Abstract
Purpose
An early adequate antifungal therapy based on the knowledge of local epidemiology can reduce the candidemia-attributable mortality and the length of hospitalization. We performed a retrospective study to analyze the epidemiology of candidemia and the antifungal consumption in our hospital.
Methods
We analyzed Candida spp. isolated from the blood, and their susceptibility profile from 2005 to 2016 in Careggi University Hospital, Florence, Italy. We also performed a stratified analysis by clinical setting where Candida spp. were isolated (Medical Wards, Surgery, Intensive Care Unit-ICU). Then, we retrospectively reviewed the annual consumption of antifungal agents and calculated the defined daily dosing for 10,000 hospital days.
Results
The rate of candidemia was higher in ICU than other settings and Candida albicans was the first cause of candidemia (61.2%). After adjustment for hospital days, the rate of C. albicans showed a statistically significant parabolic trend (p < 0.001), with a peak of incidence in 2010. After 2010, we observed a reduction of candidemia due to both C. albicans and non-albicans species. Between 2005 and 2015, we reported an increasing increased use of echinocandins. As far as resistance profile is concerned, only one Candida glabrata isolate was resistant to caspofungin (1.9%) and 30% of C. glabrata were resistant to fluconazole.
Conclusions
Our data describe C. albicans as the first cause of candidemia in all the studied settings and the low rate of echinocandin resistance, despite their increased use over the study period. ICU was confirmed as the setting with the highest incidence of candidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida spp. is the first cause of invasive fungal infections (IFI) in hospital patients and the fifth cause of nosocomial bloodstream infections (BSI) in Intensive Care Unit (ICU) [1]. In European and US studies, the incidence of candidemia was between 1.2 and 25 cases per 100,000 persons, with lower incidence rate in Europe [2,3,4]. An Italian retrospective study reported a statistically significant increase of candidemia between 2008 and 2012 in hospital patients (0.4–1.68 per 10,000 patients-day) and Candida albicans was the most isolated species [5]. The increasing incidence of the non-albicans Candida spp. probably correlated with the rise of the associated risk factors: central venous catheter and parenteral nutrition for Candida parapsilosis, neutropenia for Candida krusei, and prior treatment with an azole for Candida glabrata [6, 7]. Candidemia was associated with high mortality, high costs and prolonged hospitalization [8]. In the ICU, the candidemia-attributable mortality was between 5 and 71% [9, 10], but it could be reduced by an early antifungal treatment [11,12,13]. If a candidemia is suspected, an echinocandin is the initial recommended treatment [14,15,16]. So far, the rate of echinocandin-resistant Candida spp. was lower than 2–3%, except for C. glabrata (8–13%) [17, 18].
The knowledge of the local epidemiology is helpful to choose the adequate antifungal therapy and reduce mortality. We performed a retrospective study to analyze the epidemiology of candidemia and the consumption of antifungal agents in our hospital, between 2005 and 2016.
Methods
Data collection
Careggi Hospital is a large tertiary care teaching hospital in Florence, Italy. In 2016, the facilities are as follows: 1297-bed units, 111-sub-intensive and 70-intensive bed units, 133-day hospital and 51-day surgery bed units. In the last 4 years, the bed units were reduced to about 300.
We performed a retrospective study about Candida spp. isolated from the blood, and their susceptibility profile from January 2005 to December 2016 in the Laboratory of Microbiology and Virology of Careggi Hospital, Florence. We excluded the isolates of the same patient if they had the same susceptibility profile and if the isolation was obtained within 30 days after the first positive blood culture. Caspofungin susceptibility was performed from 2012; micafungin and anidulafungin susceptibility was performed from 2013. The in vitro susceptibility to antifungal drugs was performed using Sensititre® YeastOne®. We applied the antifungal breakpoints recently revised by Clinical and Laboratory Standards Institute (CLSI) for all samples [19].
We associated Candida isolates with the setting where they were isolated (Medical Wards-MW, surgery, Intensive Care Unit-ICU) and we excluded those obtained from Neonatal Intensive Care Unit. Between 2005 and 2007, we were not able to associate 49 Candida isolates to corresponding wards (missing data). We also extracted the hospital days for year from the Careggi Hospital database.
