Abstract
Reduction of the retrogradation of Injeolmi (IM) during storage for 3 days at 4 °C due to the addition of 0.8 % (w/w) wheat flour was analyzed. The optimum reaction temperature for the hydrolytic enzyme activity of α-amylase in the wheat flour was 30 °C for gelatinized waxy rice. Activity sharply increased until 7 h and then leveled off. The amylose content of IM remained almost constant, while that of wheat flour-added Injeolmi (WFIM) increased by 24 % during storage. Average amylopectin chain lengths of the starches in IM and WFIM increased from 27.91 to 30.36 and from 29.57 to 57.98, respectively, during storage. In the X-ray diffraction pattern, IM starch showed the characteristic peak located at 17°, while no distinct diffraction peak was observed for WFIM starch after storage. Enthalpy changes (ΔH) indicated by differential scanning calorimetry of IM and WFIM were 1.1060 and 0.6159 J/g, respectively. The hardness of IM significantly increased from 149.27 to 2202.52 g f during storage while that of WFIM increased only from 112.14 to 128.08 g f which was 5.82 % compared to that of IM.
Similar content being viewed by others
Introduction
Waxy rice cake, Injeolmi, is one of the most popular rice cakes in Korea, and has been made and enjoyed at festivals and feasts since ancient times. However, Injeolmi requires daily production due to rapid retrogradation. The retrogradation of waxy rice starch during storage hinders its use in industrialized foods. The rapid retrogradation progress of Injeolmi is mainly caused by its high moisture content, high starch concentration, and low fat content compared to bread and baked products (Lee and Maeng 1987).
Waxy rice, also called glutinous or sweet rice, is characterized chiefly by its lower levels of amylose contained in the starch. Starch retrogradation is a non-equilibrium, thermo-reversible crystallization process governed by the consecutive three-step mechanism of nucleation, propagation, and maturation. Short-term development of retrogradation or crystallization in starch gels is attributed to the gelation and crystallization of the amylose fraction (Sievert and Wursch 1993), while the long-term changes occurring during the storage of starch gels have been attributed to the amylopectin fraction (Chang and Lin 2007). Enthalpy for melting recrystallized starches correlated negatively with amylose content (Sasaki et al. 2000). Amylopectin chains with a degree of polymerization (DP) of 6–9 and DP > 25 inhibited retrogradation, whereas relative amounts of DP from 12 to 22 were positively correlated with retrograded amylopectin enthalpies (Vandeputte et al. 2003).
A large number of studies have shown retarded retrogradation of Injeolmi during storage through the use of natural additives such as tapioca, chopping jujube, Baekbokryung powder, mulberry leaf powder, citrus mandarin powder, macerated tea leaves, surichwi, and waxy barley (Cha and Lee 2001; Cho et al. 2006; Cho et al. 2008; Kang and Hong 2009; Kim and Song 2010; Lee et al. 1990; Lee and Cho 2001; Yoon and Koh 1998). However, despite the efforts of many investigators, there have not been industrial applications.
Wheat flour contains several technologically impotent enzymes such as amylases, proteases, lipoxygenase, polyphenol oxidase, and peroxidase (Rani et al. 2001). α-Amylase is located mainly in the pericarp, with small quantities present in the alcurone layer and seed coat (Kruger and Tipples 1980). α-Amylase activity is increased in wheat flour mill streams (Rani et al. 2001).
The main focus of the present study was to analyze the degree of reduction in the retrogradation of Injeolmi during storage for 3 days at 4 °C occurring when 0.8 % (w/w) wheat flour was added.
Materials and methods
Materials
Waxy rice (Hwaseon waxy rice of the japonica cultivars, harvested in Hwaseong, Gyeonggi-do, Korea in 2012) was purchased from a local market in Cheonan City, Korea. Wheat flour (all-purpose flour) and salt were purchased from Cheiljedang Company (Seoul, Korea) and Daesang Company (Seoul, Korea), respectively. Amylose contents of the waxy rice and wheat flour were 1.83 and 25.0 %, respectively.
Manufacture of wheat flour-added Injeolmi (WFIM)
A 5 kg of waxy rice was weighed and soaked for 5 h, dehydrated for 30 min, and ground with a roll-miller (KM-18, Kyungchang Machine Co., Gwangju-si, Gyeonggi-do, Korea). The moisture content of rice powder was 38.4 % (wet basis), which spread over a stainless steel steam pan and cooked for 30 min at 100 °C. The pre-cooked waxy rice powder was then mixed with 5 % (v/w of soaked waxy rice) water, in which 1 % (w/v of soaked waxy rice) salt was completely dissolved. The pre-cooked waxy rice dough was spread over a stainless steel steam pan and cooked for 20 min at 100 °C for gelatinization of starch thoroughly. The cooked waxy rice dough was immediately moved to a tray and cooled to 55 °C, which was the center temperature of the steamed waxy rice dough, and then mixed with 0.8 % wheat flour (w/w of soaked waxy rice). It was punched for 13 min (KM89, Kyungchang Machine Co.) at 55 °C, and then the Injeolmi dough was extruded with an extruder (KH-204, Kyungchang Machine Co.).
