Abstract
In this study, removal of copper from synthetic aqueous solution was done using adsorption process. The batch study was done at room temperature and analyzed using Freundlich and Langmuir isotherms. The Box–Behnken design method of response surface methodology was used to know the interactions between independent variables (pH of aqueous solution, contact time, adsorbent dose, and initial copper concentration) and response variable (copper removal). The activated Ganga sand was used as an adsorbent to make treatment in a cost-effective way. The Ganga sand was activated by using a base solution of 14 N sodium hydroxide. The synthetic aqueous solution was having copper concentration of 100 ppm. The optimum conditions for independent variables to achieve maximum removal of copper from synthetic aqueous solution were also determined. The optimum conditions of aqueous solution for pH, contact time, adsorbent dose, and initial copper concentration were obtained as 8.7, 150 min, 3.5 g/L, and 34 ppm respectively. At this optimum condition, 97.7% of copper removal was achieved. Both isotherms (Freundlich and Langmuir isotherms) were found fitted well with a good correlation coefficient (R2 > 0.95). The kinetic behavior for the responsible adsorption process was analyzed using pseudo-second-order kinetic equations. The results suggested that Ganga sand can be used as a promising economical and conventional adsorbent for copper removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The existence of heavy metals in industrial wastes is of immense distress due to their natural toxicity and hazardous effect. Copper is a type of heavy metal and has various utilization in industries like electroplating, paper, refineries, fertilizers etc. (Karthikeyan et al. 2005). Although copper is a vital element essential for the human body, still it becomes toxic if it is consumed beyond a certain limit. Excessive consumption of copper has short- and long-term influence on humans and may cause serious problems like jaundice, diarrhea, vomiting, nausea, liver and kidney damage, and DNA mutation (Al-Harahsheh et al. 2015). The permissible and maximum limit of copper in drinking water is 0.05 mg/L and 1.5 mg/L, respectively, as per the Bureau of Indian Standard (BIS 2012). EPA (2002) has recommended 1.3 mg/L of copper in drinking water, while WHO recommended 2.0 mg/L. It was also observed that heavy metal has high resistance against biodegradation (Sudha Rani et al. 2018).
There are many active methods used for the dilution of copper from aqueous solution, such as adsorption (Daneshyar et al. 2017), bio-filtration (Majumder et al. 2015), chemical precipitation (Gurmen et al. 2009), electrocoagulation (De Mello Ferreira et al. 2013), ion exchange (Veli and Pekey 2004), reverse osmosis (Sudilovskiy et al. 2008), and ultrafiltration (Molinari et al. 2004). However, these methods remove copper up to some extent but have some limitations like uneconomical, excessive use of reagents, sludge production, incomplete pollutant removal, and high energy requirements to drive the process. The adsorption process has more acceptance among all the other methods as it is more economical, easy to operate, quick and significantly less sludge production or any harmful by-products. These days, low-cost natural materials like silica mud, ash, and agricultural wastes were used for sorbent materials (Al-Harahsheh et al. 2015). Adsorption of copper from an aqueous solution can be achieved with different adsorbents like chemically activated sawdust (Sciban et al. 2006), anaerobic granular biomass (Hawari and Mulligan 2006), waste iron oxide (Huang et al. 2007), modified activated carbon using sodium acetate (Mugisidi et al. 2007), spent activated clay (Weng et al. 2007), modified coal fly ash (Pizarro et al. 2015; Attari et al. 2017), activated carbon (Daneshyar et al. 2017), carbon foam (Lee et al. 2017), spent-grain (Lee et al. 2017), and laterite soil (Sudha Rani et al. 2018). In many researches, activated carbon was widely accepted adsorbent for the removal of many pollutants along with copper with high removal efficiency, and this is due to its higher surface area. But in many methods, it becomes exceptionally lengthy and uneconomic to synthesize activated carbon. Fly ash emerged as a promising adsorbent, but since it has leaching effect, it requires numerous treatment. Some researchers reported that the fly ash contains high concentration of heavy metals, so there is chances of increasing other heavy metals to the treated water (Sharma and Kalra 2006). It has been found that numerous studies has been done to investigate whether soil and other minerals deposits can remove heavy metals from aqueous solution (Appel et al. 2008; Kul and Koyuncu 2010). Some researcher used laterite soil to remove copper from aqueous solution with 90% efficiency (Sudha Rani et al. 2018). The aforementioned studies suggest and inspire to use soil or mineral deposits to use as base material for adsorbent. The primary material used for the synthesis of adsorbent is Ganga sand, which is easily and abundantly available in the plain region of India and also at a low cost.
