Abstract
Nanocrystalline metal oxides including TiO2, Fe2O3, and ZnO and their combinations were impregnated on activated carbon (AC) and characterized by XRD, FTIR, and FESEM analyses. The results showed the size of most Fe2O3/AC, TiO2/AC, TiO2/Fe2O3/AC, ZnO/AC, and ZnO/Fe2O3/AC particles are in the range of 25–60 nm. BET analysis verified the high surface area of the six adsorbents (201–448 m2/g). The adsorption results confirmed that the modification could improve the adsorption capacity and removal efficiency as the maximum monolayer adsorption capacity and cyanide removal efficiency were observed for ZnO/Fe2O3/AC (101.0 mg/g, 82.5%), TiO2/Fe2O3/AC (96.2 mg/g, 75.1%), ZnO/AC (91.7 mg/g, 73.5%), TiO2/AC (90.9 mg/g, 72.4%), Fe2O3/AC (86.2 mg/g, 69.2%,), and AC (78.1 mg/g, 66.3%), respectively. Moreover, the study of different isotherm models including Langmuir, Freundlich, and Redlich–Peterson indicated that the Langmuir model was the most suitable one for the six adsorbents with 0.56 < RL < 0.64. The kinetic modeling of experimental data revealed the cyanide adsorption on all adsorbents followed the pseudo second-order model confirming chemisorption can be a main mechanism of adsorption. The regeneration and reusability results showed modified AC adsorbents have more reusable and stable structure than AC to be used as adsorbents in industrial wastewaters. The performance of adsorption process was compared with different methods of cyanide removal. The results approved that adsorption process as a cost-effective and simple design method using bioadsorbents can be highly effective in full-scale applications for the removal of high concentration of cyanide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free and metal cyanide complexes are used or produced in many industries such as metal plating, mining and coke-processing. Analysis of the wastewaters from 90% of the gold mining and finishing metal industries represents considerable amount of cyanide. It is a toxic compound (Yu 2015) that can pose serious health problems to humans as well as aquatic organisms (Eskandari et al. 2019; Kuyucak and Akcil 2013; Yeddou et al. 2010). Therefore, wastewaters containing cyanide must be treated prior discharging into the environment.
Wastewaters containing cyanide could be treated by various physical, chemical, and biological methods. The environmentally friendly biological treatments degrade cyanide using microorganisms under aerobic and anaerobic conditions (Dash et al. 2009b; Eskandari et al. 2019). However, the process usually suffers from the slow rate of biodegradation (Aram et al. 2020); thus, the method could be used for the treatment of effluents with low cyanide concentrations (Kim et al. 2016). Chemical processes are another option utilizing reagents to degrade cyanide. Nevertheless, chemical reactions usually produce other hazardous materials which should be consequently subjected to a secondary treatment (Hijosa-Valsero et al. 2013). Physical treatments such as adsorption can be used for cyanide separation from the industrial effluents (Deegan et al. 2011; Komkiene and Baltrenaite 2016). Commercial adsorbents like activated carbon, which have large surface area and highly porous structure (Guo et al. 2015), could be applied for cyanide separation from the waste streams (Behnamfard and Salarirad 2009; Halet et al. 2015).
Recently, activated carbon impregnated with different metals have been used to boost the contaminant removal efficiency owing to the chemisorption arising from interaction between complex ions and AC (Adhoum and Monser 2002; Mor et al. 2017; Valizadeh et al. 2016). There are limited publications, which assessed cyanide adsorption on Ag-, Cu-, and Ni-impregnated activated carbon, but surprisingly enough nearly, no previous study was found regarding to cyanide removal using Fe2O3-, TiO2-, ZnO-impregnated AC (Agarwal and Balomajumder 2015; Gicheva and Yordanov 2013; Yeddou et al. 2011). Besides, to our knowledge, no attempt has been made to investigate the efficiency of combined metal oxides (TiO2/Fe2O3, ZnO/Fe2O3) impregnated AC.
The research focuses on cyanide removal from aqueous solution by adsorption on AC, Fe2O3-, TiO2-, ZnO-impregnated AC, and combined metal oxides (TiO2/Fe2O3, ZnO/Fe2O3)-impregnated on activated carbon. Therefore, the adsorbents were synthesized and characterized by XRD, FTIR, FESEM, BET, and elemental mapping analyses. Moreover, the adsorption equilibrium data was fitted to the well-known Langmuir, Freundlich, and Redlich–Peterson isotherm models. Besides, the kinetic behaviors of the adsorption process were evaluated using pseudo first-order, pseudo second-order and the Weber-Morriss models. The mechanism of adsorption process was also investigated, and the performance of different methods of cyanide removal based on the removal time, removal efficiency, cost and other criteria was compared to determine the best promising technology for purification of cyanide wastewaters.
Materials and methods
Reagents and materials
All of the chemicals including sodium cyanide, silver nitrate, iron (III) chloride (Merck, 99% purity), titanium tetrachloride, activated carbon, 4-dimethylaminobenzylidinerhodanine (Merck, 99% purity), sodium hydroxide, hydrochloric acid, zinc acetate, and ammonium hydroxide were from analytical regent grade.