Packaging and storage
Samples were packed in laminated PET/AL/PE (SR Technopack Co., Cheonan, Korea) plastic film bags (29 × 41 cm) and stored in temperature-controlled chambers (SI-900R, JEIO TECH., Seoul, Korea) at 4 °C for 3 days.
Starch isolation
Waxy rice starch was isolated using the alkaline method, and the isolated starch was dried until reaching 5 % moisture content (wet basis) using a vacuum freeze drier (FD5518, Ilshin Lab. Co., Seoul, Korea).
α-Amylase activity
Wheat flour (1 g) was extracted for 2 h at room temperature with 5 mL of 50 mM phosphate buffer (pH 7.0) and centrifuged at 8,000×g for 10 min, after which the supernatant was used as crude enzyme extract.
Amylase activity was assayed by measuring the release of reducing sugars using the 3,5-dinitrosalicylic acid (DNS) method. Gelatinized waxy rice starch (1 g/100 mL, 250 μL) was incubated with 200 μL of phosphate buffer and 50 μL of the enzyme extract for 10 min. The reaction was stopped by the addition of 500 μL of DNS reagent. Color was developed by heating the tubes for 5 min in a boiling water bath, followed by subsequent cooling at room temperature. The solutions were prepared up to the required volume, and the optical density was measured at 575 nm with maltose as a standard. The results were expressed as μmol maltose liberated in 1 min at 20, 30, 40, 50, and 60 °C by 1.0 mL of the enzyme solution. Analyses were carried out in triplicate for the amylase activity assays.
Amylose content
A 40 mg sample (isolated starches from IM and WFIM) was placed in a 25 mL volumetric flask, after which 10 mL of 0.006 M I 2 in 90 % dimethyl sulfoxide (DMSO) was added, and then cooled to room temperature for 24 h. The resultant starch solution (1 mL) was placed in a 50 mL volumetric flask, and then adjusted to 50 mL with distilled water. This mixture was stirred well and then allowed to stand for 30 min. The absorbance of sample was scanned from 600 nm using a UV/visible spectrometer (OPTIZEN POP, Mecasys Co., Ltd. Daejeon, Korea). The amylose contents of the samples were determined as the absorbance at 600 nm based on the standard curve of pure potato amylose (A-0512, Sigma-Aldrich Co., St. Louis, MO, USA).
β-Amylolysis limits and average amylopectin chain length
A 5 g sample was added to 2.5 mL of 0.2 M phosphate buffer (pH 6.0), and then distilled water was added to 250 mL. This mixture was heated in a hot water bath (95 °C) for 15 min, and then centrifuged at 3,000×g for 30 min. The decanted portion was separated into a new tube, and the precipitates were mixed with another 2.5 mL aliquot of 0.2 M phosphate buffer (pH 6.0), adjusted to 250 mL with distilled water, heated, and centrifuged. The decanted portion was transferred to a new flask, washed twice with 70 % methanol, and then centrifuged at 3,000×g for 10 min. It was then dried in an oven at 50 °C for 24 h to obtain purified amylopectin.
Purified amylopectin (200 mg), 3 % NaCl, and 10 ml of 0.37 M sodium metaperiodate were mixed in a 100-mL erlenmeyer flask, and allowed to react with shaking at 25 °C for up to 25 h. After reaction, the sample was mixed with 1 mL of ethylene glycol at room temperature for 1 h and titrated with 0.01 N of Ba(OH)2 using methyl red as an indicator. The average chain length of amylopectin was calculated using the following equation:
X-ray diffraction (XRD)
The X-ray patterns of the starches were measured with an X-ray diffractometer (Ultima IV, Rigaku corp., Kyoto, Japan). Each sample was exposed to X-ray beams with the generator running at 40 mA and 40 kV. The scanning region of the diffraction angle (2θ) was from 5 to 40°.
