Abstract
Aquatic ecosystem is greatly affected by metal pollution and global climate change. Mercury (Hg) is one of the most common metal pollutants that pose harmful effects to organisms. In this study, we evaluated the effects of water temperature and Hg2+ on hematological parameters, such as red blood cells (RBCs), hematocrit (Ht) and hemoglobin (Hb) and some indexes involved in energy metabolism, including hexokinase (HK), pyruvate kinase (PK), malate dehydrogenase (MDH), lactate dehydrogenase (LDH), glucose (GLU), electron transport system (ETS) and Na–K-ATPase in grass carp, Ctenopharyngodon idella. Fish (45.37 ± 3.58 g) were acclimated to 15, 20, 25, 30 or 35 °C and co-exposed to 0.000 or 0.039 mg/L Hg2+ for 4 weeks. Three-way ANOVA revealed that all variables were significantly affected by water temperature, Hg2+ concentration, exposure time and their interactions, except the RBCs value corresponding Hg*Time condition. Based on the significant changes of hematological parameters in Hg2+-free groups, the best health status in fish was approximately at 25 °C, appreciating physiological dysregulation in fish under too low (15 °C)/high (35 °C) temperature, especially at 35 °C. Although our data provide evidences that increased temperatures can potentiate Hg2+ toxicity, the combined effects of temperature and metals on aquatic organisms are complex and unpredictable, so we should not ignore the role of environmental factors (such as temperature) while evaluating the harmful effects of metals on aquatic ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, temperature rise related to climate change caused the structural and functional changes of aquatic ecosystem (Val et al. 2016), that led to the decrease in dissolved oxygen, the acceleration of eutrophication and the decrease in aquatic biodiversity. Besides, the threat of environmental pollutants to aquatic organisms was increasingly serious, so the coupling stress of temperature fluctuation and water pollution on aquatic organisms has attracted more and more attention (Balbus et al. 2013; Lee et al. 2014; Abdel-Tawwab and Wafeek 2017). As far as the harmful effects on water ecosystem, metal mercury (Hg) is one of the most studied and most concerned pollutants and has been considered an environmental stressor (Li et al. 2014). In China, environmental concentrations of mercury were ranging from 0.031 to 5.7 μg/L in the natural rivers (Biscere et al. 2015). Once the Hg enters the aquatic ecosystem through various ways, it readily bioaccumulates in fish tissue, and exerts various deleterious effects on fish health, and eventually affects human health through the food chain (Berntssen et al. 2004). Additionally, water temperature can modulate the toxicity of Hg in the aquatic system (Ando et al. 2011; Maulvault et al. 2016; Sumner et al. 2019; Waheed et al. 2020). Therefore, it is necessary to study the combined effects of temperature and mercury exposure to understand the physiological mechanism of fish under multiple stressors.
Biomarkers could be used as tools to evaluate physiological changes of organisms, such as hematological indicators and enzymes related to energy metabolism (Lermen et al. 2004; Li et al. 2011b; Abdel-Tawwab and Wafeek 2017). Some researchers confirmed that hematological parameters could be used as indicators of fish health status under endogenous or exogenous stressors (Li et al. 2011a; Perveen et al. 2019). Metal exposure increases the energy requirements of fish to meet the needs of detoxification and repair process, so the liver must increase glycogen/glucose transformation (Carvalho and Fernandes 2008, 2019). In anaerobic glucose metabolism, some enzymes play important roles, such as hexokinase (HK), pyruvate kinase (PK), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH). Moreover, ATPase, also known as adenosine triphosphatase, and electron transport system (ETS) are considered to be important indexes reflecting the energy metabolism of organism (Li et al. 2015; Wen et al. 2017; Fonseca et al. 2019). Although these parameters are often used as interesting biomarkers to evaluate the impact of multiple stressors (Li et al. 2011c), the studies related to the impact of climate change coupled with metal stress on fish is still limited.
