilizers [21, 32] have been studied. However, there is a very scarcity of studies that demonstrated the effect of growing accumulator plants on the distribution of Zn and Cu in various soil phases. In addition to assessing the effectiveness of accumulator plants, this study hypothesizes that cultivating these plants will enhance the bioavailability of Zn and Cu in the soil. The objectives of the present investigation are (1) to evaluate the efficiency of sunflower, marigold, and spinach plants for their phytoextraction ability of Zn and Cu in contaminated soil and, (2) to study the changes in the distribution of Zn and Cu in soil as affected by phytoextraction by accumulator plants.
Materials and Methods
Collection and Processing of Soil Sample
A bulk surface (0–15 cm) soil sample was collected from a metal-contaminated site (30° 58′ 20.53″ N, 75° 39′ 01.64″ E), located adjacent to Budhanala of Ludhiana district, Punjab, India which flows across the town and receives industrial effluents of the city [42]. The major industries in Ludhiana city include electroplating and dying industries [42]. During the rainy season, due to heavy water flow the bank of the Budhanala remains inundated for 2–3 months which makes the adjacent site of the Budhanala contaminated with heavy metals. The collected soil sample was air-dried, ground, and passed through a 2 mm sieve. The processed soil sample was mixed thoroughly and used for the pot experiment. Initial physicochemical characteristics of the bulk soil sample were determined. The initial physicochemical properties of the soil and methods followed for analysis are presented in Table 1.
Initial Properties of the Experimental Soil
The collected soil is neutral in pH having a loam texture. The soil is very high in organic carbon (OC) i.e. 1.95%. The maximum permissible limit of Zn and Cu in soil is 300 and 140 mg kg−1, respectively [14, 18]. However, if the Cu concentration of the soil when exceeds 60–125 mg kg−1, it becomes toxic even for tolerant plants. Thus, it can be inferred that the soil is contaminated with both Zn and Cu, as their contents in the soil were 715 and 82.2 mg kg−1, respectively (Table 1).
Pot Experiment
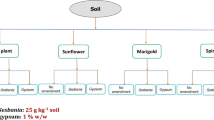
A greenhouse pot experiment was conducted for evaluating the efficiency of sunflower (Helianthus annus, vart. Surya), French marigold (Tagetes patula, vart. Pusa arpita), and spinach plant (Spinacia oleracea, vart. Pusa all green) for their phytoextraction ability in a Zn and Cu contaminated soil and distribution of Zn and Cu in various soil solid phases after harvest of the plants. Treatments comprised of the above three accumulator plants along with one control treatment i.e. soil without a plant. Each treatment was replicated thrice. The pots were arranged following a completely randomized design (CRD). The used plants are well adapted to the local soil and climatic conditions. Four kg of soil in each pot was taken and homogenously fertilized with recommended doses of N–P2O5–K2O for sunflower, marigold, and spinach, separately. Doses of fertilizer used for growing sunflowers, marigolds and spinach were 26.78–35.71–26.78, 44.64–33.48–33.48, and 16.7–16.7–33.3 mg kg−1 soil N–P2O5–K2O, respectively by use of laboratory grade urea, KH2PO4 and KCl. The pots were properly labeled and irrigated to bring optimum moisture conditions for seed sowing. Then 10, 15, and 30 seeds of sunflower, marigold, and spinach, respectively were sown. The uniform population of 2, 5, and 20 plants of sunflower, marigold, and spinach, respectively was maintained after thinning. Pots were regularly irrigated with deionized water as needed. Sunflower and Marigold plants were harvested before flowering i.e. 45 and 75 days after sowing, respectively. Spinach plants were harvested after two cuts at 60 days after sowing. After harvesting all three crops, soil from each pot was removed, and thoroughly mixed, and approximately 100 g of soil was collected from each pot to determine DTPA extractable Zn and Cu concentration in soil. The distribution of metals in different soil fractions was also assessed in post-harvest soil.