Differential scanning calorimetry (DSC)
Thermal properties of the starches were determined using a DSC (DSC-2010, TA Instruments, New Castle, DE, USA). Samples (3.0 mg, dry basis) were put into aluminum pan, and distilled water was added to give a water-to-flour (dry solid) ratio of 2.5:1 (w:w). The pan was hermetically sealed and allowed to stand for 2 h prior to thermal analysis. Thermal scanning was done from 10 to 140 °C at a heating rate of 10 °C/min. The themogram was recorded using an empty aluminum pan as the reference. The onset temperature (T o), peak temperature (T p), and gelatinization enthalpy (ΔH) were determined from the endothermic peak.
Texture property
A Textural analyzer (TA-XT2, Stable Microsystem Ltd, Surrey, UK) was used to perform an instrumental evaluation of sample texture. Samples (15 × 15 × 15 mm) were prepared using a stainless frame device and then were placed on the platform. A cylinder-type stainless steel probe with a diameter of 50 mm was used to compress each sample to 60 % of its original height with 60 mm/min of moving speed. All textural analyses were replicated ten times.
Statistical analysis
The Statistical Analysis Program System for Windows (SAS Institute Inc., Cary, NC) was employed for statistical analysis of the experimental results using the t test at a level of 5 %.
Results and discussion
α-Amylase activity in wheat flour
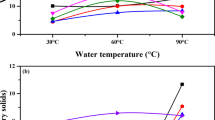
The hydrolytic enzyme activity of α-amylase in wheat flour was measured against gelatinized waxy rice starches at different reaction temperatures and times, as shown in Figs. 1 and 2. The optimum reaction temperature was found to be 30 °C in the gelatinized waxy rice starch, as represented by the reaction rate of 14.25 U/mL. The hydrolytic enzyme activity of the α-amylase in wheat flour on the gelatinized waxy rice starch at 30 °C sharply increased until 7 h of reaction, and then leveled off. The degradation of starch by α-amylase leads to changes in starch structure, and the production of low and intermediate molecular weight dextrin (Xu et al. 2014). There are two types of dextrin. Spring dextrin (SD) is a linear, poly-diverse saccharide featuring a repeating (1–4)-a-d-glucose unit. SD, with low molecular weight, disturbed the short-term retrogradation of amylose by non-bonding interactions, and it has also been reported to impact the long-term retrogradation of wheat and corn starches (Xu et al. 2012, 2013). Different from SD, blanched limit dextrin (BLD) can be made by treating gelatinized starch with α-amylase and β-amylase. It is composed of a linear chain of α-(1–4)-d-glucose residues connected together with α-(1–6)-linkages. BLD was reported to significantly reduce wheat and waxy rice starch retrogradation (Xu et al. 2014).
Amylose content
Processing to retard retrogradation (wheat flour-added processing) was performed under the following conditions. Addition of 0.8 % of wheat flour was performed when the temperature of the dough center reached 55 °C with a punching time of 13 min, as determined based on the optimization of preliminary experiments. The ranges of interest of the added wheat concentration, dough center temperature for wheat addition, and punching time investigated were 0–1.0 %, 45–95 °C and 0–13 min, respectively.
Amylose contents of the IM and WFIM during 3 days of storage at 4 °C are shown in Fig. 3. The amylose contents of starches isolated from waxy rice varieties in Korea range from 1.1 to 7.0 % (You et al. 2014; Lee 2013). During storage, the amylose contents of IM and WFIM increased from 3.49 to 3.55 % and from 3.70 to 4.41 %, respectively. The amylose content of IM remained almost constant, while that of WFIM increased by 24 % during storage. We suggest that the WFIM starch was hydrolyzed at α-(1–4)- and α-(1–6)-linkages by amylases. As a similar result, the amylose content of starch from the Te-quing rice cultivar increased by 26.5 to 28.1 % during hydrolysis by porcine pancreatic α-amylase (Man et al. 2013).
β-Amylolysis limits and average amylopectin chain lengths
The β-Amylolysis limit (%) of IM increased from 39.65 to 42.18, while that of WFIM decreased from 38.31 to 37.50 during storage (Table 1). β-Amylolysis limits contain all the branch points of the intact amylopectin molecules. Based on the average amylopectin chain length (CL) of intact molecules and the extent to which the intact molecules were hydrolyzed by β-amylase (β-amylolysis limit), one can calculate the portion of original molecules from the non-reducing terminus to the first branch points encountered by the enzyme (Thomson 2000).
CL of the IM and WFIM starches increased from 27.91 to 30.36 and from 29.57 to 57.98, respectively. The increment of CL in WFIM starch was much higher than that in IM starch. This result confirmed that the degree of retrogradation (DR) of the gelatinized waxy rice starch after storage had a significant negative correlation (p < 0.05) with the logarithmic value of the respective degrees of polymerization. The increment of DR of waxy corn starch to the decrement of CL was profoundly lower than that of waxy rice starch (Chang and Lin 2007). This suggests that the DR of waxy starch, with different molecular sizes and after gelatinization and aging, depends on not only the molecular weight of amylopectin, but also on the molecular structure of the starch.