Water temperature and environmental contaminants are two important environmental factors in aquatic ecosystem, because their fluctuation can affect the physiological-biochemical functions of aquatic organisms (such as fish) (Sokolova and Lannig 2008; Abdel-Tawwab and Wafeek 2014; Philippe et al. 2018). The rising temperature leads to reduce dissolved oxygen availability to aquatic organisms, which makes water flow rate across the gills increasing to meet the metabolic demand, resulting into increased toxicant flow across the gills, and thus become prone to increased toxicity (Kumar et al. 2019; Waheed et al. 2020). Besides this, the rising temperature can influence the toxicity of metals through degradation and volatilization rates, affecting their absorption and desorption processes and bioaccumulation rates in exposed organisms (Kuz’mina and Ushakova 2013; Kumar et al. 2019). The increasing temperature enhances the toxicity of contaminants in fish through regulation of the metabolic rate and endocrine process (Sokolova and Lannig 2008; Philippe et al. 2018), such as cadmium toxicity in Danio rerio (Park et al. 2020), mercury toxicity in Oreochromis niloticus (Waheed et al. 2020), copper toxicity in Prochilodus lineatus (Carvalho and Fernandes 2019), trichlorfon toxicity in Cyprinus carpio (Woo and Chung 2020), arsenic toxicity in Pangasianodon hypophthalmus (Kumar et al. 2019) and so on. Moreover, temperature and contaminations exposure in fish may generate reactive oxygen species (ROS), such as hydrogen peroxide, superoxide and the hydroxyl radical (Sappal et al. 2015a; Kumar et al. 2017; Park et al. 2020; Woo and Chung 2020). The physiological responses of animals to stress can lead to imbalance of internal environment and increase of energy consumption, which can be evaluated by blood indexes and energy metabolism parameters.
In consideration of the above, we hypothesize that (I) exposure of fish to increasing temperature or Hg2+ stress may disrupt hematological indicators and impair energy metabolism by regulating the activities of related enzymes; and (II) combined stress of them may aggravate these harmful effects. In order to confirm these hypothesis, grass carp (Ctenopharyngodon idella), as a model of freshwater fish, was exposed to increasing temperature and different levels of Hg2+ in an experimental system, the endpoints of physiological changes were evaluated after 7, 14 and 28 days of exposure, respectively. This study is the first approach to determine the combined effects of different temperatures and Hg2+ exposure on hematology and energy metabolism in grass carp.
Materials and methods
Chemicals and test fish
Commercial HgCl2 from Sigma-Aldrich Chemical Co. (USA) was dissolved in deionized water, and supplied as toxicant to grass carp. The juvenile grass carp Ctenopharyngodon idella (45.37 ± 3.58 g,16.80 ± 1.21 cm) procured from a local hatchery (Wuhan, China), was carefully transported to glass aquaria filled with dechlorinated water. Continuous aeration and maintenance of ambient temperature (25 ± 1 °C) were ensured using aerators and filters and heaters. The fish were fed with commercially available fish food (Tongwei, China). Feed remains and excretory wastes were siphoned off regularly to avoid contaminant-related stress in fish.
Experimental design
Fish were maintained for 2 weeks at 25 ± 1 °C and then were divided into five groups with water temperature 15, 20, 25, 30 and 35 °C for acclimating 1 month. The water temperature was kept constant by the automatic temperature control system (Haisheng, China). Then each group was divided into two subgroups, one was exposed to 0.039 mg/L Hg2+ (based on the pre-experiment, 96 h-LC50 of HgCl2 to juvenile grass fish is 0.39 mg/L), and the other group was Hg-free used as the control. During the experimental period, 50% of test solution was changed daily to maintain the same temperature and corresponding concentration of Hg2+. Each test condition was repeated by triplicate and 15 fish was stocked in one aquarium. Experimental period lasted 4 weeks. On the n 7th, 14th and 28th day, the blood and liver tissue were collected from three fish of each aquarium randomly, blood samples were analyzed immediately, while the liver tissues were immediately frozen and stored at − 80 °C, and were used for further studies. All procedures and animal handling were in accordance with the guidelines: OECD Guideline for Testing of Chemicals, No.204: “Fish, Prolonged Toxicity Test: 14-day Study” and No.215: “Fish, Juvenile Growth Test”. And the study was approved by the animal ethics committee of Shandong University.
Hematological parameters
During the process of blood collection, MS-222 (0.1%, 3-aminobenzoic acid ethyl ester methanesulfonate, CAS:886-86-2) was used to anesthetize the fish. 5 μl heparin (10 μg/ml) was used for capillary collection from the caudal vein, and the related hematological parameters (RBCs-red blood cells, Ht-hematocrit and Hb-hemoglobin) were measured by the methods of Abdel-Tawwab and Wafeek (2017). RBCs were counted under the light microscope using a haemocytometer. In order to determine the Ht value, the blood was transferred to draw-in microhematocrit tubes and centrifuged for 5 min at 10,000 g. Hb levels were determined colorimetrically by measuring the formation of cyanomethemoglobin.