Preparation of metal oxides-impregnated activated carbon
Synthesis of Fe2O3-impregnated activated carbon was performed by chemical co-precipitation method with some modifications (Wang et al. 2011a). Initially, AC was suspended in deionized water, heated up to 70 °C, and mixed with aqueous FeCl3 solution. The molar ratio of AC to FeCl3 was maintained unit through the mixing. Consequently, the solution was adjusted to pH 2.5 ± 0.5 (using NaOH and HCl) and well mixed at 70 °C for 4 h. The mixture was filtered and washed with deionized water until appeared clear filtration. The materials were dried at 80 °C for 2 h using an electronic oven and then heated in a muffle furnace at 400 °C for 2 h in limited air. The procedure of TiO2-impregnated AC preparation was similar to that of Fe2O3-impregnated AC. The only difference was adding of TiCl4 solution instead of FeCl3 solution. For preparation of (TiO2/Fe2O3)-impregnated AC, FeCl3 and TiCl4 solutions were simultaneously added into the activated carbon suspension.
The sol–gel method was used for the synthesis of ZnO/Fe2O3-impregnated AC (Maya-Treviño et al. 2014). Zinc acetate was mixed with deionize water, and the mixture was dispersed using an ultrasonic water bath for 3 h during the synthesis process. FeCl3 was added to zinc acetate mixture and after some mixing, AC was added. The pH of solution was adjusted to seven using NH4OH solution. The mixture was made under ultrasonication over 3 h and kept at ambient temperature (25 °C) for 24 h. Then, it was put in an oven at 80 °C for 12 h and finally heated in a muffle furnace at 400 °C for 2 h in limited air. The procedure of ZnO-impregnated activated preparation was almost similar to that of ZnO/Fe2O3-impregnated activated carbon. The only difference was omitting the addition of FeCl3 solution.
Characterization
Different analyses were applied to characterize the adsorbents. X-ray diffraction (XRD) (BrukerD-8 Advance, Germany) determined the crystalline phase. Fourier transform infrared (FTIR) (Jasco, 6300, Japan) spectroscopy was performed in the range of 400–4000 cm−1 to further assess the chemical composition of synthesized nanostructures. The nanostructure morphology of the six adsorbents was investigated with field emission scanning electron microscopy (FESEM; Hitachi S-4160, Japan), operating at an accelerating voltage of 15 kV. The samples were initially coated with a thin layer of gold film to avoid charging effects and evaporating in air at room temperature. ImageJ software which analyzes measured area, mean standard deviation, minimum and maximum of selection or entire image and measure lengths, angles and the diameter of particles was adopted to identify the size distributions of the synthesized adsorbents. Brunauer–Emmett–Teller (BET) surface area and pore volume measurement were analyzed by measuring N2 adsorption at 77 K (Belsorp mini, Japan).
Adsorption experiments
Equilibrium experiments
Initially, cyanide stock solution (500 mg/L) was prepared by dissolving 0.25 g sodium cyanide in 500 mL distilled water. The stock solution was added to several flasks and diluted with predetermined amount of distilled water to provide solutions with desired cyanide concentrations that ranged from 50 to 500 mg/L (pH 10). Subsequently, 1.5 g/L of the adsorbent was added to each cyanide solution and the samples incubated at 25 °C and 100 rpm for 72 h. The amount of adsorbents concentration was optimized according to screening experiments and literature reviews (Moussavi and Khosravi 2010). The adsorbents were separated by centrifugation at 8000 rpm for 10 min. The cyanide concentration was determined by a standard titration procedure (No. 4500-CN-D) (Federation and Association 2005). The adsorption capacity at equilibrium qe (mg/L) calculated by:
where C0 and Ce are the initial and equilibrium cyanide concentrations (mg/L), respectively, and \(v\) is the total solution volume (L), and W is the mass of the adsorbents (Behnamfard and Salarirad 2009).
Isotherm models
Equilibrium data was fitted by well-known isotherms including Langmuir, Freundlich and Redlich–Peterson models. The models, parameters, linear and nonlinear forms of these isotherms are shown in Table 1. Excel software was applied to determine the parameters and detect the best isotherm for cyanide adsorption.
Kinetic experiments
Batch experiments were conducted by adding 1.5 g of adsorbents to 100 mg/L cyanide solution and incubation of the mixture at 25 °C and 100 rpm. The adsorbents were separated by centrifugation of the samples at 8000 rpm for 10 min. The liquid samples were taken periodically and then subjected to cyanide analysis (Federation and Association 2005). The amount of adsorbed cyanide at each time was calculated according to the following equation:
where C0 and Ct are the cyanide concentrations (mg/L) at initial time and t, respectively (Behnamfard and Salarirad 2009).
Kinetic models
The kinetic models equations, corresponding linear expressions, and the method of parameters estimation of pseudo first order, pseudo second order and Weber-Morriss are demonstrated in Table 2. Parameters of kinetic equation determined by Excel software.
