Abstract
The antimicrobial activities of materials derived from Cassia obtusifolia seeds were evaluated against the seven food-borne bacteria. The active constituent of C. obtusifolia seeds was isolated using various chromatographic techniques and characterized as 1,2-dihydroxyanthraquinone (alizarin). The purified alizarin exhibited weak activity against Bacillus cereus (clean zone diameter, 11.0 mm), and moderate activity against Staphylococcus epidermidis (17.5 mm) and Salmonella enterica (16.2 mm) at 2.0 mg/disc. When compared with alizarin analogues, alizarin-3-methyliminodiacetic acid exhibited the strongest antimicrobial activity against B. cereus, S. intermedius, S. epidermidis, and S. enterica at 2.0 mg/disc, whereas the other analogues exhibited no antimicrobial activity against the seven food-borne bacteria. Taken together, these results indicate that alizarin isolated from C. obtusifolia seeds and its structural analogues may be useful as natural preservatives.
Similar content being viewed by others
Introduction
Food-borne illnesses resulting from the consumption of food which is contaminated with food-borne pathogenic bacteria had a major impact on public health around the world (White et al. 2002; Bajpai et al. 2012, 2013). A diversity of chemical food preservatives have been used for the prevention of food-borne illness (Şahin et al. 2004; Kim et al. 2009). Accordingly, many people have expressed interest in the use of plant-derived antimicrobial constituents as natural preservatives for foods (Lee 2002; Ultee et al. 2002). The antimicrobial activities of a wide variety of plant volatile oils and their components have been evaluated (Dorman and Deans 2000). Considering that a number of different chemical compounds are present in essential oils, it is likely that their antimicrobial activities are not attributable to one particular mechanism, but several targets in the cell (Skandamis and Nychas, 2000). The susceptibility of bacteria to the antimicrobial activity of plant essential oils has been shown to increase with decreases in the storage temperature, the pH of the food and the amount of oxygen within the packaging (Tassou et al. 1995; Skandamis and Nychas 2000; Tsigarida et al. 2000; Burt 2004). The reason for this is that the hydrophobicity of essential oil increases at low pH, enabling it to dissolve in the cell membrane lipids of the target bacteria more readily (Juven et al. 1994).
Cassia obtusifolia L. (Leguminosae) is a traditional medicine used in China, Japan, and Korea to treat eye inflammation, lacrimation, and photophobia (Zhu 1998). In addition, it has been reported that C. obtusifolia and its constituents have anti-Helicobacter pylori effect, oestrogenic activity, antimicrobial activity and inhibit histamine release from mast cells (Kitanaka et al. 1998; Bhamarapravati et al. 2003; Sung et al. 2004). Thus, in this study, we isolated the active constituents of C. obtusifolia seeds and evaluated them against seven food-borne bacteria.
Materials and methods
Chemicals
Alizarin-3-methyliminodiacetic acid, anthraquinone, 1,4-dihydroxyanthraquinone, and tetracycline were provided from Sigma-Aldrich (USA). All other compounds were of reagent grade.
Isolation and identification
C. obtusifolia seeds were purchased from a local market in Jeonju (Korea). The extraction and partition of C. obtusifolia seeds were carried out with a method modified from Jeon et al. (2009). Briefly, dried and ground seeds of C. obtusifolia (3 kg) were extracted twice with methanol (10 L) at 30 °C for 2 days, and then filtered. The combined extracts were evaporated at 35 °C to yield about 217 g (7.23 %), after which the extract (217 g) was partitioned consecutively with hexane (43.21 g), chloroform (41.97 g), ethyl acetate (24.44 g), and water (107.38 g) portions for subsequent bioassays against food-borne bacteria. The organic solvent portions were evaporated to dryness by a rotary vacuum evaporator at 35 °C, while the water portion was freeze-dried.