Energy metabolism indicators measurement
Frozen liver tissues were thawed in ice, homogenized in ice-cold 0.86% physiological saline with 10× volume, and then centrifuged to obtain the supernatant (3500 rpm, 4 °C, 10 min) for further measurement. The activities of HK, PK, MDH, LDH and Na–K-ATPase were analyzed by using the detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), as well as the contents of glucose (GLU). The ETS activity was measured according to the previous method (Wen et al. 2017). In brief, the samples were homogenized in homogenizing buffer (0.1 M Tris–HCl). After centrifugation for 10 min (4 °C, 3000 g), 100 μl supernatant from each extract was added into 300 μl substrate solution. The reaction was started by p-iodonitrotetrazolium to proceed at 20 °C for 10 min and then stopped by quench solution. The resulting increase in absorbance was measured at 490 nm. The amount of formazan formed was calculated using e = 15,900/M cm. The formation of 2 mmol formazan equals the consumption of 1 mmol O2 in the ETS.
Data statistical analyses
All values were expressed as mean ± standard deviation (SD). Three-way analysis of variance (3-way ANOVA, including temperature, mercury concentration, time and interaction between them) followed by Tukey multiple comparisons test was employed to calculate significant differences of treatment groups compared to controls, by using SPSS version 22.0 software (IBM SPSS Statistics, Chicago, IL, USA). As the key factor of individual variations, the important parameters are defined by principal component analysis (PCA), by using Statistic 6.0 (StatSoft, Tulsa, OK, USA). Besides, integrated biomarker response (IBR) was calculated based on methods by Broeg and Lehtonen (2006) and presented in site star plots.
Results and discussion
Effects on the hematological parameters
The hematological parameters were measured in fish under various experimental stress (Table 1). Based on our results, the values of RBCs, Ht and Hb increased significantly with the temperature rising to 25 °C, and a downward trend was shown subsequently. Also, the hematological parameters were higher in Hg2+-free fish than those in Hg2+-exposed one and almost all the hematological indexes of fish under 15 °C and 35 °C showed obvious inhibition, compared to the 25C group. Three-way ANOVA indicated that all the hematological parameters were significantly influenced by temperature, Hg2+ exposure, experimental time and their interactions, except the RBCs value corresponding Hg*Time condition (Table 2).
Because a close relationship between fish and water environment is present, the hematological and biochemical variables are often used as useful biomarkers to understand the health status and pathological effects of fish (Borges et al. 2007; Li et al. 2011a; Perveen et al. 2019). The measured hematological parameters were higher in Hg2+-free fish, indicating the health status was better in Hg2+-free fish than that in Hg2+-exposed ones. Moreover, the normal metabolic activities of fish depend upon ambient temperature of the environment, but alteration in the temperature beyond certain limit induces physiological disturbances and eventually toxicity to the fish (Abdel-Tawwab and Wafeek 2017; Kumar et al. 2018), so all the hematological indexes of fish under 15 °C and 35 °C showed obvious inhibition. Exposure to metals can lead to hemodilution or changes in blood concentration permeability in fish, which is an important indicator of anemia (Talas and Gulhan 2009). Consistent with other studies (Abdel-Tawwab and Wafeek 2017; Carvalho and Fernandes 2019), the hematological indexes decreased significantly in fish under mercury exposure and/or extreme temperature, which showed a serious physiological stress. In teleost, the kidney and spleen are the main organs that produce RBCs (Kori-Siakpere and Ubogu 2008). In this study, the decrease in RBCs value may be caused by the damage of kidney in fish under experimental stress. Other studies have also shown that metal exposure (such as cadmium and copper, etc.) can damage mature erythrocytes, leading to a decrease in RBCs values (Adhikari et al. 2004; Carvalho and Fernandes 2006). Ht and Hb are also important hematological parameters, which could be used to reflect the influence of renal function, as head kidney is the main hematopoietic organ (Arnaudova et al. 2008; Li et al. 2011a). In our study, the decrease of Ht and Hb values may be caused by the dysfunction of hemodilution and osmoregulation, as well as blood loss. However, some studies have shown that temperature change has no significant influences on the values of Ht and Hb of fish (Lermen et al. 2004), and other reports have found that only Ht has a negative correlation with temperature rise, while RBCs and Hb have no significant alteration in fish (Carvalho and Fernandes 2006). Therefore, although the hematological parameters are sensitive, further studies need to be carried out before they are used as a biomarker for monitoring environmental pollution.