Response surface methodology (RSM) was used to get the optimal environments for adsorption parameters for the removal of copper. RSM is a statistical tool created using multivariate nonlinear analysis, which has been acknowledged broadly for the optimization of any adsorption process (Zhao et al. 2011; Xu et al. 2012). RSM is mainly used to signify the effect of individual parameters and to establish the relationship between these independent variables.
The main objective of this study was to remove copper from synthetic aqueous solution using adsorption process. To achieve the main objective, this work was divided into some parts, these are (1) the effects of important variables related to adsorption process on copper removal. (2) The optimum conditions were found for the selected variables in order to achieve the maximum copper removal efficiency. (3) The kinetics and isotherms were plotted to know the responsible mechanism for the removal of copper.
The experimental work of this research has been carried out in the environmental laboratory of National Institute of Technology-Patna (NIT-Patna), India, under the period of February 2019 to January 2020.
Materials and methods
Materials and instrument
The chemicals used in this work were of analytical grade and bought from Merck (India). Milli-Q water was used for the preparation of all kinds of reagents and solutions. The atomic absorption spectrophotometer (AAS) manufactured by Electronic Corporation of India Limited was used to analyze copper concentration in water. Other instruments like digital pH meter (Oakton pH 700), Orbital shaker, and hot air oven were used in this study and calibrated time to time. The base material for the adsorbent was Ganga sand, which was collected from the bank of river Ganga at Gandhi Ghat (Patna, India) with GPS coordinates 25°37′15″ N and 85°10′28″ S.
Activation of Ganga sand as an adsorbent
Appropriate amount of the Ganga sand was collected from the above-mentioned location and transported immediately to the Environmental Engineering Laboratory of N.I.T. Patna. The sand was washed with tap water to make it free from organic and other impurities. The washed sand was dried in the oven at 60 °C for 2 h to remove moisture. The dried sand was sieved through 125 micron sieve.
The activation of Ganga sand was done by mixing 14 N NaOH solution with dried sand (finer than 125 micron size) in the ratio of 4:3 by volume. The mixing was done continuously for 15 min. The obtained slurry mixture was dried in the oven for 24 h at a temperature of 105 °C. Then the dried mixture was crushed and sieved through 72 micron sieve. The sieved sand was washed with distilled water to remove any residue. Finally, the washed sand was dried in oven at 105 °C until it became free from moisture. The final obtained sand (dried and moisture free) was kept in a container and named as activated Ganga sand.
Preparation of synthetic aqueous solution
Synthetic aqueous solution of 100 ppm of copper was prepared, and determination of copper in aqueous solution was as per the Standard Method for the Examination of Water and Wastewater (APHA 2005). It was prepared by dissolving 0.100 gm of copper-turning in 12 ml of Nitric acid. When copper was dissolved entirely in acid, then it was diluted in a 1000 ml volumetric flask with Milli-Q water. One ml of this solution gives 100 µg of Cu2++ concentration. The solution of different concentrations of copper for analysis was prepared by diluting this stock solution. Synthetic aqueous solution was prepared from the stock solution that contains only Cu2++ to avoid the interaction of any other ions or cations. The standards of 0, 0.5, 1.0, 2.0, 5.0, 7.0, and 10 ppm copper concentration were prepared for the calibration curve.
