Abstract
Purpose
Mycobacterium abscessus, and rapidly growing mycobacteria in general, are rare but increasing causes of central nervous system (CNS) infections. The aim of this study is to highlight the importance of considering these microorganism in the differential diagnosis of CNS infections, obtaining a prompt diagnosis, and improving clinical outcomes.
Methods
Case report and literature review.
Results
We report a case of meningeal infection in a patient who underwent decompressive craniectomy after a craniofacial trauma. The diagnosis was made analyzing a sample obtained during a second operation of cranioplasty. A regimen of amikacin, clarithromycin, and imipenem/cilastatin was started. In the following days, the patient experienced a variety of side effects. So, first clarithromycin was replaced with linezolid, then amikacin was stopped and cefoxitin added to the therapy and at the end all the antibiotics were withdrawn. The patient was discharged in good conditions and a clinical interdisciplinary follow-up was started. After 12 months, the patient is still doing well. After a literature analysis, 15 cases of M. abscessus CNS infections were identified. Various modes of acquisition, underlying disease and therapeutic schemes were evident.
Conclusions
Considering the results of the literature analysis and the increasing incidence of M. abscessus, all specialists involved in the management of CNS infection should be aware of the importance of atypical microorganisms in differential diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacterium abscessus is a rapidly growing mycobacteria (RGM) ubiquitous in soil and water. After recent changes in taxonomy, three subspecies are now identified based on whole-genome sequencing: M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. Massiliense [1].
RGM have been described as a causative agent of skin and soft-tissue infections after trauma or surgery, pulmonary infection in patients with cystic fibrosis and chronic lung diseases, and disseminated disease in immunocompromised hosts especially in AIDS patients [2]. Both, idiopathic and post-surgical CNS infections, are rare; however, in the past two decades, RGM have been increasingly reported as a cause of chronic meningitis, development of brain abscesses, and neurosurgical device infection. Most likely, this can be attributed to the rise in prosthetic device surgeries and an increasing number of patient who are immunosuppressed. RGM infections are difficult to diagnose due to clinical and microbiological factors. Clinically, the low prevalence of RGM infections puts them rarely in the differential diagnosis of CNS infections, and subsequently, samples collected are not often sent to the laboratory for mycobacterial research. Microbiologically, only highly qualified laboratories are able to isolate and identify RGM using culture and molecular techniques.
We describe a case of intracranial infection due to M. abscessus following a bifrontal decompressive craniectomy after trauma that was diagnosed incidentally during a second operation to repair the skull base.
Case report
A 46-year-old man was evaluated in the emergency room following an alleged assault, which took place in a public park. He had no comorbidities, neither structural lung diseases nor diabetes or other causes of immuno-suppression. His initial evaluation revealed a non-focal exam with a Glasgow Coma Scale (GCS) of 14 and obvious signs of facial trauma. Upfront imaging was not performed; however, after a few hours, the patient decompensated neurologically, requiring intubation. Brain-computed tomography (CT) at this time demonstrated multiple, displaced cranial and skull base fractures, frontal contusions, and an acute subdural hemorrhage (ASDH) with 5–6 mm of midline shift with early uncal herniation (Fig. 1a). Because of the ASDH, the brain swelling, and the mass effect of the frontal contusions, bifrontal decompressive craniectomy was performed emergently with intracerebral hematoma evacuation and duraplasty. It was not possible to obtain an appropriate intact bone operculum due to extreme fragmentation of the bone.
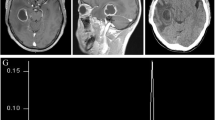
Radiological aspects of the present case. a Preoperative CT scan showing displaced cranial and skull base fractures, frontal contusions, and an acute subdural hemorrhage (ASDH) with midline shift. b Postoperative CT scan showing bifrontal decompression. c 30 days postoperative CT scan showing pneumocephalus. d Post-cranioplasty CT scan showing closure of the skull base defect, without pneumocephalus
The patient experienced an uncomplicated recovery with the only focal neurology deficit anosmia. Imaging 5 days after the operation (Fig. 1b) demonstrated appropriate decompression and improved midline shift; however, there was new frontal lobe herniation through the cribriform defect. As the patient was asymptomatic and without radiographic or clinical signs of cerebrospinal fluid (CSF) leak, we decided to let him recover in a rehabilitation institute with the plan of close follow-up and repair of the defect at the time of the cranioplasty.