Effects on the energy metabolism index
In our study, a series of parameters related to energy metabolism were determined in all fish, including the activities of enzymes (HK, PK, MDH and LDH) related to anaerobic glucose pathway, as well as GLU content, activities of Na–K-ATPase and ETS in fish liver (Table 3). And all the measured parameters were significantly influenced by temperature, Hg2+ exposure, experimental time and their interactions, after statistic calculation with three-way ANOVA. Besides the activities of Na–K-ATPase and ETS, those values of all enzymes (HK, PK, MDH and LDH) involved in the anaerobic glucose pathway increased along with the rising temperature up to 35 °C, and the higher values were observed in Hg2+-exposed fish groups. Although there was no significant increase in hepatic GLU in fish from 15 to 25 °C, the highest value of hepatic GLU was found in Hg2+-exposed fish groups at 35 °C.
In this study, the content of hepatic GLU and the activities of enzymes involved in anaerobic glucose pathway were significantly affected, which possibly because the hepatic glycogen/glucose were mobilized to meet the excessive energy demand for detoxification and repair processes under environmental stress (Fonseca et al. 2019). In glycolytic pathway, the hexokinase (HK) is located at the beginning of glycolysis sequence, while the pyruvate kinase (PK) plays a role in the terminal sequence (Carvalho and Fernandes 2008). Together with the available results (Abdel-Tawwab and Wafeek 2017; Fonseca et al. 2019), our results showed that the activities of HK and PK increased with the increase in temperature, indicating that the ability of glycolysis and activation of anaerobic metabolism pathway of grass carp increased under environmental stress, which would lead to the accumulation of lactic acid in tissue. Some researcher reported that cadmium exposure led to the decrease of PK activity in fish, which may be due to the reduction in glycolysis ability caused by excessive cadmium accumulation in fish liver(Abdel-Tawwab and Wafeek 2014). In addition, other studies showed that copper and zinc inhibited PK activity in bass, suggesting that these metals were involved in the direct competition of essential bivalent cations for binding sites of related proteins, leading to the change of enzyme conformation (Isani et al. 1994).
Under normal conditions, the energy metabolism of fish requires oxygen to avoid excessive accumulation of metabolites (such as lactic acid). In this study, the anaerobic metabolism in grass carp was increased under environmental stress, which led to need more energy to repair and maintain the destruction of internal environment balance (Lermen et al. 2004; Wen et al. 2017). The main function of LDH is to transform lactate into pyruvate, which is a necessary step to produce cell energy (Diamantino et al. 2001). The reversible reaction of oxidative dehydrogenation of malic acid to oxaloacetic acid catalyzed by MDH is one of the important enzymes in the tricarboxylic acid cycle (TAC), which plays an important role in the complete oxidation or mutual transformation of nutrients in vivo (Monteiro et al. 1998). Therefore, under the environmental stress, the energy of cells needs to be satisfied by anaerobic oxidation, so LDH and MDH activities are higher.
In the present study, the enhanced hepatic GLU was determined, which was directly related to gluconeogenesis (Fonseca et al. 2019; Kumar et al. 2019). Due to mercury exposure, metabolic demands caused by impaired insulin secretion may increase, leading to hypoxia, so as to maximize the use of stored carbohydrates (Navas-Acien et al. 2006).
The increase in environmental stress may affect the mitochondria and mitochondrial enzyme system in fish. Since ETS activity value can be used to estimate metabolic activity, the increase in ETS activity reflects the biogenesis, density increase and biochemical differentiation of mitochondria (Stackley et al. 2011; Simcic et al. 2015). Many studies have shown that metal stress can affect the mitochondrial electron transport chain system in fish (Sappal et al. 2015a, b, 2016). In this experiment, the combined effect of mercury and temperature led to the increase in ETS activity in fish, which is related to increased energy demand, while ATP is the direct energy donor, and the change of ATPase led to the impact of energy supply in fish under environmental stress (Oruc et al. 2002; Agrahari and Gopal 2008). Na, K-ATPase is responsible for establishing the electrochemical gradients of Na+ and K+ on the plasma membrane, and is considered as a potential biomarker of environmental stress (Staurnes et al. 1994; Akhtar et al. 2013). In this study, the increase in activity can be partly explained by the increase in the frequency of action potential generated by the passive ion flow through the ion channel. Thus the changes of all energy metabolism related indexes indicate that the normal energy metabolism of fish is seriously affected by the combined effect of temperature and mercury.