Soil Analysis
The total metal content in the soil was determined by digesting the soil with an HF–H2SO4–HClO4 mixture [47]. For that 0.1 g soil was taken in a Teflon beaker and 4–5 drops of 18 N H2SO4, 1 mL concentrated HClO4, and 5 mL 48% HF were added. Then the beaker was heated at 225 °C on a hot plate covering the 9/10th portion of the beaker with the lid till the volume was reduced to 2–3 mL. The entire cycle of acid addition and heating was repeated until the soil completely dissolved. Then the content was transferred to a 100 mL volumetric flask after repeated washing by double distilled water and finally, the volume was made up to the 100 mL mark. The content was filtered through a Whatman no. 42 filter paper and the concentration of Zn and Cu in the extracts was determined by an inductively coupled plasma mass spectrometer (ICP-MS) (Perkin Elmer NexIon 300). DTPA extractable Zn and Cu were determined by the method outlined by Lindsay and Norvell [31]. The Zn and Cu concentration in the extract was determined by ICP-MS (Perkin Elmer NexIon 300).
Fractionation of soil Zn and Cu was done following a short sequential extraction technique proposed by Golui and co-workers [19], by which four conceptual fractions of Zn and Cu were removed from the soil (Fig. 1). These four fractions were water-soluble and exchangeable (W + E), carbonate bound (Carb), iron and manganese oxide bound (Fe + Mn), and organically bound fraction (Org). The details of the methodology are as follows:
-
Step 1: Water-soluble + Exchangeable: One gram of processed soil was extracted with 20 mL of 0.5 M Ca(NO3)2 (50 mL centrifuge tube) by continuous shaking for 16 h at room temperature.
-
Step 2: Carbonate bound: Residue from the previous step was extracted with 8 mL of 1 M NaOAc (pH 5) by continuous shaking for 6 h at room temperature.
-
Step 3: Fe/Mn oxides bound: Residue from the previous step was extracted with 20 mL of 0.04 M NH2OH-HCl in 25% acetic acid (v/v) for 6 h at 96 °C with occasional shaking.
-
Step 4: Organically bound: Residue from previous step was extracted with 3 mL of 0.02 M HNO3 + 5 mL of pH 2, 30% H2O2 (v/v) for 2 h at 85 °C, with occasional shaking; an additional 3 mL of pH 2, 30% H2O2 for 3 h at 85 °C with occasional shaking; and a further addition of 5 mL of 3.2 M NH4OAc in 20% HNO3 (v/v) with continuous shaking for 0.5 h at room temperature following dilution to 20 mL with deionized water.
Between each step, the supernatant was separated from the solid phase by centrifuging at 5000–10,000 rpm for 20 min. The supernatant was then filtered through the Whatman No. 42 filter paper and the content was analyzed for Zn and Cu from each step of extraction using ICP-MS (Perkin Elmer NexIon 300). Additionally, the residual fraction (Res.) of Zn and Cu were computed by subtracting the sum of the four fractions determined by the method proposed by Golui et al. [19] from total soil Zn and Cu concentration.
Estimation of Shoot Biomass Yield
Sunflower, marigold, and spinach plants after harvest were washed by tap water follower by dilute HCl (0.01 N) and double-distilled water. The plant samples were then dried in a hot air oven at 65 °C to a constant weight following drying in the open air for a few days. The weight of the oven-dried shoot sample was taken and shoot biomass yield was recorded.
Estimation of Zn and Cu in Plant
The washed and oven-dried plant samples were ground, and 0.5 g of the ground sample was digested with HNO3 (Supra pure) in a microwave digester (Multiwave ECO, Anton Paar). The digested plant sample was then filtered through a Whatman no. 42 filter paper in a 50 mL volumetric flask and finally, volume was made up to 50 mL mark by double distilled water. Zinc (Zn) and Cu concentration in the digested plant samples was determined in ICP-MS (Perkin Elmer NexIon 300). A standard reference material (tomato leaves, SRM 1573a) from the National Institute of Standards and Technology, USA was used in triplicate for the validation of analysis by ICP-MS. The recoveries of metals in the SRM were: 91.2 ± 0.44% for Zn and 92.0 ± 4.02% for Cu.