Follow-up brain CT at 30 days revealed continued herniation through the cribriform defect with pneumocephalus (Fig. 1c). Subsequently, the patient was readmitted to the hospital for skull base repair and cranioplasty. He remained afebrile without signs of infection, CSF leak, or meningitis. During the operation, a dural defect with underlying yellowish, necrotic-like material was identified and evacuated from the floor of the anterior cranial fossa. In addition, specimen was sent to microbiology for gram stain and culture. The skull base was reconstructed with a split frontal bone flap and the durotomy repaired with 5–0 prolene stitches and fibrin glue sealing. Finally, a polyether–ether–ketone (PEEK) patient specific implant (PSI) was placed over the calvarium. A spinal drain was placed prior to conclusion of the operation to maximize healing results. The postoperative course was uneventful and the spinal drain was removed on postoperative day 5. Postoperative CT and MRI imaging (Fig. 1d) showed closure of the skull base defect and was without pneumocephalus.
The intraoperative swab cultured for common bacteria and fungi resulted negative. Nevertheless, due to the macroscopic necrotic aspect of the sample, the microbiologist decided to extend the analysis to mycobacteria and the molecular analysis by molecular amplification and sequencing of the hsp65 gene [3, 4] resulted positive for M. abscessus (Fig. 2). Culture analysis was negative for mycobacteria. A CSF examination did not detect any sign of infection, so no further microbiological investigations were performed.
The patient was then transferred to our infectious diseases unit, where three deep sputum samples for detection of mycobacteria were collected. Acid-Fast Bacilli (AFB) smear and culture were negative, while molecular analysis by real time PCR [5] resulted positive for atypical mycobacteria; however, due to the very low amount of bacterial DNA, it was not possible to amplify and sequence the hsp65 gene. These results suggested a probable previous colonization of patient’s airways by nontuberculous mycobacteria. HIV antibody testing was negative and a chest CT scan showed no abnormalities of lung parenchyma.
Although the patient was asymptomatic, given the summation of microbiology results and known relationship between skull base fractures and risk of infection, antimycobacterial therapy was initiated. According to what expressed in an official ATS/IDSA statement on diagnosis, treatment and prevention of nontuberculous mycobacterial diseases [6] therapy was started with a regimen of amikacin 10 mg/kg single dose daily, clarithromycin 500 mg two times daily and imipenem/cilastatin 500 mg four times daily. On day 5 of treatment, the patient developed a QTc interval prolongation, hence linezolid 600 mg two times per day replaced clarithromycin. In the following days, the patient complained of slow but progressive hearing loss and sense of ear padding. An audiometric test was performed, identifying bilateral, asymmetric neurosensory hearing impairment. On day 17 of treatment, amikacin was stopped and cefoxitin 3 g four times daily added to the ongoing therapy. After 6 weeks of therapy, the patient developed a generalized maculopapular rash and progressive thrombocytopenia. At this time, all antibiotics ceased and then patient clinically improved.
Balancing the amount of drugs adverse effects experienced by the patient, the shortage of oral antibiotic options available with related needing of prolonged hospitalization for intravenous therapy administration and the complete absence of clinical signs and symptoms, after multidisciplinary discussion, it was decided to refrain from continuing antimycobacterial treatment. The patient was subsequently discharged. He is now on month 12 after therapy interruption, in good clinical condition. He continues to follow with neurosurgeons, otolaryngologists and infectious disease physicians.
Discussion
CNS infections caused by RGM, while rare, pose a clinical challenge in the differential diagnosis of meningitis and shunt-related infections. As described in literature, the most commonly isolated species is M. fortuitum [7,8,9,10,11], followed by M. mucogenicum and M. abscessus [2, 10].