Chemometrics
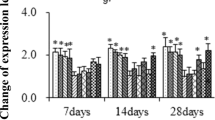
Based on bilinear decomposition of the detective data, the multivariate data are transformed into a new data set by principal component analysis (PCA). The new variables in the data set are orthogonal and have the largest interpretation. Corresponding to the first (50.61%) and the second principal component (20.64%), all indicators in this study were distinguished on the diagram, as well as water temperature, Hg2+ exposure and experimental time (Fig. 1). Additional, their correlation was confirmed and quantified by Spearman test (Table 4). According to the results of statistical analysis, the measured indexes were divided into two parts, some of which were negatively correlated with experimental stress, such as RBCs, Hb and Ht, while other parameters were positively correlated with physiological stress induced by experimental condition. In our study, IBR index was used to compare the effects of different treatments on fish liver, which could describe the overall stress responses of organisms by combining different parameters. Obviously, under the same experimental status of other factors, the increase in temperature enhanced the physiological stress of mercury on fish, and the largest IBR value appeared in Hg2+-exposed fish group under 35 °C, meaning the most serious stress (Fig. 2).
Ordination diagram of PCA of experimental factors (Temp., time, Hg) and the measured parameters in fish of tested groups. Temp.—experimental temperatures, Hg—with/without Hg2+ exposure, Time—experimental time, RBCs—red blood cells, Ht—hematocrit, Hb—hemoglobin, HK—hexokinase, PK—pyruvate kinase, MDH—malate dehydrogenase, LDH—lactate dehydrogenase, GLU—glucose, ETS—electron transport system
A star plot of all tested groups according to the IBR values. 15C—Hg2+-free group at 15 °C, 15E—Hg2+group at 15 °C, 20C—Hg2+-free group at 20 °C, 20E—Hg2+group at 20 °C, 25C—Hg2+-free group at 25 °C, 25E—Hg2+group at 15 °C, 30C—Hg2+-free group at 30 °C, 30E—Hg2+group at 30 °C, 35C—Hg2+-free group at 35 °C, 35E—Hg2+group at 35 °C
Conclusion
Overall, physiological alteration in hematology and energy metabolism in grass carp occurred in Hg2+-exposed and Hg2+-free group indicated the influences of the fluctuations in water temperature. The overall inhibition in hematological parameters and the activities involved in anaerobic glucose pathway in Hg2+-exposed fish at 35 °C showed a disturbance of internal circulation and insufficient energy supply through glucose metabolism, which may be caused by the enhanced toxic effects of Hg2+. Therefore, the present study shows that under the background of temperature rise caused by global climate change, waterborne mercury pollution could cause more unpredictable interference to freshwater ecosystem, and the impact mechanism would be more complex. The data obtained in this study can be used as potential biomarkers in the aquatic ecotoxicological studies of temperature change coupled with mercury pollution to develop suitable monitoring and early warning plans.
References
Abdel-Tawwab M, Wafeek M (2014) Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein-cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J Therm Biol 45:157–162
Abdel-Tawwab M, Wafeek M (2017) Fluctuations in water temperature affected waterborne cadmium toxicity: hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 477:106–111
Adhikari S, Sarkar B, Chatterjee A et al (2004) Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol Environ Saf 58(2):220–226
Agrahari S, Gopal K (2008) Inhibition of Na + -K + -ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic Biochem Physiol 92(2):57–60
Akhtar MS, Pal AK, Sahu NP et al (2013) Thermal tolerance, oxygen consumption and haemato-biochemical variables of Tor putitora juveniles acclimated to five temperatures. Fish Physiol Biochem 39(6):1387–1398
Ando M, Seoka M, Mukai Y et al (2011) Effect of water temperature on the feeding activity and the resultant mercury levels in the muscle of cultured bluefin tuna Thunnus orientalis (Temminck and Schlegel). Aquacult Res 42(4):516–524
Arnaudova D, Arnaudov A, Tomova E (2008) Selected hematological indices of freshwater fish from Studen Kladenetsh Reservoir. Bulg J Agric Sci 14(2):244–250
