Abstract
Background:
One of the long-standing problems of myoblasts in vitro expansion is slow cell migration and this causes fibroblast population to exceed myoblasts. In this study, we investigated the synergistic effect of laminin and epidermal growth factor (EGF) on co-cultured myoblasts and fibroblasts for cell attachment, proliferation and migration.
Methods:
Skeletal human muscle cells were cultured in four different conditions; control, EGF, laminin (Lam) and laminin EGF (Lam + EGF). Using live imaging system, their cellular properties; attachment, migration and growth were exposed to Rho kinase inhibitor, Y-27632, and EGF-receptor (EGF-R) inhibitor, gefitinib were measured.
Results:
Myoblast migration and proliferation was enhanced significantly by synergistic stimulation of laminin and EGF (0.61 ± 0.14 µm/min, 0.008 ± 0.001 h−1) compare to that by EGF alone (0.26 ± 0.13 µm/min, 0.004 ± 0.0009 h−1). However, no changes in proliferation and migration were observed for fibroblasts among the culture conditions. Inhibition of Rho kinase resulted in the increase of the myoblast migration on the laminin-coated surface with EGF condition (0.64 ± 0.18 µm/min). Compared to the untreated conditions, myoblasts cultured on the laminin-coated surface and EGF demonstrated elongated morphology, and average cell length increase significantly. In contrast, inhibition of EGF-R resulted in the decrease of myoblast migration on the laminin coated surface with EGF supplemented condition (0.43 ± 0.05 µm/min) in comparison to the untreated control (0.53 ± 0.05 µm/min).
Conclusion:
Laminin and EGF preferentially enhance the proliferation and migration of myoblasts, and Rho kinase and EGF-R play a role in this synergistic effect. These results will be beneficial for the propagation of skeletal muscle cells for clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adult skeletal muscles are constantly subjected to the risk of injury from weight bearing, exercise, and trauma. Hence, regeneration of injured muscle fibre is very important in the body. A population of cells intimately associated with the muscle fibre has been reported to be responsible for skeletal muscle regeneration. In 1961, Mauro [1] first coined the term “satellite cells” to the cell that he observed between the sarcolemma of the muscle fibre and the basement membrane. The regenerative capacity of satellite cells in skeletal muscle injury was well established and have been demonstrated in isolated cell population in vitro [2,3,4]. However, in vitro expansion of myoblasts, construction of tissue engineering scaffold and its integration with native tissues are still challenging.
Laminin role in enhancing proliferation and differentiation was shown consistently with various type of cells. Laminin effect on cell differentiation and proliferation was apparent in Schwann cells as shown by McGarvey et al. [5]. In skeletal myoblast, enhance differentiation and proliferation of murine myoblast was revealed by Ocalan et al. [6]. Investigation [7] by time-lapse observation revealed that human myoblasts on the laminin coated surface exhibited rapid migration with morphological changes. Myoblast migration was further encouraged on the laminin coated surface when EGF existed. These results indicated that the stimulation by laminin coating and EGF supplementation made a synergistically function in promoting myoblast properties. However, the potential signalling pathway involved in enhancement of myoblast migration in laminin and EGF synergy is still not clear.
Cellular migration is vital for tissue development, wound repair, inflammatory response, immune surveillance, and plays a role in pathological processes such as metastasis [8]. Cell migration is controlled by various set of intracellular signalling molecules, including tyrosine phosphorylation, GTPases activation, phospholipid biosynthesis and adhesion complex integrin. One particular group of proteins, the Rho GTPases has been shown to regulate actin cytoskeletal organization and thus cellular migration [9, 10]. Rho GTPases are frequently overexpressed in invading tumor cells when the tumor cells increase cell motility to cross tissue boundaries during invasion [11]. Since their discovery in the late eighties, the role of Rho GTPases in the regulation of cell migration has been extensively studied and has mainly focused on the hallmark family members Rho, Rac, and Cdc42 [12]. The activated Rho-GTPases will then interact with downstream effectors such as ROCK to trigger responses including actin cytoskeletal rearrangements, gene transcription regulation, membrane trafficking, cell cycle regulation, and the control of apoptosis [9]. Inhibition of ROCK by Y-27632 has been shown to increase myoblast migration accompanied with loss of direction [13]. Y-27632 is a cell-permeable, highly potent and selective inhibitor of ROCK. Y-27632 inhibits both ROCKI and ROCKII by competing with ATP for binding to the catalytic site [14].
Receptor tyrosine kinase of the EGF receptor (EGF-R) is another potential regulator of cellular migration [15, 16]. EGF-R signalling pathway has been shown to regulate multiple cellular functions such as tissue morphogenesis and wound repair, because these events require the key cell responses of survival, proliferation and migration. EGF-R has been shown to be one of the regulatory mechanism influencing intestinal epithelial cells migration via MAPK signalling [17] and endothelial cells [18]. Down regulation of EGF-R has been shown to decrease the differentiation ability of myoblast [19] and EGF-R inhibitor exhibits slow migration in vascular smooth muscle cells [20].