Experimental methodology
The selected independent variables in this study were pH of the solution, dose of adsorbent, initial copper in the aqueous sample, and contact period at a fixed temperature of 25 ± 2 °C. The pH of the solution, contact time, initial copper concentration, and adsorbent dose were varied from 4 to 12, 20 to 180 min, 10 to 150 ppm, and 0.5 to 4 g/L, respectively. The preliminary analysis for each independent variable was investigated to know effect on the response variable (copper removal). A batch study in conical flask was done to perform the preliminary analysis. Three sets of preliminary test was done for each independent variable and reported the average values of results to ensure a minimum error in experimental readings. The obtained results were applied to statistical analysis to find the optimum conditions for independent variables. The experiments were performed at obtained optimum conditions to remove copper from aqueous solution. The Box–Behnken design (BBD) of response surface methodology (RSM) was used for optimization method. The statistical analysis was done with the Design expert 12.0 software. The pH of the solution was fixed using 1 N NaOH and 1 N HCl solution. All the experimental analyses were done at a constant temperature of 25 ± 2 °C. The percentage of copper adsorbed or removed through the adsorption process was calculated through the following equation
where Ci represents the initial concentration (ppm) of copper in an aqueous solution, while Cf represents the final residue concentration of copper after the adsorption process.
Experiment design and data analysis
While dealing with RSM problems, the relationships between variables and responses are unidentified, so an appropriate approximation between the factors and response is to be established. The rigorously used approximating functions are polynomials (Nair et al. 2014). The quadratic second-order equation as described below in Eq. 2 with interaction terms was used to establish relation between the variables and response. The pH of the solution, contact time, initial copper concentration, and adsorbent dose were varied from 4–12, 20–180 min, 10–100 ppm, and 0.5–4 g/l, respectively. Design expert 12.0 software (Stat Ease Inc. Minneapolis, 175, USA) was used to perform statistical analysis with the BBD model.
where Y, Xi, and Xj represent the predicted response and independent variables, respectively, βo, βi, βii, and βij are regression coefficients for intercept, linear, quadratic, and interaction coefficients, respectively, k is the number of variables studied.
Results and discussion
Effect of pH on copper adsorption
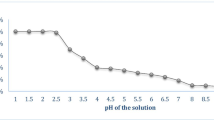
The behavior of pH on the adsorption process was determined under a fixed condition by performing preliminary test. The pH of solution was varied in the range of 2–13 with the help of NaOH and HCl solution, while keeping all other parameters constant. The other parameters like contact time, initial copper concentration and adsorbent dose were fixed to 120 min, 100 ppm, and 1 g/L, respectively. Then it was stirred in a shaker for a time period of 120 min. After it, sample was taken out and filtered on filter paper (Whatman No- 42). Then the filtered solution was analyzed on AAS at a wavelength of 324 nm for residue copper. The variation of pH for adsorbent is shown in Fig. 1a. The optimum pH of 7 was observed at which more than 98% copper was removed from the aqueous solution by this adsorbent. This shows that the neutral region of pH is the most favorable for the adsorption process in this work. It was also observed that the efficiency of the process was more in the basic region compared to the acidic region and this may be due to the reason that in an acidic medium, most of the ions get used in neutralizing themselves and very few sites have remained for the adsorption process.
Effect of contact time on copper adsorption
To determine the effect of contact period on the copper adsorption, the same procedure was followed as in the previous section with the only change being that here contact period was varied, keeping all other parameters constant. From the previous section, it was concluded that at a pH of 7, efficiency was highest, so here pH was fixed to 7. Based on experimental data, the graph is shown in Fig. 1b, and it was observed that a significant copper removal can be achieved at very less contact time of 15 min. But equilibrium time was achieved at 120 min after which only minor changes in the removal efficiency have been noted.
Effect of initial copper concentration on copper adsorption
The same procedure was followed here with Cu2++ as a variable. Here pH was fixed to 7 and contact time for 120 min. The effect of initial Cu2++ on this adsorbent can be observed from the graph shown in Fig. 1c. Up to a concentration around 100 ppm, it can be seen that good efficiency (up to 98%) was observed after that efficiency dropped to 90% at 150 ppm. So, here it can be noted that up to 100 ppm of Cu2++ the adsorption rate was quite high after which it started to fall.