Focusing on CNS infections by M. abscessus, only 15 cases have been reported (Table 1): 14 adult cases and 1 pediatric case in a 16-month-old female with type 1 neurofibromatosis [12]. The first case involving a 59-year-old female with chronic M. abscessus complex meningitis secondary to a neck stab wound was described in 2001 [13]. In the same year, Liebeskind et al. reported a case of a 35-year-old man with M. abscessus complex meningitis and endocarditis [14]. Both patients expired, despite combination antimycobacterial therapy for 3 and 2 months, respectively. The third case involved a 28-year-old female who developed meningoencephalitis by M. abscessus related to dissemination from a post-orbital surgery infection with prosthesis implantation; she was successfully treated by prosthesis removal and long-term, suppressive, combined antibiotic therapy [8]. Eight other cases were described in a series from Taiwan in 2012: three had otolaryngological diseases, four had received neurosurgery and one had disseminated disease. Three patients expired and among the five who survived, all received a clarithromycin-based combination therapy with a median duration of 12 months [15]. More recently, M. abscessus was reported as the causative agent of ventriculoperitoneal shunt (VPS) infections in three patients. Two patients expired despite externalization of the shunt and combined antimycobacterial therapy [16, 17]. The third case [18] involved a 30-year-old man with a VPS implanted in childhood because of hydrocephalus; after two failed attempts to treat the infection by shunt externalization and prolonged, combined antimycobacterial treatment, fragments of the original VPS were detected in the brain, neck and chest wall tissues. In this case, only fragment removal, combined with a new course of antimycobacterial therapy led to cure with no relapse, possibly due to an ability of M. abscessus to produce biofilm.
Interestingly, all reported cases had a subacute presentation, with slow onset over several weeks and with nonspecific signs consisting of nausea, headache and gradual global deterioration. Fever was present in less than half of the patients. According to literature, four main ways of acquisition of CNS infections by M. abscessus may be identified: post-traumatic (e.g. stab wound) [10], spreading from a contiguous source of infection (e.g. chronic otitis media) [13, 15], following neurosurgical procedures (including ventriculoperitoneal shunt implantation) [15, 18] or as part of a disseminated infection [14, 15].
There is a lack of sufficient evidence regarding the optimal management of M. abscessus CNS infections. Use of corticosteroids is controversial and was reserved to cases with meningeal inflammation and increased intracranial pressure [10, 12]. With regard to choice of antimycobacterial drugs, the mainstay is a clarithromycin-based combination therapy, due to the high rates of reported sensitivity to this agent [2]. Parenteral combination therapy with amikacin plus carbapenem was generally used during hospitalization and was later shifted to oral antibiotics (mostly clarithromycin plus moxifloxacin) to complete the treatment course. Even duration of treatment is controversial and varied among the cases reported, ranging from 2 months [15] to 3 years [18], with a median of 12 months. Removal of any prosthetic devices including ventriculoperitoneal shunts is considered of crucial importance, due to recurrence of the infection in previous cases of incomplete foreign body removal [10, 18].
Although M. abscessus is a rare pathogen of the CNS, death occurred in about half of patients despite prolonged and combined antimycobacterial treatment. Among reasons for the high rate of adverse outcome, delay in diagnosis may play a crucial role. The prolonged interval between symptoms onset and diagnosis may be due to the fact that cultures for acid-fast bacilli are not routinely requested and clinical suspicion often arises only after failure to respond to standard antibiotic treatment.
Different from other reported cases, meningeal infection in our patient run asymptomatically and was diagnosed incidentally during a second operation, which may represent in our opinion a timeframe of subclinical infection. Despite rapid diagnosis and prompt initiation of treatment, serious adverse events experienced by the patient (QTc prolongation and ototoxicity) forced us at first to switch to a suboptimal non-clarithromycin-based regimen and then to interrupt prematurely the ongoing therapy, leading to an insufficient total duration of about 6 weeks and to a subsequent high risk of relapse. Moreover, the presence of a PEEK prosthesis represents a potential focus of persistent low-grade infection and, therefore, an adjunctive risk factor for relapse. Balancing good clinical conditions with serious subjective antibiotic intolerance and high risk related to a third operation to remove the implant, we decided to act conservatively, to withhold antibiotic therapy and to strictly follow-up the patient with a combined approach by neurosurgeons, infectious disease physicians and otolaryngologists.