Quantification of Phytoextraction Efficiency
The phytoextraction efficiency of the accumulator plants was quantified by estimating the metal uptake by the accumulator plant from the individual pots. The metal uptake by the accumulator plants was estimated by multiplying the concentrations of Zn and Cu in the shoot of the accumulator plants with their respective oven-dried shoot weight following the following formula [47].
where uptake of metal by shoot was expressed as mg pot−1, the concentration of metal as mg kg−1, and shoot biomass yield as g pot−1.
Statistical Analysis
Significant effects of the treatments on shoot biomass yield of the accumulator plants, Zn and Cu concentration in the shoot of the accumulator plants, DTPA extractable Zn and Cu, and distribution of metals (Zn and Cu) in different soil fractions were determined by one-way ANOVA. The pair-wise mean comparison was done by the “post-hoc” Duncan’s Multiple Range Test (DMRT) at a 5% level of significance. All the statistical analyses were performed with SPSS 25.0 software (SPSS Inc., Chicago, IL).
Results and Discussion
Shoot Biomass Yield, Concentration, and Uptake of Zn and Cu in the Accumulator Plants
All the accumulator plants differ significantly concerning their shoot biomass yields (Fig. 2). The highest biomass yield of accumulator plants was obtained with marigold (30.1 g pot−1), followed by sunflower (16.3 g pot−1) and spinach (7.75 g pot−1). Regarding shoot Zn concentration, the spinach presented the highest shoot Zn concentration, whereas, the marigold had the lowest (Table 2). In the case of Cu, marigold again recorded the lowest shoot Cu concentration, the sunflower, and spinach being similar in their shoot Cu concentration (Table 2). Zinc uptake by accumulator plants varied significantly, where Zn uptake by spinach and marigold was statistically at par, whereas significantly higher uptake of Zn was observed in the shoot of sunflower (Table 2). All accumulator plants differed significantly for their shoot Cu uptake. The sunflower recorded the highest value in comparison to the other two accumulator plants. The order of Cu uptake followed as sunflower > marigold > spinach.
The shoot metal concentrations depend upon the plant species grown. Plants differ in their ability to uptake a particular metal in their above-ground portion of the plants. Metals are generally taken up by plants via membrane transporter proteins [43]. Hence, the concentration of metal varies with the type of accumulator plants. The uptake of metals depends on a discrete number of proteins. In fact, for most metals, multiple transporters exist in plants. The transporter proteins vary with respect to their transport rate, substrate affinity, and substrate specificity. Furthermore, the abundance of each transporter varies with tissue type and environmental condition. Thus, the transporter proteins are species and condition-dependent, which might explain the reason for differential Zn and Cu concentrations in sunflower, marigold, and spinach plants in the present investigation. For assessing the efficacy of accumulator plants for phytoextraction of trace elements, biomass yield is important along with the concentration of trace elements in accumulator plants. Hence, uptake of these pollutant elements by accumulator was worked out. In the present study, the highest concentration of Zn is associated with spinach (Table 2). But, if the data of the shoot biomass were observed the order of the shoot biomass yield was marigold > sunflower > spinach. Uptake of Zn, which is the product of Zn concentration in shoot and shoot biomass was found to be highest for Sunflower (Table 2). The order of accumulator plants based on phytoextraction of Zn follows sunflower > marigold = spinach. Although the biomass yield of spinach was lower than that of marigold, the comparable uptake of Zn by spinach was solely due to the very high Zn content in the spinach plant. The highest uptake of Zn by sunflowers was due to its higher shoot Zn concentration and shoot biomass yield.
Copper concentrations in the shoots of sunflower and spinach were found to be statistically similar and higher than that of marigolds (Table 2). However, the highest uptake of copper was recorded by sunflowers. The order of phytoextraction/uptake of Cu by the accumulator plants follows Sunflower > marigold > spinach (Table 2). Although the concentration of Cu was greater in spinach than in the marigold, the phytoextraction ability of the marigold was found to be higher than in spinach. This is due to the higher shoot biomass production of marigolds. In the case of both Zn and Cu, the highest efficiency of phytoextraction was found in the case of sunflower and in both cases, spinach proved to be lower in efficiency of phytoextraction than the other two accumulator plants due to its lower biomass production.