Effect of adsorbent dose on copper adsorption
For this analysis, the adsorbent dose was varied, keeping other parameters constant as per the result of the above section, i.e., pH equals 7, contact time for 120 min and Cu2++ as 100 ppm. The adsorbent dose was varied from 0.5 to 4 gm/L. The maximum copper removal (more than 98%) was obtained at an adsorbent dose of 4 gm/L. But at a dose of 2 gm/L, decent (around 92%) efficiency was observed, which indicates that if the adsorbent considered from an economic point of view, we can go with a dose of 2 gm/L, but if we have to only deal with the efficiency of copper removal, then we would adopt 4 g/L dose of the adsorbent.
Optimization of the adsorption process
During preliminary analysis, the copper removal was optimized by varying only one parameter while keeping all other parameters fixed. For optimizing adsorption process, all the variables should be considered together by varying all the parameters in a single operation. To do so, a software named Design expert 12.0 was used. There was five option (minimum, maximum, within range, none, and target) provided by the mentioned software to set each variable and response for the optimization process. The pH, initial Cu2++, contact time, and adsorbent dose were taken as independent variables.
Since the synthesis of an adsorbent is a cost-effective process, it requires to take consideration of the adsorbent dose. The optimization was done based on two criteria. In the first criteria, the adsorbent dose was given priority which means there was a limit for the use of adsorbent. The desired goal (copper removal) was fixed at a maximum, adsorbent dose at a minimum, while pH, contact time, and initial Cu2++ were set to within range. The optimum conditions for pH, contact time, initial Cu2++, and adsorbent dose were achieved as 9.6, 142 min., 100 ppm and 1.704 g/L, respectively. Copper removal was found 83% at the obtained optimum condition.
While in second criteria, focus was on maximizing the copper removal irrespective of adsorbent dose which means adsorbent dose can go as high to get equilibrium of copper removal. In the first criteria, the only change was the adsorbent dose, which was set to maximum. The optimum values for pH, Contact time, initial Cu2 + and adsorbent time were 8.7, 150 min., 34 ppm and 3.5 g/L. The copper removal was found 99% at obtained optimum conditions which is higher than copper removal from first criteria.
In order to ensure the above-predicted result by the software, there were three confirmative experiments done in the laboratory whose average value came 80.3% and 97.7%, respectively, for both the condition.
Isotherm of the adsorption process
At equilibrium, adsorption isotherm established a relationship between adsorbate and adsorbent. The isotherm was carried out by performing Langmuir and Freundlich models. Langmuir model works with the assumption that if there is no interaction between the adsorbed molecules, then the monomolecular layer is formed (Aksu 2002). Equilibrium data were described using the Langmuir constant (Ka), and the maximum monolayer adsorption capacity Qm (mg/g) associated with the attraction of the binding sites (L/mg) was calculated using the Langmuir adsorption isotherm equation given as:
The above equation of the Langmuir model can be written in linear form as Eq. 4 given below:
where Ce is the equilibrium concentration (mg/L), qe the amount of solute (Cu2++) adsorbed per unit of adsorbent (mg/g), Qm monolayer adsorption capacity (mg/g), and ka is the adsorption intensity. The values of Qm and ka can be calculated by the help of slope and intercept of the Ce/qe Vs Ce plot as shown in Fig. 2a.
The adsorption process which occurs on a heterogeneous surface is explained through the Freundlich isotherm model. It deals with the interaction between the liquid and solid phase capacity at equilibrium, which is based on the multilayer adsorption. This isotherm assumes that the distribution of the adsorption sites is exponential with respect to adsorption’s heat (Kalavathy M. et al. 2009). The Freundlich isotherm model is explained through Eq. 5 given as:
The above equation can be written in linear form as Eq. 6 given below:
where Kf is the empirical constants related to bonding energy or as an indicator of adsorption capacity and 1/n measures the adsorption intensity.
Using Eq. 4 and 6, the graph has been drawn to study the Freundlich and Langmuir isotherms, which is shown in Fig. 2. Freundlich isotherm is best fitted with R2 = 0.9963. Langmuir Isotherm with R2 = 0.9927 is also acceptable. The constant of both the model is mentioned in Table 1. The monolayer adsorption capacity (Qm) derived from Langmuir Isotherm was found to be 80 mg/g, and this validates the acceptance of the adsorbent for the removal of copper.