Balbus JM, Boxall AB, Fenske RA et al (2013) Implications of global climate change for the assessment and management of human health risks of chemicals in the natural environment. Environ Toxicol Chem 32(1):62–78
Berntssen MHG, Hylland K, Julshamn K et al (2004) Maximum limits of organic and inorganic mercury in fish feed. Aquacult Nutr 10(2):83–97
Biscere T, Rodolfo-Metalpa R, Lorrain A et al (2015) Responses of two scleractinian corals to cobalt pollution and ocean acidification. PLoS ONE 10(4):e0122898
Borges A, Scotti LV, Siqueira DR et al (2007) Changes in hematological and serum biochemical values in jundia Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere 69(6):920–926
Broeg K, Lehtonen KK (2006) Indices for the assessment of environmental pollution of the Baltic Sea coasts: integrated assessment of a multi-biomarker approach. Mar Pollut Bull 53(8–9):508–522
Carvalho CS, Fernandes MN (2006) Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture 251(1):109–117
Carvalho CD, Fernandes MN (2008) Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp Biochem Phys A 151(3):437–442
Carvalho CD, Fernandes MN (2019) Effects of copper toxicity at different pH and temperatures on the in vitro enzyme activity in blood and liver of fish, Prochilodus lineatus. Mol Biol Rep 46(5):4933–4942
Diamantino TC, Almeida E, Soares AMVM et al (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45(4–5):553–560
Fonseca JD, Marangoni LFD, Marques JA et al (2019) Energy metabolism enzymes inhibition by the combined effects of increasing temperature and copper exposure in the coral Mussismilia harttii. Chemosphere 236:124420
Isani G, Cattani O, Carpene E et al (1994) Kinetic-properties of liver and muscle pyruvate-kinase of a marine teleost, sea bass (Dicentrarchus-Labrax L). Comp Biochem Phys B 107(4):617–624
Kori-Siakpere O, Ubogu EO (2008) Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp (Osteichthyes: Clariidae). Afr J Biotechnol 7(12):2068–2073
Kumar N, Krishnani KK, Meena KK et al (2017) Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171:265–274
Kumar N, Krishnani KK, Brahmane MP et al (2018) Temperature induces lead toxicity in Pangasius hypophthalmus: an acute test, antioxidative status and cellular metabolic stress. Int J Environ Sci Te 15(1):57–68
Kumar N, Gupta SK, Bhushan S et al (2019) Impacts of acute toxicity of arsenic (III) alone and with high temperature on stress biomarkers, immunological status and cellular metabolism in fish. Aquat Toxicol 214:105233
Kuz’mina VV, Ushakova NV (2013) Influence of temperature and pH on the effects of zinc and copper on proteolytic activities of the intestinal mucosa of planktivorous and benthophagous fishes and their potential preys. Toxicol Environ Chem 95(1):150–162
Lee S, Ji K, Choi K (2014) Effects of water temperature on perchlorate toxicity to the thyroid and reproductive system of Oryzias latipes. Ecotoxicol Environ Saf 108:311–317
Lermen CL, Lappe R, Crestani M et al (2004) Effect of different temperature regimes on metabolic and blood parameters of silver catfish Rhamdia quelen. Aquaculture 239(1–4):497–507
Li ZH, Velisek J, Grabic R et al (2011a) Use of hematological and plasma biochemical parameters to assess the chronic effects of a fungicide propiconazole on a freshwater teleost. Chemosphere 83(4):572–578
Li ZH, Zlabek V, Turek J et al (2011b) Evaluating environmental impact of STPs situated on streams in the Czech Republic: an integrated approach to biomonitoring the aquatic environment. Water Res 45(3):1403–1413
Li ZH, Zlabek V, Velisek J et al (2011c) Antioxidant responses and plasma biochemical characteristics in the freshwater rainbow trout, Oncorhynchus mykiss, after acute exposure to the fungicide propiconazole. Czech J Anim Sci 56(2):61–69
Li ZH, Chen L, Wu YH et al (2014) Effects of mercury on oxidative stress and gene expression of potential biomarkers in larvae of the Chinese rare minnow Gobiocypris Rarus. Arch Environ Contam Toxicol 67(2):245–251
Li ZH, Li P, Shi ZC (2015) Chronic exposure to Tributyltin induces brain functional damage in juvenile common carp (Cyprinus carpio). PLoS ONE 10(4):e0123091
Maulvault AL, Custodio A, Anacleto P et al (2016) Bioaccumulation and elimination of mercury in juvenile seabass (Dicentrarchus labrax) in a warmer environment. Environ Res 149:77–85