Distribution and Availability of Zn and Cu in the Soil as Affected by Accumulator Plants
Distribution of Zn in different soil fractions followed the order as Fe + Mn > Res > Carb >> W + E (Table 3; Fig. 3a). The share (%) of W + E, Carb, Fe + Mn, and Org fractions in soil under sunflower crop was 1.04, 18.7, 41.9, and 9.70%, respectively; the corresponding values for marigold and spinach were 1.02, 19.2, 42.1, 9.60% and 0.99, 18.9, 41.9, 11.3%, respectively (Fig. 3a). The effect of accumulator plants on W + E-Zn was significant. Only the sunflower and marigold treatments increased the W + E fraction by 9.85 and 6.91%, respectively in comparison to the control treatment. Consequent to the growth of accumulator plants, Carb-Zn (4.17–6.29%) decreased in the soil in comparison to the control treatment. The highest decrease in the Carb fraction was associated with sunflowers. The Fe + Mn bound fraction did not differ significantly after the growing of accumulator plants. Only spinach led to a significant 14.1% increase in Org fraction over the control treatment. The residual fraction of Zn did not vary as a result of the growing of accumulator plants. Only the sunflower treatment resulted in a significant 4.11% decrease in DTPA-Zn, whereas, the rest two treatments recorded statistically at par value with the control treatment.
Distribution of Cu in different soil fractions followed the order of Org > Res > Carb > Carb > W + E (Table 4). The share (%) of W + E, Carb, Fe + Mn, and Org fractions in soil under sunflower crops was 0.24, 8.11, 12.2, and 40.6%, respectively; the corresponding values for marigold and spinach were 0.24, 7.79, 13.0, 39.7% and 0.24, 8.11, 13, 45.4%, respectively (Fig. 3b). The W + E, Carb, and Fe + Mn fraction of Cu did not differ significantly as a result of the growth of accumulator plants. A significant 1.63% increase in Org-Cu was observed in spinach treatment over that of control treatment. In contrast to this, a reduction in Org was observed in sunflower and marigold treatment. Opposite to the result of Org, in Res significant 21.2 and 22.3% increase was observed in comparison to the control treatment. Only spinach treatment led to a significant 8% increase in the DTPA-Cu, in comparison to the control treatment.
The evaluation of metal distribution in various soil solid fractions is useful in the prediction of metal solubility, mobility, bioavailability, and thus toxicity [26]. The exchangeable fraction of Zn is the most labile form in the soil and has the most adjacent correlation with Zn uptake by plants [7, 30]. Due to cation exchange capacity and available exchange site for Zn in organic matter, organically bound Zn is also available to plants [27]. However, the bioavailability of Zn is reduced in its carbonate and Fe–Mn oxides binding form in the soil [49]. In the present investigation, the fractionation of Zn in soil shows that a major portion is associated with the Fe + Mn oxide bound fraction, followed by the residual fraction, carbonate bound fraction, organically bound fraction, and water-soluble + exchangeable fraction (Table 3). The effect of different accumulator plants on the redistribution of zinc in different soil fractions was different. In sunflower and marigold-grown soil, there was an increase in the W + E fraction of Zn, while a decrease in carbonate bound fraction of Zn was observed in comparison to the control treatment (Table 3). There was an increase in the organically bound fraction of Zn in soil under spinach, while a significant decrease in the carbonate bound fraction of Zn was noted. The dynamics of metals in different soil fractions depend upon plant type. For instance, root exudates of oats can solubilize the heavy metals bound to carbonate and oxides and able to bring them to the exchangeable form, enhancing the bioavailability of heavy metals [38]. The soil pH of the rhizosphere gets reduced by the amino acids secreted by the roots of ryegrass increasing the organic Zn of rhizosphere soil than that of non-rhizosphere soil [57]. Reduction of carbonate bound Zn in sunflower, marigold, and spinach-grown soil was accompanied by enrichment of water-soluble + exchangeable fraction and the organically bound fraction of Zn, which indicates the transformation of the sparingly soluble form (carbonate bound fraction) of Zn. This might be made feasible by the organic ligands released by the roots of the accumulator plants, which might form stable complexes with Zn and increase the solubility of this metal in soil [52]. However, enrichment of dissolved Carb-Zn in W + E fraction in the case of sunflower and marigold treatment, and Org fraction in case of spinach treatment may be related to variation in root exudates of these plants as in the case of Oat and ryegrass mentioned earlier in this section. Overall, the variation in the redistribution of Zn in different soil fractions in soils grown with different accumulator plants might partly be due to the differences in their biomass yield and the rate and composition of their root exudates. The data of the distribution of Zn in different soil fractions in different treatments revealed that all the accumulator plants increase the bioavailability and toxicity of Zn. However, the increase in bioavailability and toxicity is more in the case of sunflower and marigold as compared to the spinach treatment as the dissolved Carb fraction enriched the more labile fraction i.e. W + E fraction in sunflower and marigold treatment. In other words, sunflower and marigold plants widen the scope for phytoextraction for subsequently grown crops than that of spinach plants does.