Kinetic behavior of adsorption process
The kinetic behavior of the process, i.e., the sorption velocity of Cu2++ on the activated Ganga sand adsorbent, was analyzed through three different kinetic equations. These three equations have been discussed briefly in this section. The pseudo-first-order equation for the adsorption of liquid/solid system centered on solid capacity is given as (Balouch et al. 2013).
When Eq. 7 is integrated for the boundary condition (t = 0, qt = 0 and t = t, qt = qt), we get
where qe (mg/g) is the sum of adsorbate adsorbed on the adsorbent’s surface, qt (mg/g) is the total of adsorbate on the surface of the adsorbent for time t (min), and K1 is the equilibrium rate constant (min−1). Equation 8 was used for the analysis of pseudo-first-order kinetics, result of which was plotted and is shown in Fig. 3a. Using the slope of this plot, K1 and qe were determined and compared with the experimental data in Table 2.
A pseudo-second-order equation was also used to test the experimental data. The equation is given as (Ho and Mckaf 2000).
If Eq. 9 is integrated for the boundary condition (t = 0, qt = 0 and t = t, qt = qt,) and rearranged, then this equation forms as
where h is the initial sorption rate, i.e., h = K2qe2, and K2 is termed as rate constant (g/mg/min). Equation 10 was used for the analysis of pseudo-second-order kinetics, result of which was plotted and is shown in Fig. 3b. Using the slope of this plot, K2 and qe were determined and compared with the experimental data in Table 2.
The intraparticle diffusion model was used to identify the mass transfer steps in the Cu2++ ions onto the adsorbent (Hu et al. 2013). The intraparticle diffusion model was governed using the following equation (Karthikeyan et al. 2005).
where Kp (mg/g t1/2) is the rate constant for intraparticle diffusion and C is the constant of intercept. The intraparticle diffusion was plotted and is shown in Fig. 3c.
On the analysis of the three mentioned kinetics model, the nonlinear form of the pseudo-second-order model fitted the best for this adsorption process. The value of K2 and qe was calculated, and the value of this calculated qe (49 g/mg/min) was much nearer to the experimental value (48.82 g/mg/min). The coefficient of correlation (R2) of the pseudo-second-order model is 0.999. This value is very much close to 1, and it indicates that kinetic data followed the pseudo-second-order model, which suggests a chemisorption process (Reddad et al. 2002).
As per literature, if the regression of the intraparticle diffusion plot, i.e., qt Vs t1/2 is linear and passes through the origin, then the intraparticle diffusion will be the rate-controlling step (Svilović et al. 2010). But according to Fig. 3c, the plot was multilinear in this case, which indicates that the adsorption process was being affected by more than one process.
Statistical analysis by design expert 12.0 software
In order to test the model, statistical analysis techniques were used to evaluate the suitability of the model, experimental error, and significance of the terms in the model. In this work, it was done using software named Design Expert 12.0, which evaluates the RSM program. The independent variable which includes pH, contact period, initial Cu2++, and adsorbent dose were nominated as X1, X2, X3, and X4, respectively. The experiment range was determined from the result of preliminary analysis and reported in term of low, medium and high level. The low-level, medium- and high-level experimental range for X1, X2 and X3 was 4, 8, 12; 20, 100, 180; 10, 55, 100, and 0.5, 2.25, 4.0, respectively.