X-ray diffraction (XRD)
The X-ray diffraction patterns of IM and WFIM are presented in Fig. 4. The crystal type of native waxy rice starch was found to be A-type. However, after gelatinization, the XRD patterns formed a new crystalline structure. The gelatinized IM and WFIM starches displayed a mixture of B- and V-type polymorphic forms in the X-ray diffractograms. Shi and Gao (2011) reported the native A-type crystallinity of starch to be destroyed after gelatinization. After 3 days of storage at 4 °C, the IM starch showed the characteristic peak located at 17°, which is the distance between the 100 crystallographic planes in the hexagonal unit cell of B-amylose (Nishiyama et al. 2009). In contrast, no distinct peak was observed for the WFIM starch after storage. This indicated that the WFIM starch was not retrograded, a result which agreed well with the previous research (Xu et al. 2014).
Differential scanning calorimetry (DSC)
Table 2 shows the DSC properties of IM and WFIM starches during storage for 3 days at 4 °C. The onset temperature (T o), peak temperature (T p), and enthalpy (△H) of the IM starch increased from 61.42 to 72.77 °C, 74.09 to 77.04 °C, and 0.6582 to 1.1060 J/g, respectively. These results coincided with the report that transition temperatures and endothermic enthalpy of gelatinized waxy rice starch increases with increase of the degree of crystallite and the stabilization of double helical structures during storage (Shi and Gao 2011). In contrast, the onset temperature and peak temperature of WFIM starch decreased from 69.56 to 64.44 and 75.67 to 74.65 °C, respectively, while enthalpy increased from 0.4086 to 0.6159 J/g during storage. We suggest that the gelatinized rice starch was hydrolyzed in WFIM during storage, while the same phenomena occurred due to enzymatic hydrolysis (Shrestha et al. 2012).
Texture property
As shown in Table 3, the hardness value of IM significantly increased from 149.27 to 2202.52 g f over the storage period. In contrast, that of WFIM had only a small increase from 112.14 to 128.08 g f, representing only a 5.82 % increase compared to that of IM. Therefore, it appeared that the gelatinized waxy rice starch was hydrolyzed in the WFIM during storage. Through the process of retrogradation, gelatinized starch is transformed from an amorphous state to a more ordered or crystalline state. Starch retrogradation occurs readily during the storage of heat-processed starchy food, such as IM, as a spontaneous process reaching a metastable state of lower free energy (Yu et al. 2009). Retrogradation, however, often exerts unacceptable influences on the texture, such as the hardening of IM. The DP of amylopectin is negatively correlated with the hardness of retrograded waxy rice (Villareal et al. 1993).
The adhesiveness values of IM and WFIM increased from 57.75 to 158.60 erg, and from 32.65 to 114.22 erg, respectively. Adhesiveness is more of a surface characteristic and depends on the combined effect of adhesive and cohesive forces, as well as the viscosity and viscoelasticity (Adhikari et al. 2001). The springiness value of IM drastically decreased from 0.86 to 0.25 during storage, while that of WFIM only decreased from 0.83 to 0.79. Springiness is the perception of gel rubberiness in the mouth and is a measure of how much the gel structure is broken down by the initial compression. High springiness is the expression of when the gel structure is broken only into a few large pieces during the first compression, whereas low springiness results in the production of many small pieces (Huang et al. 2007).