The distribution of Cu in various soil fractions followed the order: organically bound > residual > Fe + Mn oxide bound > carbonate bound > water soluble + exchangeable. The dominance of Cu in the organically bound fraction is ascribed to its extraordinary affinity for organic matter due to its unique electronic configuration [36, 59]. Significant redistribution of Cu in different soil fractions was not observed in the case of all the treatments, except in the case of the Org-Cu form. Spinach led to an increase in Org-Cu, while, a decrease in Org-Cu was observed in the case of sunflower and marigold treatment. It shows that relatively spinach is more efficient in increasing the bioavailability and toxicity of Cu than that of Sunflower and marigold. Generally, plants are excluders of Cu and Cu-accumulators are uncommon [40]. In the present investigation, however, a much higher concentration of Cu was found in the accumulators than the average concentration of Cu found in non-accumulator plants (1 mg kg−1) [48]. Uptake of comparatively less amount of Cu by the accumulator plants might be the major reason for not getting many variations of the metal distribution in different soil solid phases.
Conclusions
The study was conducted to evaluate the efficiency of sunflower, marigold, and spinach plants for their phytoextraction ability in Zn and Cu-contaminated soil, and to record the effect of phytoextraction on bioavailability and toxicity of the metals in the soil. Sunflower demonstrated the highest uptake of both Zn and Cu, followed by marigold and spinach. Despite lower biomass yield, spinach showed comparable Zn uptake due to its exceptionally high Zn content. Marigold demonstrated higher phytoextraction efficiency than spinach, despite lower shoot metal concentrations, due to its higher shoot biomass production. Accumulator plants influenced the distribution of Zn and Cu in different soil fractions. The results of the fractionation study revealed that sunflower and marigold plants are more potent than spinach in increasing the bioavailability and toxicity of Zn and Cu for succeeding crops. More or less the distribution of Cu in different soil solid fractions remained unaffected as a result of the growing of accumulator plants. It is revealed that all accumulator plants in the present study increased the bioavailability and toxicity of Zn, with sunflower and marigold having a more pronounced effect than spinach. The study emphasizes the importance of considering both biomass yield and metal concentrations when evaluating accumulator plants for phytoextraction. The findings contribute to understanding the complex interplay between plant species, metal uptake, and soil metal distribution, providing valuable insights for future phytoextraction studies and environmental management practices.