Then, we retrospectively reviewed the annual consumption of antifungal agents in the same period: caspofungin and fluconazole from 2005 to 2016, anidulafungin from 2009 to 2016 and micafungin from 2010 to 2016. For every antifungal agent, we calculated the defined daily dose (DDD) for 10,000 hospital days, in accordance with the Guidelines for ATC classification and DDD assignment (WHO collaborating Centre for Drug Statistic Methodology; http://www.whocc.no). No data were available about the reason for the use of antifungal agents (e.g., prophylaxis, therapy or pre-emptive therapy). It was an observational retrospective study based on anonymized microbiological data. No clinical and personal data of patients were used. Thus, the study did not require the patient’s consent and the approval by the Institutional ethics committee.
Statistical analysis
Data were analyzed using Statistical Analysis System (SAS) version 9.3. For categorical variables, number and percentage are reported. To assess association between counting variables (expressed as rates) and years a log-linear regression model was used. Statistical significance level is fixed to 5%. Wald test was used for calculation of p values, and 95% CI were used for descriptive purposes.
Results
In Fig. 1, we report the most common isolates from blood cultures from 2005 to 2016. Coagulase-negative staphylococci were not considered due to the impossibility to clearly attribute a contaminant or pathogenic role. Escherichia coli was the most common isolated pathogen during the entire study period, followed by Staphylococcus aureus. Candida spp. ranked between the third and the fifth position. Of note, a sharp increase of BSI due to Klebsiella was observed.
In the study period, we obtained 1091 isolates of Candida spp.: the more frequent species were C. albicans (n = 668, 61.2%), followed by C. parapsilosis (n = 183, 16.8%), and C. glabrata (n = 141, 12.9%), without statistical significant difference in trend (Fig. 2). In Fig. 2, we report the annual distribution of Candida spp. and in Fig. 3 the distribution of Candida spp. for years and for setting. After adjustment for hospital days, the rate of C. albicans showed a statistically significant parabolic trend (p < 0.001, confidence interval 95% 0.05–0.22), with a peak incidence in 2010 (Fig. 4a).
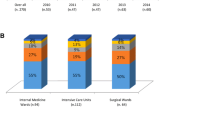
After adjustment for hospital days for year, we observed a higher rate of candidemia in ICU setting (mean 14.5 for 10,000 hospital days) than in MW (mean 2.8 for 10,000 hospital days). The significant parabolic trend was confirmed for C. albicans (Fig. 4b) only for ICU (p < 0.001, CI 95% 1.08–2.39), while the trend of non-albicans Candida spp. was parabolic but not statistically significant. In ICU, the rate of non-albicans Candida spp. was statistically significantly higher than in MW (p < 0.001), in particular C. parapsilosis (p < 0.05).
We also analyzed the MIC value of fluconazole from 2005 to 2016 and echinocandins from 2012 to 2016. In Table 1, we report the distribution of Candida spp. based on MIC values and in Table 2, we report the MIC50 and MIC90 values. We observed that all isolates of C. glabrata had high MIC values for fluconazole (MIC50 16 µg/ml, MIC90 128 µg/ml), with 33% (35/105) of them non-susceptible. For C. parapsilosis, we observed decreasing fluconazole MIC values (p < 0.001) over the study period. As for echinocandins, C. parapsilosis had higher MIC values than other Candida spp., but all within the susceptibility cut-off value without resistant isolate (caspofungin MIC50 0.25 µg/ml, MIC90 1 µg/ml; micafungin and anidulafungin MIC50 1 µg/ml, MIC90 2 µg/ml). Only one C. glabrata isolate was resistant to caspofungin (MIC 1 µg/ml) (1/53, 1.9%). The caspofungin MIC values for C. albicans decreased from 2011 to 2015 (p < 0.05).
Analyzing the consumption of fluconazole and echinocandin from 2005 to 2016, we observed a progressive increase of the echinocandin use until 2011, followed by a sharp increase in 2012. Starting from 2015, we observed a reduction of echinocandins use. Fluconazole was the most commonly utilized antifungal drug until 2012 (Fig. 5).
Discussion
The increasing numbers of risk factors for Candida infections in hospital population and the high rate of attributable mortality are of concern for clinicians. The knowledge of local epidemiology is helpful for clinicians to prescribe empiric therapy, awaiting the microbiological results, and to reduce the mortality with early therapy. We performed an epidemiological study in an Italian large tertiary care teaching hospital to describe the local epidemiology of candidemia and to oversee the presence of drug resistance, according to the use of echinocandins.
The number of candidemia was higher in MW (602), but after adjustment for hospital days, the rate is higher in ICU than in other settings (14.5 versus 2.8 for 10,000 hospital days in MW), as described in another Italian study, in which the incidence of candidemia in ICU was 1.95 per 1000 patient/days versus 0.17 in the other wards considered together [5].