The hexane portion (10 g) was consecutively chromatographed on a silica gel column (Merch 70–230 mesh, 590 g, 6.5 × 68 cm), and then sequentially eluted using a stepwise gradient of hexane–ethyl acetate (10, 20, 30, 50, and 80 %). The biologically active fraction (4.1 g) was then chromatographed on a silica gel column and eluted with hexane: ethyl acetate (2:1). The column fractions were analysed by thin layer chromatography (TLC, hexane: ethyl acetate, 2.5:1) and fractions exhibiting similar TLC patterns were combined for bioassay against food-borne bacteria. The active fraction (2.7 g) was rechromatographed on a silica gel column and sequentially eluted with hexane:ethyl acetate (7:3). To further separate the active substance, prep high-performance liquid chromatography (Prep. HPLC, Waters Delta Prep 4000, Milford, MA) was conducted. For HPLC, a Bondapak C18 (Waters, 29 × 300 mm) column was used. Methanol:water (3:7), which was used as the mobile phase, was applied at a flow rate of 8 mL/min, and detection was carried out at 260 nm. Finally, the active principle was isolated, and the structure was determined by various instrumental analyses. 1H and 13C NMR spectra were recorded with a JNM-LA 400F7 spectrometer (JEOL, Japan; 1H-600 MHz; 13C-150 MHz), and the UV spectra were obtained using an Uvikon 922 spectrometer (Kontron, Germany). The mass spectra were obtained on a JEOL GSX 400 spectrometer (FEOL, Japan).
Microorganisms and culture conditions
Antimicrobial activities of alizarin isolated from C. obtusifolia and its derivatives were evaluated against seven food-borne bacteria, which were obtained from the Korean Culture Center of Microorganisms (Seoul, Korea). These included the four Gram-positive bacteria Bacillus cereus (ATCC14579), Listeria monocytogenes (ATCC 15313), Staphylococcus intermedius (ATCC29663), and Staphylococcus epidermidis (ATCC 12228), and the three Gram-negative bacteria Salmonella enterica (ATCC 43971), Salmonella typhimurium (IFO 14193), and Shigella sonnei (ATCC 25931). The tested bacteria were cultured in nutrient broth (NB, Difco, USA) at 37 °C for 24 h.
Bioassay
The antimicrobial activity of each sample against the food-borne bacteria was tested by the paper disc agar diffusion method. To assay the antimicrobial activity against the tested microorganisms, one loopful of each type of bacteria was suspended in 1 mL of sterilized physiological saline (Lee and Ahn 1998). The 0.1 mL of test bacterial suspension was seeded on a Mueller–Hinton agar (MHA, Difco, USA) plates. Each test sample was then dissolved in 0.1 mL of methanol solution and applied to a paper disc with a Drummond glass microcapillary (8 mm diameter and 1 mm thickness; Tokyo Roshi Kaisha, Japan). After vaporization of the solvents, the paper discs were placed on the surface of the agar which had been incubated with the test bacteria. All test plates were then incubated at 37 °C for 24 h under aerobic conditions. The control discs received 0.1 mL of methanol. All growth inhibition tests were performed in triplicate. The range of antimicrobial activity was classified as follows: potent activity, more than 30 mm; strong activity, 21–30 mm; moderate activity, 16–20 mm; weak activity, 10–15 mm; little or no activity, less than 10 mm.
Results and discussion
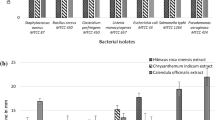
The antimicrobial activity of the methanol extract of C. obtusifolia seeds against seven food-borne bacteria was determined by the paper disc agar diffusion method. During routine screening tests, the methanol extract of C. obtusifolia showed little inhibitory activity at 10 mg/disc against S. epidermidis and S. enterica, but showed no inhibitory activity at all against B. cereus, S. intermedius, L. monocytogenes, S. typhimurium, and S. sonnei (Table 1). The methanol extract was further separated into five fractions and tested at 10 mg/disc. The chloroform fraction showed little inhibitory activity against B. cereus, and weak inhibitory activity against S. epidermidis and S. enterica (Table 1). Accordingly, the chloroform fraction was selected for isolation of the biologically active constituent from C. obtusifolia seeds. Bioassay-guided fractionation of the chloroform fraction afforded an active component, which was identified based on the spectroscopic date produced by IR, UV, 1H-NMR, and 13C-NMR. The biologically active component was characterized as 1,2-dihydroxyanthraquinone (alizarin) (C14H8O4, MW 342.26); EI MS (70 eV) m/z (% relative intensity) M+ 240 (100), 212 (15), 184 (9), 155 (4), 138 (10), 128 (11), 92 (4), 77 (6), 51 (4); 1H NMR (CDCOCD3, 400 MHz, δ ppm) 8.27 (m, 1H), 8.16 (m, 1H), 7.93 (m, 2H), 7.73 (d, 1H, J = 12 Hz), 7.28 (d, 1H, J = 6.9 Hz); 13C NMR (CD3OD3, 100 MHz, δ ppm) 189.8, 181.1, 152.7, 135.7, 134.9, 134.4, 127.6, 127.6, 126.1, 121.7, 121.5, 121.2, 117.1. The spectroscopic data obtained from the analysis of alizarin were compared with the data from previous studies (Jeon et al. 2009). Anthraquinones are known to complex irreversibly with nucleophilic amino acids in proteins, often resulting in subsequent inactivation of the proteins and loss of function (Stern et al. 1996; Arvind et al. 2004; Mbaveng et al. 2008) (Fig 1).