References
Adhikari B, Howes T, Bhandari BR, Truong V (2001) Stickiness in foods: mechanism and test methods: a review. Int J Food Prop 4:1–33
Cha GH, Lee HG (2001) Sensory and physicochemical characteristics and storage time of Daechu-Injeulmi added with various levels of chopping jujube. J Food Cook Sci 17:29–42
Chang YH, Lin JH (2007) Effects of molecular size and structure of amylopectin on the retrogradation thermal properties of waxy rice and waxy corn starches. Food Hydrocoll 21:645–653
Cho TO, Seo HJ, Kim JS, Hong JS (2006) Effect of kneading, ingredients and enzymatic hydrolysis on retrogradation of Injulmi. Korean J Food Cook Sci 22:282–290
Cho TO, Kim HJ, Hong JS (2008) Quality characteristics of waxy barley Injeulmi prepared with Baekbokryung powder. Korean J Food Cook Sci 24:157–163
Huang M, Kennedy JF, Li B, Xu X, Xie BJ (2007) Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: a texture profile analysis study. Carbohydr Polym 69:411–418
Kang YS, Hong JS (2009) Quality characteristics of Injeulmi made with different ratios of mulberry leaf powder. Korean J Food Cook Sci 25:275–282
Kim CW, Song E (2010) Quality characteristics of Gamgyul-Injeulmi with citrus mandarin powder during storage. Korean J Food Nutr 23:247–257
Kruger JE, Tipples KH (1980) Relationships between falling number, amylograph viscosity and α-amylase activity in Canadian wheat. Cereal Res Commun 8:97–105
Lee YT (2013) Properties of normal and glutinous black rice flours prepared by different milling methods. Food Eng Prog 17:339–345
Lee SM, Cho JS (2001) Sensory and mechanical characteristics of Surichwi-injeulmi by adding Surichwi contents. J Food Cook Sci 17:1–6
Lee CH, Maeng YS (1987) A literature review on Korean rice-cakes. Korean J Food Cult 2:117–132
Lee MG, Kim SS, Lee SH, Oh SL, Lee SW (1990) Effects on retrogradation of Injeulmi (Korean glutinous rice cake) added with the macerated tea leaves during storage. J Korean Soc Appl Biol Chem 33:277–281
Man J, Yang Y, Zhang C, Zhang F, Wang Y, Gu M, Liu Q, Wei C (2013) Morphology and structural characterization of high-amylose rice starch residues hydrolyzed by porcine pancreatic α-amylase. Food Hydrocoll 31:195–203
Nishiyama Y, Putaux JI, Montesanti N, Hazemann JL, Rochas C (2009) B-A allomorphic transition in native starch and amylose spherocrystals monitored by in situ synchrotron X-ray diffraction. Biomacromolecules 11:76–87
Rani KU, Prasada Rao UJS, Leelavathi K, Haridas Rao P (2001) Distribution of enzymes in wheat flour mill streams. J Cereal Sci 34:233–242
Sasaki T, Yasui T, Matsuki J (2000) Effect of amylose content on gelatinization, retrogradation and pasting properties of starches from waxy and non-waxy wheat and their FI seeds. Cereal Chem 77:131–145
Shi MM, Gao QY (2011) Physicochemical properties, structure and in vitro digestion of resistant starch from waxy rice starch. Carbohydr Polym 84:1151–1157
Shrestha AK, Blazek J, Flanagan BM, Dhital S, Larroque O, Morell MK, Gilbert EP, Gidley MJ (2012) Molecular, mesoscopic and microscopic structure evolution during amylase digestion of maize starch granules. Carbohydr Polym 90:23–33
Sievert D, Wursch P (1993) Amylose chain association based on differential scanning calorimetry. J Food Sci 58:1332–1345
Thomson DB (2000) On the non-random nature of amylopectin branching. Carbohydr Polym 43:223–239
Vandeputte GE, Vermeylen R, Geeroms J, Delcour JA (2003) Rice starches III. Structural aspects provide insight in amylopectin retrogradation properties and gel texture. J Cereal Sci 38:61–68
Villareal CP, Juliano BO, Hizukuri S (1993) Varietal differences in amylopectin stailing of cooked waxy milled rices. Cereal Chem 70:753–758
Xu J, Zhao W, Ning Y, Jin Z, Xu B, Xu X (2012) Comparative study of spring dextrin impact on amylose retrogradation. J Agric Food Chem 60:4970–4976
Xu J, Fan X, Ning Y, Wang P, Jin Z, Lv H, Xu B, Xu X (2013) Effect of spring dextrin on retrogradation of wheat and corn starch gels. Food Hydrocoll 33:361–367
Xu J, Wang Q, Bashari M, Chen F, Wang P, Cui L, Yuan J, Xu X, Fan X (2014) Branched limit dextrin impact on wheat and waxy starch gels retrogradation. Food Hydrocoll 39:136–143
Yoon GS, Koh HY (1998) Preparation of waxy barley cake and its quality characteristics. J Korean Soc Food Sci Nutr 27:890–896
You SY, Lim ST, Lee JH, Chung HJ (2014) Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr Polym 112:729–735
Yu S, Ma Y, Sun DW (2009) Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J Cereal Sci 50:139–144
Acknowledgments
This study was carried out with the support of the cooperative Research Program for Agriculture Science and Technology Development (PJ008540022014) of the Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HS., Kim, KM., Han, GJ. et al. Effect of wheat flour addition on retardation of retrogradation in waxy rice cake, Ingeolmi . J Korean Soc Appl Biol Chem 58, 285–291 (2015). https://doi.org/10.1007/s13765-015-0054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0054-6