Migration of myoblasts is considering as an important general mechanism to ensure the efficiency of productivity of myoblasts during in vitro expansion and therapeutic efficiency after transplantation. Enhancement of migration was shown to reduce the myoblast terminal differentiation that in turns facilitates in vitro expansion [7]. High migration of myoblasts in 3D tissue construct was also shown to facilitate endothelial cells infiltration that helps vascularization [21, 22]. Several approaches have been employed to enhance the myoblast migration. Previous study [7] by time-lapse observation revealed that the human myoblasts on the laminin coated surface exhibited rapid migration with the frequent morphological changes. Myoblast migration was further encouraged on the laminin coated surface when EGF existed, while no significant enhancement of migration rate was recognized when supplemented with EGF alone. These results indicated that the stimulation by laminin coating and EGF supplementation made a synergistically function in promoting myoblast properties.
In this study, the synergistic role of laminin and EGF on regulating the biological properties of myoblast and fibroblast in a co-culture system was evaluated. It was assumed that EGF-like structure in laminin may facilitate the expression and/or activation of EGF-R that in turns function synergistically to regulate the expression of functional proteins involved myoblasts. Cellular behaviour and morphological analysis of myoblasts were studied in the presence of specific inhibitors to elucidate the signalling mechanism involved in the enhancement of myoblast migration by laminin and EGF. Two different potential signalling pathways i.e. EGF-R signalling and Rho/ROCK pathway were evaluated using Gefitinib and Y-27632, respectively.
2 Materials and methods
The redundant human skeletal muscle samples were collected from the consented patients in Universiti Kebangsaan Malaysia Medical Centre. Universiti Kebangsaan Malaysia Research and Ethics Committee (UKMREC) approved this research with approval code of UKM FPR.4/244/FRGS/2/2013/SG05/UKM/03/1.
2.1 Human skeletal muscle cells
Skeletal muscle, which contains mixture of myoblasts and fibroblasts, were isolated by digestion with 0.75% Trypsin–EDTA (Sigma Aldrich, St. Louis, MO, USA). The cells were seeded into T75 flask (Greiner Bio-One, Monroe, NC, USA) and cultured in F10: Dulbecco’s Modified Eagle Medium (DMEM; Sigma) containing 10% foetal bovine serum (FBS; Biowest, Riverside, MO, USA) and incubated at 37 °C in 5% CO2 incubator.
2.2 Laminin coating and EGF supplementation culture conditions
Laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane (1 mg/ml, Sigma-Aldrich) was diluted with phosphate-buffered saline (PBS; Sigma) to a concentration range of 50 μg/ml. The laminin was added to 12 well plates (Greiner Bio-One, Kremsmünster, Austria) and incubated for 30 min at 37 °C, 5% CO2. The excess laminin was removed, and plates were subsequently stored at 4 °C and washed with PBS prior to use. The cells were treated with 0.05% Trypsin–EDTA (Sigma) and incubated for 5 min. The cell suspension was centrifuged at 5000 rpm for 5 min at 37 °C. The cells undergoing three passages and the seeding density were fixed at 2500 viable cells/cm2. The culture was conducted in 12-well plate in the plain-coated surface (Control), plain-coated surface with EGF (EGF), laminin-coated surface without EGF supplemented (Lam) and laminin-coated surface with EGF (Lam + EGF). Supplementation of human recombinant EGF (Biovision, Milpitas, CA, USA) in the medium (100 ng/ml EGF when added, Biovision).
2.3 Single cell live imaging
The cellular behaviours of individual myoblasts were observed using a live cell imaging tool microscopy, Nikon A1R microscope (Nikon, Tokyo, Japan). The images were captured every 20 min on the bottom surface of each well. The singly occurring myoblast cells on the captured images were selected randomly and traced in a time-lapse manner.
2.4 Immunocytochemical staining of desmin and EGF-R
Cells were fixed with 4% paraformaldehyde (Sigma Aldrich) in DPBS (Sigma) for 20 min, and permeabilised with 0.5% Triton X-100 (Sigma). Cells were incubated with 10% goat serum (Sigma) for 1 h before incubated with primary antibody: anti-desmin mouse monoclonal antibody (1:500; Agilent Dako, Santa Clara, CA, USA) and anti-EGF-R (1:500; Abcam, Cambridge, UK) for 2 h at 37 °C and cells were washed before incubated with secondary antibody Alexa Fluor 594-conjugated anti mouse IgG antibody (1:300; Invitrogen, Carlsbad, CA, USA) for 2 h. Then, cells were counterstained for 20 min with 4′, 6-diamidino-2-phenylindole (DAPI) (1:15,000; Invitrogen) for nuclei staining and Alexa Fluor™ 488 Phalloidin (Invitrogen) for cytoskeleton. The images of culture surface were captured from four different positions in each vessel every 24 h using fluorescence microscope (A1R Nikon). The number of cells were counted manually on the images obtained at a given culture time (t).