References
Achiba WB, Gabteni N, Lakhdar A, Du Laing GD, Verloo M, Jedidi N, Gallali T (2009) Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric Ecosyst Environ 130:156–163. https://doi.org/10.1016/j.agee.2009.01.001
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf 174:714–727. https://doi.org/10.1016/j.ecoenv.2019.02.068
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120. https://doi.org/10.1016/j.jenvman.2012.04.002
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54(5):464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Bower CA, Reitemeier RF, Fireman M (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73(4):251–262. https://doi.org/10.1097/00010694-195204000-00001
Brun LA, Le Corff J, Maillet J (2003) Effects of elevated soil copper on phenology, growth and reproduction of five ruderal plant species. Environ Pollut 122(3):361–368. https://doi.org/10.1016/s0269-7491(02)00312-3
Chahal DS, Sharma BD, Singh PK (2005) Distribution of forms of zinc and their association with soil properties and uptake in different soil orders in semi-arid soils of Punjab, India. Commun Soil Sci Plant Anal 36(19–20):2857–2874. https://doi.org/10.1080/00103620500306031
Chao TT (1984) Use of partial dissolution techniques in geochemical exploration. J Geochem Explor 20(2):101–135. https://doi.org/10.1016/0375-6742(84)90078-5
Chatterjee S, Singh L, Chattopadhyay B, Datta S, Mukhopadhyay SK (2012) A study on the waste metal remediation using floriculture at East Calcutta Wetlands, a Ramsar site in India. Environ Monit Assess 184(8):5139–5150. https://doi.org/10.1007/s10661-011-2328-8
Clevenger TE (1990) Use of sequential extraction to evaluate the heavy metals in mining wastes. Water Air Soil Pollut 50(3–4):241–254. https://doi.org/10.1007/BF00280626
Datta SP, Subba Rao A, Ganeshamurthy AN (1997) Effect of electrolytes coupled with variable stirring on soil pH. J Indian Soc Soil Sci 45:185–187
de Melo WJ, de Stéfani AP, Peruca M, de Melo GM, Peruca de Melo V (2007) Nickel in a tropical soil treated with sewage sludge and cropped with maize in a long-term field study. Soil Biol Biochem 39(6):1341–1347. https://doi.org/10.1016/j.soilbio.2006.12.010
Devi R, Behera B, Raza MB, Mangal V, Altaf MA, Kumar R, Kumar A, Tiwari RK, Lal MK, Singh B (2022) An insight into microbes mediated heavy metal detoxification in plants: a review. J Soil Sci Plant Nutr 22:914–936. https://doi.org/10.1007/s42729-021-00702-x
European Union. 2002. Heavy Metals in Wastes. European Commission on Environment. http://ec.europa.eu/environment/waste/studies/pdf/heavymetalsreport.pdf. Accessed on 3 Feb 2023
Fitamo D, Itana F, Olsson M (2007) Total contents and sequential extraction of heavy metals in soils irrigated with wastewater, Akaki, Ethiopia. Environ Manag 39(2):178–193. https://doi.org/10.1007/s00267-006-0074-4
Garbisu C, Alkorta I (2003) Basic concepts on heavy metals oil bioremediation. Eur J Miner Process Environ Prot 3:58–66
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3(1):1–18. https://doi.org/10.15666/aeer/0301_001018
Golui D, Dali M, Singh R, Datta SP, Mandal J, Ray P et al (2022) Assessing soil degradation and risk in relation to metal pollution in Hindon river water irrigated soils of western Uttar Pradesh of India. Water Air Soil Pollut 233(5):168. https://doi.org/10.1007/s11270-022-05640-7
Golui D, Datta SP, Dwivedi BS, Meena MC, Trivedi VK, Jaggi S et al (2021) Assessing geoavailability of zinc, copper, nickel, lead and cadmium in polluted soils using short sequential extraction scheme. Soil Sediment Contam An Int J 30(1):74–91. https://doi.org/10.1080/15320383.2020.1796924
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Private Limited, New Delhi
Jalali M, Moharami S (2010) Redistribution of cadmium, copper, lead, nickel, and zinc among soil fractions in a contaminated calcareous soil after application of nitrogen fertilizers. J Plant Nutr Soil Sci 173(2):237–244. https://doi.org/10.1002/jpln.200800143