The antimicrobial activities of alizarin were assessed by the paper disc agar diffusion method at 2.0 mg/disc and compared with those of tetracycline, which served as a positive control (Table 2). Alizarin, isolated from C. obtusifolia seeds, demonstrated weak activity against B. cereus (clean zone diameter, 11.0 mm), and moderate activity against S. epidermidis (17.5 mm) and S. enterica (16.2 mm). These results were in agreement with those of a previous study, which evaluated the antimicrobial activity of alizarin against Bacillus cereus (Lee et al., 2013). The results also indicated that the antimicrobial activity of C. obtusifolia seeds against the food-borne pathogenic bacteria could be mostly attributed to alizarin. To establish the structure–activity relationships of four structural analogues, three additional compounds (anthraquinone, 1,4-dihydroxyanthraquinone, alizarin-3-methyliminodiacetic acid) were evaluated by the agar diffusion method at 2.0 mg/disc (Table 2). Alizarin-3-methyliminodiacetic acid exhibited potent activity against S. intermedius (30.3 mm) and S. enterica (33.1 mm), strong activity against S. epidermidis (24.5 mm), and moderate activity against B. cereus (16.8 mm). However, anthraquinone and 1,4-dihydroxyanthraquinone exhibited no growth inhibitory effects against the seven food-borne bacteria. The structures of the four anthraquinones exhibited different inhibitory activities against the seven food-borne bacteria. Alizarin contains hydroxyl functional groups on the anthraquinone skeleton. Alizarin-3-methyliminodiacetic acid showed the strongest inhibitory activity against B. cereus, S. intermedius, S. epidermidis, and S. enterica. In the case of Alizarin, which has hydroxyl functional groups conjugated at positions 1 and 2, the inhibitory activity was observed against B. cereus, S. epidermidis, and S. enterica. However, 1,4-dihydroxyanthraquinone, which had hydroxyl functional groups at positions 1 and 4, showed no antimicrobial activity against any of the seven food-borne bacteria. Likewise, anthraquinone, which was a skeleton of alizarin, showed no antimicrobial activity against the seven food-borne bacteria. These results indicated that the antimicrobial activities were affected by the position of the hydroxyl functional group. Similarly, the antimicrobial activities were influenced by the position of the hydroxyl and carboxyl functional groups in the anthraquinone ring against S. aureus (Wu et al. 2006). In addition, the different positions of the hydroxyl and carboxyl functional groups in the anthraquinone ring led to the enhancement of toxicity against protozoa (Wu et al. 2006).
Based on the Material Safety Data sheet (Sigma-Aldrich), the oral lethal dose of alizarin (316 mg/kg) and alizarin-3-methyliminodiacetic acid (170 mg/kg) indicated a moderate acute toxicity to mammals (Sigma-Aldrich, USA). These findings indicate that C. obtusifolia seeds and alizarin analogues should be useful as natural antimicrobial compounds, and potentially suitable as a replacement for artificial preservatives.