According to the Box–Behnken design (BBD), a total of 29 runs which includes five replicates at the central point, were performed and details of runs with predicted and observed experimental results of responses are presented in Table 3. The observed values fitted by using second-order polynomial, as mentioned in Eq. 2 to satisfy the model. The quadratic equation for copper removal in terms of coded factors is given by Eq. 12 as shown below:
Analysis of variance (ANOVA) was done to ensure the suitability of this second-order polynomial model. Fisher test was also performed to know the consequence of each parameter. Table 4 represents the ANOVA result along with the P value and the F value of each model terms for copper adsorption. If the P > F value becomes greater than 0.05, then the model term is considered as insignificant (Nair et al. 2014). So in Eq. 12, many terms got eliminated except the term to maintain the hierarchical order and reduced to Eq. 13 as given below:
ANOVA results are generally presented through several parameters such as “P > F” value, F value, R2 value, adjusted R2, predicted R2, lack of fit (LOF), adequate precision (AP) and coefficient of variance (CV)(Nair et al. 2014). The model behavior can be predicted according to the above-mentioned parameters. The model is considered as significant if the P > F value of the ANOVA test result is found to be less than 0.05 at a 5% confidence interval but if its value is more than 0.05, then the model gets eliminated. A model will be considered a failure if the LOF (P > F value) of the ANOVA test result is less than 0.05, i.e. for a significant model, LOF should be no significant (Kumar and Quaff 2020). The LOF value was found to be 0.0768 (not significant), which is acceptable as it is greater than 0.05.
The values of R2, adjusted R2 and predicted R2 decide the overall efficiency of the model. The value of R2, adjusted R2, and predicted R2 are 0.9455, 0.8910, and 0.7044, respectively. The value of R2 (0.9455) was close to 1, which indicates a good fit. The difference between the two R2 is 0.1866, which is less than 0.2, which is required for a good fit model. The predicted R2 is in reasonable agreement with adjusted R2.
Adequate precision (AP) is also a deciding term for the adequacy of any model. It is a measurement of the signal-to-noise ratio, and this ratio should be greater than 4, which is necessary for a good fit model (Kumar and Quaff 2020). The AP was found to be 14.54, indicating an adequate signal. The CV tells about the reproducibility of the model, and in this case, its value is 9.51%. Hence, it is acceptable (less than 10% is desirable). On the basis of ANOVA results, it is established that outcomes of the statistical analysis support the acceptance of the model and the model can be considered as a good fit model.
The normal plotting of residuals can also analyze the fitting of the model. A model is said to be well fitted if the said plot has a straight line with very less scattered data (Nair et al. 2014). The normal plot of the residuals as shown in Fig. 4 has a straight line with a few scattered, which indicates that the quadratic model derived in this model is well accepted. Figure 5 shows the comparisons between the experimental and predicted models for Cu2++ removal efficiency. From this plot, it is observed that a straight line with very little difference in the actual and predicted plot which also represent a well-fitted model.
Interaction effects of adsorption parameters
The influence of independent variables and their interaction on the response can be observed as a 3-D response surface plot. The adsorption capacities of the adsorbent at different conditions were shown through a 3-D response surface plot. Figure 6a, b, c and d shows the interaction effect of independent variables on copper removal. The copper removal was found minimum at a low and high level values of independent variable there was neither increasing nor decreasing trend at some specific points, and this confirms that this was the point of maximum copper removal.
Three-dimensional response surface: Interaction effect of a contact time and pH with a copper concentration of 55 ppm and the adsorbent dose of 2.25 g/L; b copper concentration and pH with contact period of 100 min and the adsorbent dose of 2.25 g/L; c adsorbent dose and pH with contact period of 100 min and initial copper concentration of 55 ppm; d adsorbent dose and copper concentration with pH 8 and contact period of 100 min
Conclusion
The preliminary analysis suggests that the amount of copper removal depends mostly on pH of the solution affect among the all variables. The pH in the neutral region slightly inclined toward the basic region is the most favorable region for the adsorption of the copper from the solution. This may be due to the reason that in an acidic medium, most of the ions get used in neutralizing themselves and very few sites have remained for the adsorption process. The rate of adsorption was found quite high up to 100 ppm concentration Cu2++ after which it started to fall due to the high load of the adsorbate. While the contact period was effective only in the initial period afterward, the minimal effect of contact time on copper removal was observed. ANOVA results indicated that the model is significant and well fitted. The variables (pH, copper concentration, contact period, and adsorbent dose) were successfully optimized using RSM, and the optimum predicted value for copper adsorption was found to be 97.7%. The adsorption capacity at the optimization level of this process was found to be 9.7 mg/g, which a significant figure for the adsorption of copper. Isotherm and kinetic studies of the adsorption process done in this research strongly suggest the use of activated Ganga sand for the adsorption of copper ion from aqueous solution. The adsorption process followed both Freundlich and Langmuir isotherms. Kinetics of the process was governed by pseudo-second-order kinetics. It can be concluded that the activated Ganga sand may be used as an effective adsorbent and may be successfully applied for the removal of copper from aqueous solution.