Monteiro MD, Schwantes MLB, Schwantes AR et al (1998) Thermal stability of soluble malate dehydrogenase isozymes of subtropical fish belonging to the orders Characiformes, Siluriformes and Perciformes. Genet Mol Biol 21(2):191–199
Navas-Acien A, Silbergeld EK, Streeter RA et al (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Perspect 114(5):641–648
Oruc EO, Uner N, Tamer L (2002) Comparison of Na+-K+-ATPase activities and malondialdehyde contents in liver tissue for three fish species exposed to azinphosmethyl. Bull Environ Contam Toxicol 69(2):271–277
Park K, Han EJ, Ahn G et al (2020) Effects of combined stressors to cadmium and high temperature on antioxidant defense, apoptotic cell death, and DNA methylation in zebrafish (Danio rerio) embryos. Sci Total Environ 716:137130
Perveen S, Hashmi I, Khan R (2019) Evaluation of genotoxicity and hematological effects in common carp (Cyprinus carpio) induced by disinfection by-products. J Water Health 17(5):762–776
Philippe C, Hautekiet P, Gregoir AF et al (2018) Combined effects of cadmium exposure and temperature on the annual killifish (Nothobranchius furzeri). Environ Toxicol Chem 37(9):2361–2371
Sappal R, Fast M, Stevens D et al (2015a) Effects of copper, hypoxia and acute temperature shifts on mitochondrial oxidation in rainbow trout (Oncorhynchus mykiss) acclimated to warm temperature. Aquat Toxicol 169:46–57
Sappal R, MacDougald M, Fast M et al (2015b) Alterations in mitochondrial electron transport system activity in response to warm acclimation, hypoxia-reoxygenation and copper in rainbow trout, Oncorhynchus mykiss. Aquat Toxicol 165:51–63
Sappal R, Fast M, Purcell S et al (2016) Copper and hypoxia modulate transcriptional and mitochondrial functional-biochemical responses in warm acclimated rainbow trout (Oncorhynchus mykiss). Environ Pollut 211:291–306
Simcic T, Jesensek D, Brancelj A (2015) Effects of increased temperature on metabolic activity and oxidative stress in the first life stages of marble trout (Salmo marmoratus). Fish Physiol Biochem 41(4):1005–1014
Sokolova IM, Lannig G (2008) Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim Res 37(2–3):181–201
Stackley KD, Beeson CC, Rahn JJ et al (2011) Bioenergetic profiling of zebrafish embryonic development. PLoS ONE 6(9):e25652
Staurnes M, Rainuzzo JR, Sigholt T et al (1994) Acclimation of atlantic cod (Gadus-Morhua) to cold-water—stress-response, osmoregulation, gill lipid-composition and gill Na-K-atpase activity. Comp Biochem Phys A 109(2):413–421
Sumner AW, Johnston TA, Lescord GL et al (2019) Mercury bioaccumulation in lacustrine fish populations along a climatic gradient in northern ontario, Canada. Ecosystems. https://doi.org/10.1007/s10021-019-00464-9
Talas ZS, Gulhan MF (2009) Effects of various propolis concentrations on biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss). Ecotoxicol Environ Saf 72(7):1994–1998
Val J, Muniz S, Goma J et al (2016) Influence of global change-related impacts on the mercury toxicity of freshwater algal communities. Sci Total Environ 540:53–62
Waheed R, El Asely AM, Bakery H et al (2020) Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression. Sci Total Environ 718:137326
Wen B, Jin SR, Chen ZZ et al (2017) Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 481:169–176
Woo SJ, Chung JK (2020) Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp Biochem Phys C 229:108698
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
All procedures and animal handling were in accordance with the guidelines: OECD Guideline for Testing of Chemicals, No.204: “Fish, Prolonged Toxicity Test: 14-day Study,” adopted April 4, 1984, and OECD Guideline for Testing of Chemicals, No.215: “Fish, Juvenile Growth Test,” adopted January 21, 2000. And the study was approved by the animal ethics committee of Shandong University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Editorial responsibility: Jing Chen.
Rights and permissions
About this article
Cite this article
Li, ZH., Li, P. & Wu, Y. Effects of waterborne mercury at different temperatures on hematology and energy metabolism in grass carp (Ctenopharyngodon idella). Int. J. Environ. Sci. Technol. 18, 1489–1498 (2021). https://doi.org/10.1007/s13762-020-02906-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02906-7