In our study, C. albicans was the first cause of candidemia, followed by C. parapsilosis and C. glabrata, in accordance with findings from other Italian and European studies [20,21,22]. In the ARTEMIS DISK Global Antifungal Surveillance Study, reporting data about invasive Candida isolates from 39 countries, C. albicans was the main cause of candidemia, although with a decreasing trend. In contrast, the frequency of C. parapsilosis and C. tropicalis was increasing, and some countries described a shift from C. albicans to non-albicans Candida spp. [20]. In an Italian tertiary University Hospital, Bassetti et al. reported a global incidence of 0.79 episodes/1000 admission, and C. albicans was isolated in 60.3% of cases, followed by C. parapsilosis (16.7%) and C. glabrata (11.8%) [21]. In a French study, C. albicans was the predominant species (51.8%), followed by C. parapsilosis (14.5%) and C. glabrata (9.8%) [22].
In our study, we did not observe an increase of non-albicans Candida spp., neither considering the total sample nor analyzing the different settings (ICU, MW, and surgery). This finding is in contrast to data from another Italian study, reporting a shift from C. albicans to non-albicans species, with non-albicans Candida spp. causing 55.8% of candidemia and C. parapsilosis being the most frequent species [23]. In an Asiatic study, the predominant species isolated from BSI were non-albicans Candida spp. (73.14%) [24]. During our study period (2005–2016), the most commonly isolated non-albicans Candida spp. were C. parapsilosis and C. glabrata. The high rate of C. parapsilosis, observed in our ICU, was probably due to patient risk factors, such as the use of central venous catheter and parenteral nutrition.
After 2010, we observed a reduction of candidemia due to both albicans and non-albicans species, similar to the data reported by Turkish studies. Yesilkaya et al. reported a significant negative trend of candidemia episodes between 2007 and 2014, and Kazak et al. reported a decreased candidemia incidence in the period 2008–2012 compared to 1996–2001 [25, 26]. The reduction of candidemia may be attributed to the increasing use of antifungal therapy in our setting, in particular echinocandins, as empiric and pre-emptive therapy in patients at high risk. Between 2005 and 2015, we reported an increasing use of echinocandins in particular from 2012, probably due to the new ESCMID guidelines, that indicated echinocandins as recommended therapy for candidemia [14]. Our data confirmed a French study about the consumption trend over 10 years (2004–2013): echinocandin use increased significantly, and the use of fluconazole did not change from 2004 to 2013 [27]. The drug use did not affect the susceptibility profile of Candida spp. isolates. From 2015, we observed a reduction of echinocandin use, probably due to an antimicrobial stewardship programme (Antimicrobial and Infection Management—AIM) implemented in Careggi Hospital in the same period.
Another possible reason to explain the reduction of candidemia in our study may be the increasing caution in the use and management of CVC, in virtue of the implementation of procedures for the CVC insertion and management in the last 5 years.
Of note, the candidemia decreasing trend in our hospital was observed contemporary to the rise of number of K. pneumoniae BSI. From 2010 to 2016, K. pneumoniae moved from the twelfth to the fourth position in the ranking of total BSI.
As far as the resistance profile is concerned, we found a low percentage of echinocandin resistance in C. glabrata (1.9%), and no resistance in C. albicans. Previous studies reported the percentage of echinocandin resistance in C. glabrata to be between 3 and 13% [28]. A recent study conducted in another Italian large University hospital reported full susceptibility to echinocandins of non-albicans Candida spp. and one resistant C. albicans isolate (1.8%) [29].
We also reported fluconazole resistance of C. glabrata to be about 30% [28]. Data from a multicenter study in five tertiary care teaching hospitals in Italy and Spain reported fluconazole resistance of C. glabrata to be 45.6% [9].
The present study had several limitations: the study is retrospective and clinical data were not evaluated. It was not possible to associate the isolates to a clinical status or to value the risk factors associated with a single species. Further studies are needed to clarify the role of improvement in CVC management and the increasing echinocandin use in the observed trend of candidemia. To the best of our knowledge, no studies have been performed to study about the relationship between K. pneumoniae and Candida spp. epidemiological trends.
Other limitation of our study was represented by missing data of some susceptibility test results.
In conclusion, our data describe C. albicans as the first cause of candidemia in all the studied settings and the low rate of echinocandin resistance, despite their increased use over the study period. ICU was confirmed as the setting with the highest incidence of candidemia.
References
European Centre for Disease Prevention and Control. Annual epidemiological report 2014. Antimicrobial resistance and healthcare-associated infections. Stockholm: ECDC; 2015.
Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27.
Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55:1352–61.
Klingspor L, Tortorano AM, Peman J, Willinger B, Hamal P, Sendid B, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin Microbiol Infect. 2015;21:87.
Milazzo L, Peri AM, Mazzali C, Grande R, Cazzani C, Ricaboni D, et al. Candidaemia observed at a university hospital in Milan (northern Italy) and review of published studies from 2010 to 2014. Mycopathologia. 2014;178:227–41.
Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, Brandt ME, et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol. 2004;42:4468–72.
Bassetti M, Ansaldi F, Nicolini L, Malfatto E, Molinari MP, Mussap M, et al. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother. 2009;64:625–9.
Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40:839–45.
Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013;51:4167–72.
Bouza E, Muñoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008;32:S87–91.
Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–5.
De Rosa FG, Corcione S, Filippini C, Raviolo S, Fossati L, Montrucchio C, et al. The effect on mortality of fluconazole or echinocandins treatment in candidemia in Internal Medicine Wards. PLoS ONE. 2015;10:e0125149.
Bassetti M, Molinari MP, Mussap M, Viscoli C, Righi E. Candidaemia in internal medicine departments: the burden of a rising problem. Clin Microbiol Infect. 2013;19:E281–4.
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37.
Scudeller L, Viscoli C, Menichetti F, del Bono V, Cristini F, Tascini C, et al. An Italian consensus for invasive candidiasis management (ITALIC). Infection. 2014;42:263–79.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50.
Perlin DS. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drug. 2014;74:1573–85.
Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–32.
Clinical and Laboratory Standards Institute, Wayne, PA. CLSI 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. CLSI document M27-S4
Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5–10.
Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, et al. Clinical and therapeutic aspects of Candidemia: a 5 years single centre study. PLoS ONE. 2015;10(5):e0127534. https://doi.org/10.1371/journal.pone.0127534.
Tadec L, Talarmin JP, Gastinne T, Bretonnière C, Miegeville M, Le Pape P, et al. Epidemiology, risk factor, species distribution, antifungal resistance and outcome of Candidemia at a single French hospital: a 7-year study. Mycoses. 2016;59:296–303.
Caggiano G, Coretti C, Bartolomeo N, Lovero G, De Giglio O, Montagna MT. Candida bloodstream infections in Italy: changing epidemiology during 16 years of surveillance. Biomed Res Int. 2015. https://doi.org/10.1155/2015/256580.
Ng KP, Kuan CS, Kaur H, Na SL, Atiya N, Velayuthan RD. Candida species epidemiology 2000–2013: a laboratory-based report. Trop Med Int Health. 2015;20:1447–53.
Yeşilkaya A, Azap Ö, Aydın M, Akçil Ok M. Epidemiology, species distribution, clinical characteristics and mortality of candidaemia in a tertiary care university hospital in Turkey, 2007–2014. Mycoses. 2017;60:433–9.
Kazak E, Akın H, Ener B, Sığırlı D, Özkan Ö, Gürcüoğlu E, et al. An investigation of Candida species isolated from blood cultures during 17 years in a University Hospital. Mycoses. 2014;57:623–9.
Bailly S, Maubon D, Fournier P, Pelloux H, Schwebel C, Chapuis C, et al. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—trends over 10 years. J Infect. 2016;72:103–11.
Perlin DS. Mechanisms of echinocandin antifungal drug resistance. Ann NY Acad Sci. 2015;1354:1–11.
De Francesco MA, Piccinelli G, Gelmi M, Gargiulo F, Ravizzola G, Pinsi G, et al. Invasive candidiasis in Brescia, Italy: analysis of species distribution and antifungal susceptibilities during 7 years. Mycopathologia. 2017. https://doi.org/10.1007/s11046-017-0155-3 (Epub ahead of print).
Acknowledgements
In 2015, JM received a research fellowship from Società Italiana Malattie Infettive e Tropicali (SIMIT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JM received grant support from Gilead, MSD Italy, outside the submitted work. GMR received grants, personal fees and other from Pfizer, MSD Italy, outside the submitted work. AB received grants and personal fees from MSD Italy and Pfizer, grants from Gilead and Astellas, outside the submitted work. LT, EM, ER, RF, FB, GC, AF, PP and LB have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Mencarini, J., Mantengoli, E., Tofani, L. et al. Evaluation of candidemia and antifungal consumption in a large tertiary care Italian hospital over a 12-year period. Infection 46, 469–476 (2018). https://doi.org/10.1007/s15010-018-1139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1139-z