2.5 Cellular behaviour analysis
The growth rate was evaluated by counting cell number at 24 h and 72 h. The growth rate was evaluated using this following equation:
The cell migration was calculated using this following equation:
x1 = initial x coordinate, y1 = initial y coordinate, t1 = initial time; x2 = final x coordinate, y2 = final y coordinate, t2 = final time.
2.6 Inhibition of rock and EGF-R analysis
The cells were seeded on plain and laminin-coated surface with or without EGF on 12 wells culture plate and incubated for 24 h. Cells were either untreated (control) or treated with 10 mM Y-27632 and 10 µM gefitinib respectively. The images were captured at every 20 min using time-lapse confocal microscope imaging. The cells area, width, circularity and length were analysed using the NIS Element AR 3.1 software (Nikon).
2.7 Statistical analysis
All the results are expressed as means ± standard deviation (SD). Analysis of variance (ANOVA) was used in this study to test for significant differences; p < 0.05 was considered statistically significant.
3 Results
3.1 Effect of laminin and EGF on myoblast and fibroblast attachment
The attachment, proliferation and migration of myoblasts and fibroblast in laminin and EGF synergy were evaluated. As shown in Fig. 1, myoblasts in control and EGF displayed small, shiny and rounded cell bodies at 0 min then accompanied with moderate increase in contact area over time culturing. Myoblasts in Lam and Lam + EGF showed small and rounded cell bodies also at the early event, then gradually enlarged contact area due to spreading. Myoblast spreading was visible as early as 30 min in Lam + EGF compare to that Lam. At 120 min, myoblast in Lam and Lam + EGF already have elongated cell bodies. In accordance with above observation, it was also found that myoblasts in Lam and Lam + EGF predominantly formed actin cytoskeleton compare to that in control and EGF in Fig. 2A. Noticeably, the number of filopodia also can be observed in these conditions. Figure 1B show in control, EGF, Lam and Lam + EGF showed similar attachment morphology and pattern across the conditions. Initially, they appeared rounded and compact, then after 60 min, filopodia started to form accompanied with enlarged of contact area. After 2 h the actin can be observed in all conditions. Fibroblasts under all conditions are localized and did not start to migrate not like myoblasts under the laminin-coated surface with EGF.
Effect of laminin and EGF on myoblasts and fibroblast at different time interval in control, EGF, Lam and Lam + EGF conditions. A Myoblasts on laminin-coated surface with or without EGF showed small and rounded at the very early min, then an enlarged contact area with spreading initiated in the laminin coated with EGF. B Fibroblasts on the plain and laminin-coated surfaces with or without EGF showed similar attachment morphology and pattern across the conditions. (Scale bar: 50 µm)
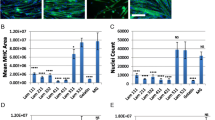
Quantitative analysis of morphoplogical and cell concentration of myoblasts and fibroblasts in laminin and EGF. A Immunocytochemistry of cells after 2 h plating in different conditions with anti-desmin mouse monoclonal antibody (red), phalloidin (green) and nuclei staining, DAPI (blue). Cells expressing desmin were identified as myoblasts; unstained cells were identified as fibroblasts. (Scale bar: 100 µm) B The cell concentration of myoblasts and fibroblasts (n = 3) at 0, 30, 60 and 120 min for attachment analysis. Myoblast attachment was significantly higher on laminin coated with or without EGF at all intervals compared to that on the plain surface with or without EGF. (At least 30 cells were measured per image). *Significant difference (p < 0.05). C Average cell area (µm2) and average cell circularity of myoblasts and fibroblasts in the plain, plain EGF, laminin and laminin EGF conditions at 0, 30, 60 and 120 min (n = 3). Average cell area and cell circularity were measured using NIS Element AR 4.2.0 (Nikon). Myoblasts on the laminin-coated surface with or without EGF had higher average cell area and lower average cell circularity compared to myoblasts on the plain surface with or without EGF. The fibroblasts showed similar trends for the average cell area and average cell circularity in all conditions
Myoblasts and fibroblasts were further analysed to evaluate the effect of laminin and EGF on their attachment profile at 5 min, 10 min, 30 min, 60 min, and 120 min as shown in Fig. 2B. Myoblast attachment was significantly higher in Lam and Lam + EGF at all intervals time compared to that in control and EGF. Whereas, fibroblasts under all conditions have no differences in attachment profile between all the conditions and measured times. To further understand the effect of laminin and EGF on cellular morphology, average cell area and circularity were determined for myoblasts and fibroblasts under different culture condition. The average cell area and circularity were determined to further analyse the myoblast and fibroblast morphology at 0, 30, 60 and 120 min shown in Fig. 2C. Myoblasts in Lam and Lam + EGF showed significantly higher average cell area in respect to myoblasts in control and EGF, at each time point measured. Myoblasts in Lam and Lam + EGF exhibited decreasing in average cell circularity compared to that in control and EGF which displayed similar cell circularity value with increasing time. Comparing with myoblasts, fibroblasts have similar trends for the average cell area and average cell circularity value in all conditions.