In conclusion, it is an imperative to be aware of the importance of atypical microorganisms in the differential diagnosis of CNS infections. M. abscessus complex, and RGM in general, should be taken into consideration when evaluating patients with CNS infections secondary to neurosurgery, trauma, otolaryngological infection, intracranial catheter placement or M. abscessus complex disease in other organs. Early clinical suspicion may lead to early microbiological detection and start of treatment, which may have an impact on both management and clinical outcome.
References
Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther 2016;14:1139–54
Brown-Elliott BA, Philley JV. Rapidly growing mycobacteria. Microbiol Spectr. 2017;5(1). https://doi.org/10.1128/microbiolspec.TNMI7-0027-2016
Odell ID, Cloud JL, Seipp M, Wittwer CT. Rapid species identification within the Mycobacterium chelonae–abscessus group by high-resolution melting analysis of hsp65 pcr products. Am J Clin Pathol. 2005;123:96–101.
Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–8.
Sali M, De Maio F, Caccuri F, Campilongo F, Sanguinetti M, Fiorentini S, Delogu G, Giagulli C. Multicenter evaluation of anyplex plus mtb/ntm mdr-tb assay for rapid detection of Mycobacterium tuberculosis complex and multidrug-resistant isolates in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2016;54:59–63.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, Subcommittee ATSMD. American Thoracic S and Infectious Disease Society of A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416.
Chan KH, Mann KS, Seto WH. Infection of a shunt by mycobacterium fortuitum: case report. Neurosurgery. 1991;29:472–4.
Madaras-Kelly KJ, DeMasters TA, Stevens DL. Mycobacterium fortuitum meningitis associated with an epidural catheter: case report and a review of the literature. Pharmacotherapy. 1999;19:661–6.
Midani S, Rathore MH. Mycobacterium fortuitum infection of ventriculoperitoneal shunt. South Med J. 1999;92:705–7.
Talati NJ, Rouphael N, Kuppalli K, Franco-Paredes C. Spectrum of CNS disease caused by rapidly growing mycobacteria. Lancet Infect Dis. 2008;8:390–8.
Viswanathan R, Bhagwati SN, Iyer V, Newalkar P. Ventriculo-peritoneal shunt infection by Mycobacterium fortuitum in an adult. Neurol India. 2004;52:393–4.
Martin JS, Zagzag D, Egan M, Milla S, Harter D, Lighter-Fisher J. Intracranial Mycobacterium abscessus infection in a healthy toddler. Pediatr Infect Dis J. 2015;34:223–4.
Maniu CV, Hellinger WC, Chu SY, Palmer R, Alvarez-Elcoro S. Failure of treatment for chronic Mycobacterium abscessus meningitis despite adequate clarithromycin levels in cerebrospinal fluid. Clin Infect Dis. 2001;33:745–8.
Liebeskind DS, Ostrzega N, Wasterlain CG, Buttner EA. Neurologic manifestations of disseminated infection with Mycobacterium abscessus. Neurology. 2001;56:810–3.
Lee MR, Cheng A, Lee YC, Yang CY, Lai CC, Huang YT, Ho CC, Wang HC, Yu CJ, Hsueh PR. Cns infections caused by Mycobacterium abscessus complex: clinical features and antimicrobial susceptibilities of isolates. J Antimicrob Chemother. 2012;67:222–5.
Levy ZD, Du V, Chiluwal A, Chalif DJ, Ledoux DE. Ventriculoperitoneal shunt infection with mycobacterium abscessus: a rare cause of ventriculitis. World Neurosurg. 2016;86:510 e1–4.
Baidya A, Tripathi M, Pandey P, Singh UB. Mycobacterium abscessus as a cause of chronic meningitis: a rare clinical entity. Am J Med Sci. 2016;351:437–9.
Montero JA, Alrabaa SF, Wills TS. Mycobacterium abscessus ventriculoperitoneal shunt infection and review of the literature. Infection. 2016;44:251–3.
Acknowledgements
We thank Dr. Angela Bohnen for her contribution to the English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
An approval by an ethics committee was not applicable.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Giovannenze, F., Stifano, V., Scoppettuolo, G. et al. Incidental intraoperative diagnosis of Mycobacterium abscessus meningeal infection: a case report and review of the literature. Infection 46, 591–597 (2018). https://doi.org/10.1007/s15010-018-1141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1141-5