References
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem 38(1):89–99. https://doi.org/10.1016/S0032-9592(02)00051-1
Al-Harahsheh MS, Al Zboon K, Al-Makhadmeh L et al (2015) Fly ash based geopolymer for heavy metal removal: a case study on copper removal. J Environ Chem Eng 3(3):1669–1677. https://doi.org/10.1016/j.jece.2015.06.005
APHA (2005) Standard methods for the examination of water and wastewater. 21st Edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Appel C, Ma LQ, Rhue RD, Reve W (2008) Sequential sorption of lead and cadmium in three tropical soils. Environ Pollut 155(1):132–140. https://doi.org/10.1016/j.envpol.2007.10.026
Attari M, Bukhari SS, Kazemian H, Rohani S (2017) A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J Environ Chem Eng 5(1):391–399. https://doi.org/10.1016/j.jece.2016.12.014
Balouch A, Kolachi M, Talpur FN et al (2013) Sorption kinetics, isotherm and thermodynamic modeling of defluoridation of ground water using natural adsorbents. Am J Anal Chem 4:221–228. https://doi.org/10.4236/ajac.2013.45028
BIS (Bureau of Indian Standards) (2012) Specification for drinking water IS 10500: 2012. New Delhi, India
Daneshyar A, Ghaedi M, Sabzehmeidani MM (2017) H2S adsorption onto Cu-Zn–Ni nanoparticles loaded activated carbon and Ni-Co nanoparticles loaded γ-Al2O3: optimization and adsorption isotherms. J Colloid Interf Sci 490:553–561. https://doi.org/10.1016/j.jcis.2016.11.068
De Mello FA, Marchesiello M, Thivel PX (2013) Removal of copper, zinc and nickel present in natural water containing Ca2+ and HCO3- ions by electrocoagulation. Sep Purif Technol 107:109–117. https://doi.org/10.1016/j.seppur.2013.01.016
Gurmen S, Ebin B, Stopi S, Friedrich B (2009) Nanocrystalline spherical iron-nickel (Fe-Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR). J Alloys Compd 480(1):529–533. https://doi.org/10.1016/j.jallcom.2009.01.094
Hawari AH, Mulligan CN (2006) Biosorption of lead(II), cadmium(II), copper(II) and nickel(II) by anaerobic granular biomass. Bioresour Technol 97(4):692–700. https://doi.org/10.1016/j.biortech.2005.03.033
Ho KS, Mckaf G (2000) The kinetics of sorption of basic dyes fi-om aqueous solution by sphagnum moss peat. Can J Chem Eng 76(4):822–827. https://doi.org/10.1002/cjce.5450760419
Hu XJ, Liu YG, Wang H et al (2013) Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep Purif Technol 108:189–195. https://doi.org/10.1016/j.seppur.2013.02.011
Huang YH, Hsueh CL, Cheng HP et al (2007) Thermodynamics and kinetics of adsorption of Cu(II) onto waste iron oxide. J Hazard Mater 144(1–2):406–411. https://doi.org/10.1016/j.jhazmat.2006.10.061
Kalavathy MH, Regupathi I, Pillai MG, Miranda LR (2009) Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Coll Surf B Biointerf 70(1):35–45. https://doi.org/10.1016/j.colsurfb.2008.12.007
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124(1–3):192–199. https://doi.org/10.1016/j.jhazmat.2005.05.003
Kul AR, Koyuncu H (2010) Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: kinetic, equilibrium and thermodynamic study. J Hazard Mater 179:332–339. https://doi.org/10.1016/j.jhazmat.2010.03.009
Kumar S, Quaff AR (2020) Treatment of domestic wastewater containing phosphate using water treatment sludge through UASB–clariflocculator integrated system. Environ Dev Sustain 22:4537–4550. https://doi.org/10.1007/s10668-019-00396-3
Lee CG, Lee S, Park JA et al (2017) Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 166:203–211. https://doi.org/10.1016/j.chemosphere.2016.09.093