3.2 Effect of laminin and epidermal growth factor (EGF) on myoblast and fibroblast growth and migration
Effect of laminin and EGF on myoblast and fibroblast growth rate was evaluated. Figure 3A demonstrates the representative images of myoblasts and fibroblast in different conditions at day 1 and day 3. As shown in Fig. 3B, myoblasts cultured in Laminin + EGF showed significantly higher growth rate (0.008 ± 0.001 h−1) compared to control and EGF (0.004 ± 0.0009 h−1 and 0.004 ± 0.0005 h−1, respectively). The growth rate of myoblast in Lam was slightly higher (0.007 ± 0.001 h−1) as compared to control but not significant. In contrast, fibroblast under different culture condition demonstrated similar growth rate, fibroblasts in control, 0.007 ± 0.0007 h−1; EGF, 0.007 ± 0.001 h−1; Lam, 0.006 ± 0.001 h−1 and Lam + EGF, 0.006 ± 0.0005 h−1. Single cell migration of both myoblasts and fibroblasts were analysed to evaluate the effect of laminin and EGF. It was observed myoblasts migration rate was significantly higher in Lam + EGF (0.61 ± 0.14 µm/min) compared to control and EGF (0.26 ± 0.13 µm/min and 0.29 ± 0.05 µm/min). Lam (0.48 ± 0.09 µm/min) showed higher migration to that in control and EGF but not significant. While, for fibroblast migration rate showed similar trend across all condition, control (0.41 ± 0.02 µm/min), EGF (0.38 ± 0.09 µm/min), Lam (0.44 ± 0.15 µm/min) and Lam + EGF (0.40 ± 0.1 µm/min).
Cellular behaviour analysis of myoblasts and fibroblasts in laminin and EGF. A Confocal images of myoblasts and fibroblasts. Cells were fixed and stained on day 1 and day 3. Cell expressing anti-desmin mouse monoclonal antibody (green) were identified as myoblasts via immunostaining; unstained cells were identified as fibroblasts with nuclei staining only, DAPI (blue). Lam + EGF shows higher number of myoblasts compared to myoblast in control and EGF at Day 3. (Scale bar: 100 µm) B Graphs show the myoblast and fibroblast growth rates and migration rates in the control, EGF, Lam and Lam + EGF conditions (n = 3). *Significant difference (p < 0.05). Myoblast in Lam + EGF has higher growth and migration rate compared to myoblast in control and EGF. Fibroblast demonstrate similar growth and migration rate for all conditions
3.3 Effect of the EGF-R inhibitor gefitinib on myoblast and fibroblast morphology and migration
To investigate the involvement of EGF-R, the expression of active EGF-R was identified via immunostaining and migratory properties after blocking the EGF-R using gefitinib. Immunostaining was performed to determine expression of EGF-R on myoblasts and fibroblasts in control, EGF, Lam and Lam + EGF conditions. Figure 4A shows cells in Lam + EGF has highest EFGR expression compared to Lam and very low expression in control & EGF. Then, the effect of gefitinib treatment on myoblasts and fibroblasts in control, EGF, Lam and Lam + EGF conditions. The immunocytochemical images of myoblasts and fibroblasts treated with gefitinib after 12 h live imaging was shown in Fig. 4B. Under gefitinib treatment in control, EGF, Lam and Lam + EGF, myoblasts (green staining) displayed flattened, spread and rounded cell appearance. Fibroblasts (red staining) in all conditions under treatment of gefitinib demonstrate more flattened, spread and bigger cell morphology. From graph shown in Fig. 4C, myoblasts in Lam (0.31 ± 0.06 µm/min) and Lam + EGF (0.43 ± 0.05 µm/min) decrease in migration rate compared to untreated control (Lam; 0.40 ± 0.09 µm/min, Lam + EGF; 0.53 ± 0.05 µm/min). While myoblast in control and EGF had no effect. However, a decrease trend in fibroblast migration was observed when treated with gefitinib for all conditions.