Jośko I (2019) Copper and zinc fractionation in soils treated with CuO and ZnO nanoparticles: the effect of soil type and moisture content. Sci Total Environ 653:822–832. https://doi.org/10.1016/j.scitotenv.2018.11.014
Kabala C, Karczewska A, Szopka K, Wilk J (2011) Copper, zinc, and lead fractions in soils long-term irrigated with municipal waste water. Commun Soil Sci Plant Anal 42(8):905–919. https://doi.org/10.1080/00103624.2011.558960
Kabata-Pendias A, Pendias H (1992) Trace elements in soil and plants, 2nd edn. CRS Press, Boca Raton, p 365
Kabata-Pendias A, Pendias H (2001) Trace elements in soil and plants, 3rd edn. CRS Press Inc., Boca Raton
Kabata-Pendias A, Pendias H (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Khoshgoftarmanesh AH, Afyuni M, Norouzi M, Ghiasi S, Schulin R (2018) Fractionation and bioavailability of zinc (Zn) in the rhizosphere of two wheat cultivars with different Zn deficiency tolerance. Geoderma 309:1–6. https://doi.org/10.1016/j.geoderma.2017.08.019
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified Plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol Adv 27(6):799–810. https://doi.org/10.1016/j.biotechadv.2009.06.003
Lajayer BA, Moghadam NK, Maghsordi MR, Gharbanpou M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26(9):8468–8484. https://doi.org/10.1007/s11356-019-04241-y
Li L, Wu H, van Gestel CAM, Peijnenburg WJGM, Allen HE (2014) Soil acidification increases metal extractability and bioavailability in old orchard soils of North East Jiaodong Peninsula in China. Environ Pollut 188:144–152. https://doi.org/10.1016/j.envpol.2014.02.003
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42(3):421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Liu J, Duan CQ, Zhu YN, Zhang XH, Wang CX (2007) Effect of chemical fertilizers on the fractionation of Cu, Cr and Ni in contaminated soil. Environ Geol 52(8):1601–1606. https://doi.org/10.1007/s00254-006-0604-7
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q et al (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121. https://doi.org/10.1016/j.ecoenv.2015.12.023
Majhi PK, Raza B, Behera PP, Singh SK, Shiv A, Mogali SC et al (2022) Future-proofing plants against climate change: a path to ensure sustainable food systems. In: Biodiversity, functional ecosystems and sustainable food production. Springer, Cham, pp 73–116
Marschner H, Rimmington G (1988) Mineral nutrition of higher Plants. Plant Cell Environ 11:147–148
McBride MB (1989) Reactions controlling heavy metal solubility in soils. Adv Soil Sci 10:1–56. https://doi.org/10.1007/978-1-4613-8847-0_1
McLaren RG, Crawford DV (1973) Studies on soil copper. 1: the fractions of copper in soils. J Soil Sci 24:443–452. https://doi.org/10.1111/j.1365-2389.1973.tb00753.x
Mench MJ, Fargues S (1994) Metal uptake by iron-efficient and inefficient oats. Plant Soil 165(2):227–233. https://doi.org/10.1007/BF00008066
Milićević T, Relić D, Škrivanj S, Tešić Ž, Popović A (2017) Assessment of major and trace element bioavailability in vineyard soil applying different single extraction procedures and pseudo-total digestion. Chemosphere 171:284–293. https://doi.org/10.1016/j.chemosphere.2016.12.090
Napoli M, Cecchi S, Grassi C, Baldi A, Zanchi CA, Orlandini S (2019) Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 219:122–129. https://doi.org/10.1016/j.chemosphere.2018.12.017
Nogueira TAR, Melo WJ, Fonseca IM, Marcussi SA, Melo GMP, Marques MO (2010) Fractionation of Zn, Cd and Pb in a tropical soil after nine-year sewage sludge applications. Pedosphere 20(5):545–556. https://doi.org/10.1016/S1002-0160(10)60044-6
PPCB (2010) Action plan for abatement of pollution in critically polluted area of Ludhiana city. Punjab Pollution Control Board. Vatavaran Bhawan, Nabha road, Patiala
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214
Rachman F, Supriatin S, Niswati A, Salam AK (2022) Lime-enhanced phytoextraction of copper and zinc by land spinach (Ipomoea reptans Poir.) from tropical soils contaminated with heavy metals. AIP Conf Proc 2563:040015. https://doi.org/10.1063/5.0103992