References
Arvind S, Reg FC, Enzo AP (2004) Identification of antimicrobial component of an ethanolic extract of the Australian medicinal plant, Eremophila duttonii. Phytother Res 18:615–618
Bajpai VK, Rahman R, Kang SC (2012) Control of salmonella in foods by using essential oils: a review. Food Res Int 45:722–734
Bajpai VK, Sharma S, Baek KH (2013) Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 32:82–90
Bhamarapravati S, Pendland SL, Mahady GB (2003) Extracts of spice and food plants from Thai traditional medicine inhibit the growth of the human carcinogen Helicobacter pylori. In Vivo 17:541–544
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods: a review. Food Microbiol 94:223–253
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant xolatile oils. J Appl Microbiol 88:308–316
Jeon JH, Song HY, Kim MG, Lee HS (2009) Anticoagulant properties of alizarin and its derivatives derived from the seed extract of Cassia obtusifolia. J Korean Soc Appl Biol Chem 52:163–167
Juven BJ, Kanner J, Schved F, Weisslowicz H (1994) Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol 76:626–631
Kim MG, Jeong EY, Lee HS (2009) Antimicrobial effects of anthracene and its derivatives against intestinal bacteria. J Korean Soc Appl Biol Chem 52:327–330
Kitanaka S, Nakayama T, Shibano T, Ohkoshi E, Takido M (1998) Antiallergic agent from natural sources: structures and inhibitory effect of histamine release of naphthopyrone glycosides from seeds of Cassia obtusifolia L. Chem Pharm Bull 46:1650–1652
Lee HS (2002) Tyrosinase inhibitors of Pulsatilla cernua root derived materials. J Agric Food Chem 50:1400–1403
Lee HS, Ahn YJ (1998) Growth-inhibiting effects of Cinnamomum cassia bark-derived materials on human intestinal bacteria. J Agric Food Chem 46:8–12
Lee NH, Lee SM, Song DH, Yang JY, Lee HS (2013) Antimicrobial effect of emodin isolated from Cassia tora Linn. seeds against food-borne bacteria. J Appl Biol Chem 56:187–189
Mbaveng AT, Kuete V, Nguemeving JR, Beng VP, Nkengfack AE, Meyer JJM, Lall N, Krohn K (2008) Antimicrobial activity of the extracts and compounds obtained from Vismia guineensis (Guttiferae). Asian J Tradit Med 3:211–213
Şahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M, Agar G, Özer H (2004) Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15:549–557
Skandamis PN, Nychas GJE (2000) Development and evaluation of a model predicting the survival of Escherichia coli O157:H7 NCTC 12900 in homemade eggplant salad at various temperatures, pHs and oregano essential oil concentrations. Appl Environ Microb 66:1646–1653
Stern JL, Hagerman AE, Steinberg PD, Mason PK (1996) Phorotannin–protein interactions. J Chem Ecol 22:1887–1899
Sung BK, Kim MK, Lee WH, Lee DH, Lee HS (2004) Growth responses of Cassia obtusifolia toward human intestinal bacteria. Fitoterapia 75:505–509
Tassou C, Drosinos EH, Nychas GJE (1995) Effect of essential oil from mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4°C and 10°C. J Appl Bacteriol 78:593–600
Tsigarida E, Skandamis P, Nychas GJE (2000) Behavior of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J Appl Microbiol 89:901–909
Ultee A, Bennik MHJ, Moezelaar R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen bacillus cereus. Appl Environ Microb 68:1561–1568
White DG, Zhao S, Simjee S, Wagner DD, McDermott WP (2002) Antimicrobial resistance of food-borne pathogens. Microbes Infect 4:405–412
Wu YW, Ouyang J, Xiao XH, Gao WY, Liu Y (2006) Antimicrobial properties and toxicity of anthraquinones by microcalorimetric bioassay. Chin J Chem 24:45–50
Zhu YP (1998) Chinese materia medica chemistry, pharmacology and applications. Harwood Academic Publishers, Netherlands
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JH., Lee, HS. Antimicrobial activities of 1,2-dihydroxyanthraquinone derivatives against food-borne bacteria. J Korean Soc Appl Biol Chem 58, 121–125 (2015). https://doi.org/10.1007/s13765-015-0014-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0014-1