Effect of inhibitor on myoblasts morphology and cellular behaviour in laminin and EGF synergy. A Immunocytochemistry of cells with anti-EGF-R (red), Alexa Fluor™ 488 Phalloidin (green) and nuclei staining, DAPI (blue). Cells in Lam + EGF shows higher expression of EGF-R compared to that in Lam, EGF and control. (Scale bar: 10 µm). B Immunocytochemistry of the cells with staining for anti-desmin mouse monoclonal antibody (red), Alexa Fluor™ 488 Phalloidin (green) and DAPI (blue) treated with gefinitib and Y-27632. In treated with gefinitib, myoblasts and fibroblasts demonstrate more flattened, spread and bigger cell morphology Whilst, myoblasts treated with Y-27632 in Lam and Lam + EGF showed thin and elongated cell body with the longest tail formation (Scale bar: 100 µm). C Migration rates of myoblasts and fibroblasts treated and untreated with gefitinib and Y-27632 in the four different conditions. No significance difference between myoblast and fibroblast in all conditions treated and untreated with gefitinib. Myoblast migration rate were significantly higher in groups treated with Y-27632 for Lam + EGF and Lam as compared to untreated with Y-27632 in Lam + EGF and Lam (n = 3). *Significant difference (p < 0.05)
3.4 Effect of laminin and EGF in Rho/Rho kinase (ROCK) signalling of myoblast and fibroblast morphology and migration
To elucidate the involvement of Rho/ROCK signalling in the synergy effect, we performed quantitative analysis of migrating myoblasts and fibroblasts comparing the effect of treatment with the ROCK inhibitor (ROCKi), Y-27632 versus normal medium in the four conditions.
The effect of Y-27632 treatment that inhibits both ROCKI and ROCKII in myoblasts and fibroblasts under plain and laminin coated with or without EGF were evaluated based on immunocytochemical images after 12 h live imaging Fig. 4B. Under Y-27632 control and EGF myoblasts (green staining) displayed wider and more flattened and shown more lamellipodia formation at the edge of cells with more membrane protrusions compared to myoblasts in Lam and Lam + EGF. Myoblasts in Lam and Lam + EGF under treatment of Y-27632 showed more slender morphology with elongated tail formation. Also, more filopodial projections can be observed on myoblasts in Lam and Lam + EGF under treatment of Y-27632. In comparison with myoblasts under treatment of Y-27632, fibroblasts (red staining) in all conditions under treatment of Y-27632 demonstrate less elongated and more flattened, spread and rounded cell appearance. Consistent with the observed differences in cell morphology, Fig. 4C shows that myoblast migration rate were significantly higher in groups treated with Y-27632 for Lam + EGF (0.64 ± 0.18 µm/h) and Lam (0.54 ± 0.12 µm/h) as compared to untreated with Y-27632 for both Lam + EGF (0.52 ± 0.12 µm/h and Lam (0.45 ± 0.16 µm/h).
We further analysed the quantitative parameters of average length, width, area and circularity of cells in describing the morphological behaviour of the migrating myoblasts under treatment of Y-27632 to elucidate the role of the Rho/ROCK pathway as a possible mechanism in laminin and EGF synergy. Quantitative morphological analysis, especially increased in cell length and decreased cell circularity, was identified when the myoblasts under treatment of Y-27632. Figure 5A shows that myoblast average length in the Lam + EGF conditions after treatment of Y-27632 (161.10 ± 56.80 µm) was increased significantly followed by myoblasts in Lam (140.28 ± 48.94 µm) compared to myoblasts in the Lam + EGF and Lam before treatment of Y-27632 (Lam; 97.03.26 ± 29.29 µm, Lam + EGF: 100.35 ± 34.44 µm). Myoblasts in control and EGF after treatment of Y-27632 showed no significant difference with myoblasts in control and EGF before treatment of Y-27632. Myoblasts average width in Lam + EGF conditions after treatment of Y-27632 (9.46 ± 3.60 µm) shown significant lower compared to myoblasts average width in Lam + EGF conditions before treatment of Y-27632 (12.84 ± 4.91 µm). Whereas, there are no significant difference between myoblasts average width in control, EGF and Lam conditions after treatment of Y-27632 compared before treatment of Y-27632. For myoblasts average circularity and area, there are no significant difference for all conditions before and after treatment of Y-27632. Further analysis with 12 h live imaging of myoblasts shown in Fig. 5B also confirm increasing trend in cell length and decreasing trend in cell width after treatment of Y-27632 compare to before treatment of Y-27632.
A Quantitative evaluation of myoblast area, length, circularity and width before and after treatment of Y-26732 in plain, plain EGF, laminin and laminin EGF conditions (n = 3) untreated or treated with Y-27632. Myoblasts length in the laminin coated with EGF conditions was increased compared to that before treatment. B Time analysis of 12 h of length and width before and after treatment of Y-27632 myoblasts in plain, plain EGF, laminin and laminin EGF conditions (n = 3) with and without Y-27632. After adding Y-27632, myoblast in laminin-coated surface with and without EGF increase from 0 to 12 h
4 Discussion
Adult skeletal muscle myoblasts exhibit a remarkable capacity for self-renewal that has led to the practical concept of myoblast transplantation for treating skeletal muscle degenerative disorders and cardiac muscles disorders. Exploring the mechanism behind myoblast migration and proliferation will help improve myoblast expansion for future clinical application. Studies on the effects of external factors on myoblast migration have suggested that myoblast migration is significantly enhanced by the synergistic effect of laminin and EGF [7]. In the present study, we compared the effect of laminin and EGF on myoblast and fibroblast co-cultures and on their migration mechanism by examining the potential involvement of two pathways; EGF-R pathway and Rho guanosine triphosphatase (GTPase) Rho kinase (ROCK) pathway in myoblast migration in the laminin and EGF synergy.