Rahman MM, Asaduzzaman M, Naidu R (2013) Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J Hazard Mater 262:1056–1063. https://doi.org/10.1016/j.jhazmat.2012.06.045
Rattan RK, Datta SP, Sanyal SK (2009) Pollutants elements and human health. In: Narayanasamy G, Rattan RK, Ganeshamurthy AN (eds) Soil quality for human health, vol 27. Bulletin of the Indian Society of Soil Science, pp 103–123
Samal SK, Datta SP, Dwivedi BS, Meena MC, Nogiya M, Choudhary M et al (2023) Phytoextraction of nickel, lead, and chromium from contaminated soil using sunflower, marigold, and spinach: comparison of efficiency and fractionation study. Environ Sci Pollut Res 30(17):50847–50863. https://doi.org/10.1007/s11356-023-25806-y
Sheoran V, Sheoran AS, Poonia P (2009) Phytomining: a review. Miner Eng 22(12):1007–1019. https://doi.org/10.1016/j.mineng.2009.04.001
Shuman LM, Wang J (1997) Effect of rice variety on zinc, cadmium, iron, and manganese content in rhizosphere and non-rhizosphere soil fractions. Commun Soil Sci Plant Anal 28(1–2):23–36. https://doi.org/10.1080/00103629709369769
Sinhal VK, Srivastava A, Singh VP (2010) EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J Environ Biol 31(3):255–259
Sung M, Lee CY, Lee SZ (2011) Combined mild soil washing and compost-assisted phytoremediation in treatment of silt loams contaminated with copper, nickel, and chromium. J Hazard Mater 190(1–3):744–754. https://doi.org/10.1016/j.jhazmat.2011.03.113
Tao S, Liu WX, Chen YJ, Cao J, Li BG, Xu FL (2005) Fractionation and bioavailability of copper, cadmium and lead in rhizosphere soil. In: Huang PM, Gobran GR (eds) Biogeochemistry of trace elements in the Rhizosphere. Elsevier, Amsterdam, pp 313–336
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851. https://doi.org/10.1021/ac50043a017
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292. https://doi.org/10.4067/S0718-95162010000100005
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38. https://doi.org/10.1097/00010694-193401000-00003
Wang G, Su MY, Chen YH, Lin FF, Luo D, Gao SF (2006) Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in Southeastern China. Environ Pollut 144(1):127–135. https://doi.org/10.1016/j.envpol.2005.12.023
Xu H, Wang HX, Liu H, Xiong ZT, Singh B (2007) Single and combined pollution of zinc and cadmium influence on root exudates and Zn, Cd forms in rye grass. Acta Sci Circum 28:2089–2095
Zheljazkov VD, Warman PR (2004) Phytoavailability and fractionation of copper, manganese, and zinc in soil following application of two composts to four crops. Environ Pollut 131(2):187–195. https://doi.org/10.1016/j.envpol.2004.02.007,PMID15234085
Zhou LX, Wong JWC (2001) Effect of dissolved organic matter from sludge and sludge compost on soil copper sorption. J Environ Qual 30(3):878–883. https://doi.org/10.2134/jeq2001.303878x
Acknowledgements
Financial support and infrastructure facility from Division of Soil Science and Agricultural Chemistry, ICAR-Indian Agricultural Research Institute, New Delhi is duly acknowledged.
Author information
Authors and Affiliations
Contributions
SKS: Conceptualization, Methodology, Data curation, Formal analysis, Visualization, Writing—original draft, Investigation. SPD: Supervision, Resources, Validation, Writing—review and editing. DG: Methodology, Data curation, Writing—review and editing. BSD: Visualization, Formal analysis and editing. MCM: Data curation, Formal analysis and editing. MN: Review and editing. MC: Review and editing. MBR: Review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samal, S.K., Datta, S.P., Golui, D. et al. Comparing the Efficiency of Sunflower, Marigold and Spinach Plants for Their Phytoextraction Ability of Zinc and Copper in Contaminated Soil. Agric Res 13, 542–552 (2024). https://doi.org/10.1007/s40003-024-00713-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-024-00713-x