Majumder S, Gangadhar G, Raghuvanshi S, Gupta S (2015) Biofilter column for removal of divalent copper from aqueous solutions: performance evaluation and kinetic modeling. J Water Process Eng 6:136–143. https://doi.org/10.1016/j.jwpe.2015.03.008
Molinari R, Argurio P, Poerio T (2004) Comparison of polyethylenimine, polyacrylic acid and poly(dimethylamine-co-epichlorohydrin-co-ethylenediamine) in Cu2+ removal from wastewaters by polymer-assisted ultrafiltration. Desalination 162:217–228. https://doi.org/10.1016/S0011-9164(04)00045-1
Mugisidi D, Ranaldo A, Soedarsono JW, Hikam M (2007) Modification of activated carbon using sodium acetate and its regeneration using sodium hydroxide for the adsorption of copper from aqueous solution. Carbon N Y 45(5):1081–1084. https://doi.org/10.1016/j.carbon.2006.12.009
Nair AT, Makwana AR, Ahammed MM (2014) The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: a review. Water Sci Technol 69:464–478. https://doi.org/10.2166/wst.2013.733
Pizarro J, Castillo X, Jara S et al (2015) Adsorption of Cu2+ on coal fly ash modified with functionalized mesoporous silica. Fuel 156:96–102. https://doi.org/10.1016/j.fuel.2015.04.030
Reddad Z, Gerente C, Andres Y, Cloirec PLE (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36(9):2067–2073. https://doi.org/10.1021/es0102989
Sciban M, Klasnja M, Skrbic B (2006) Modified hardwood sawdust as adsorbent of heavy metal ions from water. Wood Sci Technol. https://doi.org/10.1007/s00226-005-0061-6
Sharma SK, Kalra N (2006) Effect of flyash incorporation on soil properties and productivity of crops: a review aspects of flyash for its application in agriculture. J Sci Ind Res (India) 65:383–390
Sudha Rani K, Srinivas B, Gourunaidu K, Ramesh KV (2018) Removal of copper by adsorption on treated laterite. Mater Today Proc 5(1):463–469. https://doi.org/10.1016/j.matpr.2017.11.106
Sudilovskiy PS, Kagramanov GG, Kolesnikov VA (2008) Use of RO and NF for treatment of copper containing wastewaters in combination with flotation. Desalination. https://doi.org/10.1016/j.desal.2007.01.076
Svilović S, Rušić D, Bašić A (2010) Investigations of different kinetic models of copper ions sorption on zeolite 13X. Desalination 259(1–3):71–75. https://doi.org/10.1016/j.desal.2010.04.033
Veli S, Pekey B (2004) Removal of copper from aqueous solution by ion exchange resins. Fresenius Environ Bull 13(3):244–250
Weng CH, Tsai CZ, Chu SH, Sharma YC (2007) Adsorption characteristics of copper(II) onto spent activated clay. Sep Purif Technol 54(2):187–197. https://doi.org/10.1016/j.seppur.2006.09.009
Xu J, Wang L, Zhu Y (2012) Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 28:8418–8425. https://doi.org/10.1021/la301476p
Zhao G, Li J, Wang X (2011) Kinetic and thermodynamic study of 1-naphthol adsorption from aqueous solution to sulfonated graphene nanosheets. Chem Eng J 173(1):185–190. https://doi.org/10.1016/j.cej.2011.07.072
Acknowledgements
The authors wish to express their gratitude to N.I.T. Patna, Bihar (India), for its financial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Editorial responsibility: Binbin Huang.
Rights and permissions
About this article
Cite this article
Mritunjay, Quaff, A.R. Adsorption of copper on activated Ganga sand from aqueous solution: kinetics, isotherm, and optimization. Int. J. Environ. Sci. Technol. 19, 9679–9690 (2022). https://doi.org/10.1007/s13762-021-03651-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03651-1