Skeletal muscle myofibres are surrounded by an extracellular matrix (ECM), which was initially thought to act only as a scaffold for maintaining tissue structure. It has since been shown that the ECM regulates many cellular processes, including the attachment, proliferation, migration, and differentiation of precursor cells. The ECM is a complex meshwork of many different types of proteins, proteoglycans and polysaccharides, and the ratio of components differs with the tissue type. Here, we observed that myoblasts in Lam and Lam + EGF attached and spread on the tissue culture plate as early as 30 min after plating compared to that in control and EGF conditions as well as compared to fibroblasts on control, EGF, Lam and Lam + EGF. Here, laminin is shown to play a role in initial attachment and spreading of myoblasts. However, EGF supplementation had no effect on the initial cell attachment and spreading. Integrin α7 is highly expressed in skeletal and cardiac muscle, and functions as the major laminin-binding integrin. In myoblast, integrin α7 has been shown to involve in migration and cell adhesion; blocking of integrin α7 by antibody causes inhibition of myoblast migration and adhesion [23]. In addition, in other cell type, breast carcinoma cells, transfection of integrin α7 was also reported to aid cell adhesion and migration [24]. Integrin α7β1 is responsible for receptor-mediated attachment of myoblast migration via laminin-1, in which the cytoplasmic tail of the β1 integrin chain plays a major role [25]. Mediated by integrin, laminin forms bridges between the myoblast actin cytoskeleton and aids myoblast establishment of attachment stability earlier and the initiation of migration after the event. By contrast, myoblasts in control, EGF conditions and fibroblasts in all conditions would first secrete the ECM and adhere to the charged plastic surface before receptor binding to the ECM. The adhesion-promoting proteins present in serum, such as vitronectin and fibronectin, also contribute to cell adhesion to the plastic surface [26, 27]. Myoblasts in Lam and Lam + EGF have lower circularity value compared to that in control and EGF. The higher circularity values are, the higher trend for a cell to be assumed as circular shape [28]. Active migrating cells would have dynamic value of circularity while static cells would have constant circularity values.
Over the past decades, myoblasts have been tested for application in cell therapy for regenerating muscles and in gene delivery systems for treating muscle and non-muscle diseases. Culturing skeletal muscle cells, which contain a mixture of myoblasts and fibroblasts, is laborious and challenges the routine myoblast manipulation procedures [29]. The fibroblast population increases remarkably during expansion, as fibroblasts proliferate faster than myoblasts. Here, we examined the effect of laminin and EGF on the migration and growth of co-cultured myoblasts and fibroblasts. Lam + EGF conditions enhanced myoblast growth rates significantly compared to that in control and EGF. The combination of laminin coating and EGF supplementation exerted a synergistic effect on the promotion of myoblast migration. However, there were no notable differences in fibroblast growth and migration in all conditions. Type I collagen plays a major role in dermal fibroblast attachment and proliferation [30]. Previous study [7] did prove enhancement of myoblast migration and growth in skeletal myoblast cell line. The present study show combination of laminin-coated surface with supplementation of EGF in the medium demonstrate improvement of myoblast’s migration but not fibroblasts of human skeletal muscle. This shown that laminin and EGF cooperatively enhance signalling pathway involve in cell migration. We hypothesized that cross-talk occurs between the laminin and EGF intracellular receptors, and that this synergy may facilitate the signalling pathway involve in migration, such as the Rho/ROCK pathway and EGF-R pathway.
First, attempted to determine the potential role of EGF-R signalling pathway specifically in myoblast migration in laminin and EGF synergistic conditions. Upon stimulation, EGF receptor (EGF-R) activates several signalling cascade events that regulate cell proliferation, migration, angiogenesis and survival. EGF-R is a part of the ErbB family of receptors, a sub-family of four closely related receptor tyrosine kinases: EGF-R (ErbB-1), HER2/neu (ErbB-2), HER3 (ErbB-3) and HER4 (ErbB-4) [31]. Alterations in EGF-R expression or activity lead to cancer [32]. Gefitinib is a drug used to treat cancers such as breast cancer and lung cancer. It inhibits EGF-R, which is believed to be overactivated in cancer, slowing cancer cell proliferation [33]. EGF-R plays a critical role in transforming growth factor beta 1 (TGF-β1)-dependent fibroblast-to-myofibroblast differentiation [34, 35]. However, little is known of the effect of EGF-R on myoblast migration.
In the present study, we tested the effect of EGF-R inhibition on myoblast and fibroblast migration using gefitinib. Gefitinib decreased myoblast migration under laminin coated with EGF conditions. Gefitinib hinders EGF-R activity and reduces the migration of tumour cells [36] and mesothelioma cells [37]. Here, myoblasts in laminin coated with EGF conditions showed decreased cell length and became more fibroblastic compared to the untreated conditions. Downregulation of the EGF-R signalling pathway triggers myoblast differentiation [19].
We attempted to determine the potential role of ROCK specifically in myoblast migration in laminin and EGF synergistic conditions. We found that inhibition of ROCK by Y-27632, a ROCK-specific inhibitor, increased myoblast migration significantly in Lam and Lam + EGF, and there was no significant difference in control and EGF. This result was consistent with studies on other cell types [38, 39], but we observed no differences for fibroblasts in all conditions. Here, we speculate that ROCK may inhibit laminin and α7β1 integrin interaction, as laminin increases myoblast migration rates. The change in cell–matrix adhesion stability may therefore be an underlying factor in the increased velocity of ROCK-inhibited myoblasts.
To further analyse the role of ROCK in the synergy between laminin and EGF for enhancing myoblast migration, we analysed myoblast morphological changes during incubation with Y-27632. Notably, myoblasts exhibited elongated tails in Lam, and the tail elongation was prominent in Lam + EGF. ROCK plays a role in regulating cytoskeletal reorganisation [40, 41] and therefore it was not surprising that Y-27632 resulted in a change from the myoblast-like cellular morphology to stellate-like cells [13]. This is primarily due to the myoblasts losing the ability to retract their trailing edge, leaving a long tail behind the cells [42, 43]. The prominent elongation of myoblast tails in laminin coated with EGF conditions may be explained by the role of myosin in the retraction of the tail at the posterior region. Myosin is controlled by the EGF-R signalling pathway [44] and is also regulated by ROCK [45] where ROCK inactivates the EGF-R pathway [46]. The disruption of myosin regulation causes myoblasts to fail to retract their tails, which stretch to great lengths in the presence of ROCK inhibitors until they break. Thus, we demonstrate the potential role of ROCK in the synergy between laminin and EGF. Further, we evaluated the effect of 12-h exposure to ROCK inhibitor on myoblast and fibroblast cell morphology. The fibroblasts exhibited dynamic cell morphology when treated with Y-27632. This dynamic trend is well known in fibroblast proliferation and migration.
In conclusion, synergistic effect of laminin and EGF promote migration and growth of myoblast but not fibroblast. We demonstrate here, the potential involvement of ROCK signalling pathway as a mechanism behind the synergy effect of laminin and EGF in enhancing myoblast migration. The inhibition of ROCK in laminin and EGF supplemented conditions on myoblast result in morphological change and increase in myoblast migration indicates the important of ROCK in the synergistic effect. Thus, we believe outcome of current proposed study will lead to better understanding of molecular mechanism for regulating critical biological properties of myoblasts. These will help to determine the strategy in designing tissue engineering construct for myoblasts based treatment.
References
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–7.
Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–54.
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301.
McGarvey ML, Baron-Van Evercooren A, Kleinman HK, Dubois-Dalcq M. Synthesis and effects of basement membrane components in cultured rat Schwann cells. Dev Biol. 1984;105:18–28.
Ocalan M, Goodman SL, Kühl U, Hauschka SD, von der Mark K. Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev Biol. 1988;125:158–67.
Chowdhury SR, Muneyuki Y, Takezawa Y, Kino-oka M, Saito A, Sawa Y, et al. Synergic stimulation of laminin and epidermal growth factor facilitates the myoblast growth through promoting migration. J Biosci Bioeng. 2009;108:174–7.
de Lucas B, Pérez LM, Gálvez BG. Importance and regulation of adult stem cell migration. J Cell Mol Med. 2018;22:746–54.
Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12.
Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–83.
Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–21.
Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217:447–57.
Goetsch KP, Snyman C, Myburgh KH, Niesler CU. ROCK-2 is associated with focal adhesion maturation during myoblast migration. J Cell Biochem. 2014;115:1299–307.
Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, et al. Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol Pharmacol. 2000;57:976–83.
Ohnishi Y, Yasui H, Kakudo K, Nozaki M. Regulation of cell migration via the EGFR signaling pathway in oral squamous cell carcinoma cells. Oncol Lett. 2017;13:930–6.
Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017;9:52.
Miguel JC, Maxwell AA, Hsieh JJ, Harnisch LC, Al Alam D, Polk DB, et al. Epidermal growth factor suppresses intestinal epithelial cell shedding through a MAPK-dependent pathway. J Cell Sci. 2017;130:90–6.
Maretzky T, Evers A, Zhou W, Swendeman SL, Wong PM, Rafii S, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011;2:229.
Leroy MC, Perroud J, Darbellay B, Bernheim L, Konig S. Epidermal growth factor receptor down-regulation triggers human myoblast differentiation. PLoS One. 2013;8:e71770.
Savikko J, Rintala JM, Rintala S, Koskinen P. Epidermal growth factor receptor inhibition by erlotinib prevents vascular smooth muscle cell and monocyte-macrophage function in vitro. Transpl Immunol. 2015;32:175–8.
Kino-oka M, Ngo TX, Nagamori E, Takezawa Y, Miyake Y, Sawa Y, et al. Evaluation of vertical cell fluidity in a multilayered sheet of skeletal myoblasts. J Biosci Bioeng. 2012;113:128–31.
Nagamori E, Ngo TX, Takezawa Y, Saito A, Sawa Y, Shimizu T, et al. Network formation through active migration of human vascular endothelial cells in a multilayered skeletal myoblast sheet. Biomaterials. 2013;34:662–8.
Crawley S, Farrell EM, Wang W, Gu M, Huang HY, Huynh V, et al. The α7β1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res. 1997;235:274–86.
Yao CC, Ziober BL, Squillace RM, Kramer RH. α7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–603.
Alvarez B, Stroeken PJ, Edel MJ, Roos E. Integrin cytoplasmic domain-associated protein-1 (ICAP-1) promotes migration of myoblasts and affects focal adhesions. J Cell Physiol. 2008;214:474–82.
Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin-A major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–58.
Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12:313–6.
Pasqualato A, Lei V, Cucina A, Dinicola S, D’Anselmi F, Proietti S, et al. Shape in migration: quantitative image analysis of migrating chemoresistant HCT-8 colon cancer cells. Cell Adhes Migr. 2013;7:450–9.
Zahari NK, Idrus RBH, Chowdhury SR. Laminin-coated poly(methyl methacrylate) (PMMA) nanofiber scaffold facilitates the enrichment of skeletal muscle myoblast population. Int J Mol Sci. 2017;18:2242.
Busra FM, Lokanathan Y, Nadzir MM, Saim A, Idrus RBH, Chowdhury SR. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: a comparative study. Malays J Med Sci. 2017;24:33–43.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Chang TC, Chin YT, Nana AW, Wang SH, Liao YM, Chen YR, et al. Enhancement by nano-diamino-tetrac of antiproliferative action of gefitinib on colorectal cancer cells: mediation by EGFR sialylation and PI3K activation. Horm Cancer. 2018;9:420–32.
Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, et al. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem. 2013;288:14824–38.
Midgley AC, Bowen T, Phillips AO, Steadman R. MicroRNA-7 inhibition rescues age-associated loss of epidermal growth factor receptor and hyaluronan-dependent differentiation in fibroblasts. Aging Cell. 2014;13:235–44.
Hölsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blümcke I, Buslei R. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Res. 2011;17:4367–77.
Favoni RE, Pattarozzi A, Lo Casto M, Barbieri F, Gatti M, Paleari L, et al. Gefitinib targets EGFR dimerization and ERK1/2 phosphorylation to inhibit pleural mesothelioma cell proliferation. Curr Cancer Drug Targets. 2010;10:176–91.
Kroening S, Neubauer E, Wullich B, Aten J, Goppelt-Struebe M. Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: modulation by hypoxia. Am J Physiol Renal Physiol. 2010;298:796–806.
Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell–cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011;32:576–86.
Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). 2010;67:545–54.
Xu B, Song G, Ju Y, Li X, Song Y, Watanabe S. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2012;227:2722–9.
Kolega J. Fluorescent analogues of myosin II for tracking the behavior of different myosin isoforms in living cells. J Cell Biochem. 1998;68:389–401.
Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–45.
Saxena A, Denholm B, Bunt S, Bischoff M, VijayRaghavan K, Skaer H. Epidermal growth factor signalling controls myosin II Planar polarity to orchestrate convergent extension movements during Drosophila tubulogenesis. PLoS Biol. 2014;12:e1002013.
Kaneko-Kawano T, Takasu F, Naoki H, Sakumura Y, Ishii S, Ueba T, et al. Dynamic regulation of myosin light chain phosphorylation by Rho-kinase. PLoS One. 2012;7:e39269.
Nakashima M, Adachi S, Yasuda I, Yamauchi T, Kawaguchi J, Hanamatsu T, et al. Inhibition of Rho-associated coiled-coil containing protein kinase enhances the activation of epidermal growth factor receptor in pancreatic cancer cells. Mol Cancer. 2011;10:79.
Acknowledgement
The authors wish to acknowledge the sponsorship by the Ministry of Higher Education Malaysia [Fundamental Research Grant Scheme (FRGS/2/2013/SG05/UKM/03/1)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare that they have no competing interests.
Ethical statement
The study protocol was approved by Universiti Kebangsaan Malaysia Research and Ethics Committee with approval code of UKM FPR.4/244/FRGS/2/2013/SG05/UKM/03/1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mat Afandi, M.A., Maarof, M., Chowdhury, S.R. et al. Synergistic Effect of Laminin and Epidermal Growth Factor on Biological and Morphological Properties of Co-Cultured Myoblasts and Fibroblasts. Tissue Eng Regen Med 17, 835–845 (2020). https://doi.org/10.1007/s13770-020-00283-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00283-3