Statistical analysis
The validity of the adsorption isotherm and kinetic models were evaluated by average relative error (ARE), Chi-square test (X2), and correlation coefficient (R2). ARE and X2 are defined as follows (Depci 2012):
where qe expi and qe calci are the experimental and calculated values of cyanide adsorbed on activated carbon and synthesized adsorbents (mg/g), respectively. N is the number of adsorption data in the regression model.
Results and discussion
Adsorbent characterization
The XRD patterns of activated carbon and five synthesized adsorbents are shown in Fig. 1a–d. The patterns of activated carbon (Fig. 1a) indicate the peaks at 2θ = 31.0° and 51.0° set of planes of the micrographitic structure (Eskandari et al. 2017, 2019; Ouzzine et al. 2014). The XRD pattern of the Fe2O3/AC nanostructures shown in Fig. 1b proves that all the strong and sharp diffraction peaks can be indexed as the hexagonal phase of Fe2O3 (JCPDS Card Number of 01-072-0469) (Davari et al. 2017; Shen et al. 2009). The XRD analyses of TiO2/AC (Fig. 1c) and TiO2/Fe2O3/AC (Fig. 1c) reveal the broad peaks at 2θ = 25.3°, 27.4°, 36.1°, 48.2°, 55.1°, 62.9° and 70.3° correspond to the TiO2 structure. The crystalline phase of TiO2 in both TiO2/AC and TiO2/Fe2O3/AC are identified as anatase (JCPDS Card Number of 01-071-1167) and rutile (JCPDS Card Number of 00-001-1292) (Davari et al. 2017; Eskandari et al. 2017, 2019; Wang et al. 2011a). Furthermore, the XRD patterns of both ZnO/AC (Fig. 1d) and ZnO/Fe2O3/AC (Fig. 1d) are characterized by the following peaks: 2θ = 30.75°, 36.82°, 40.01°, 42.24°, 55.93°, 66.73°, 74.32° and 79.94° confirming the presence of the wurtzite hexagonal phase of ZnO- (JCPDS Card Number of 00-003-0888) (Davari et al. 2017; Eskandari et al. 2017; Hernández et al. 2007). However, there is no significant peak representing the Fe ion in the XRD pattern of TiO2/Fe2O3/AC (Fig. 1c) and ZnO/Fe2O3/AC (Fig. 1d). The low concentration of Fe2O3 doped on TiO2 and ZnO is the reason of no detecting of Fe2O3 patterns.
FTIR spectroscopy analyses of the adsorbents (Fig. 2a–d) reveal the functional groups of the surface. The absorption bands at around 3450 cm−1 represent O–H mode while the broad peaks at 1700–1720 cm−1 confirm the existence of C=O functional groups. The peaks at the range of 1100–1160 cm−1 and at approximately 1565 cm−1 belong to carbon skeleton. The intensive peaks from 600 to 700 cm−1 in Fig. 2c are related to Ti–O bond. Besides, the stretching vibrations modes of Fe–O (Fig. 2b, d) and Zn–O (Fig. 2d) bonds show a signal at 456 and 655 cm−1, respectively.
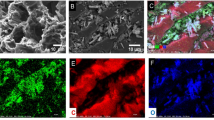
The morphology of the adsorbent is an important parameter affects the cyanide removal efficiency. FESEM micrograph of activated carbon and different impregnated activated carbon are shown in Fig. 3a–f. Comparing the FESEM images of synthesized adsorbents with AC reflects major morphological changes. It can be observed that the Fe2O3, TiO2, TiO2/Fe2O3, ZnO and ZnO/Fe2O3 nanoparticles homogeneously distributed on the AC surface. It seems that the pores of activated carbon are occupied by the corresponding metals (TiO2, Fe2O3 and ZnO) which bring about making a homogeneous coverage. The size distribution curves of different synthesized adsorbents are illustrated in Fig. 4a–e. It is found that more than 70% of Fe2O3/AC particles have the size less than 55 nm. The size of most TiO2/AC nanoparticles are in the range of 46–50 nm, while TiO2/Fe2O3/AC nanoparticles have the size of about 45 nm in average. In addition, the size of most ZnO/AC and ZnO/Fe2O3/AC nanoparticles are in the range of 25–29 nm and 40–44 nm, respectively. Further, the elemental mapping of adsorbents showed extensive dispersion of Fe, Ti, Zn and O elements confirming that Fe2O3, TiO2 and ZnO were well distributed on the surface of AC (Figs. 5, 6, 7, 8, 9).
The results of surface areas measurements (BET) of the adsorbents are given in Table 3. The surface area and pore volume of activated carbon is larger as compared with the synthesized adsorbents, which verifies the effect of nanometals loading. Decrease of the surface area is due to the entrance of nanooxides into pores of activated carbon. However, BET of the synthesized samples are still high. Moreover, lower amount of BET of TiO2/Fe2O3/AC and ZnO/Fe2O3/AC than that of TiO2/AC and ZnO/AC verifies that the pores are filled with Fe2O3 nanoparticles, which could have significant influence on adsorption capacity. The results also prove that the amounts of surface area and total pore volume are dependent on the types of materials, which are modified on activated carbon surface. Besides, the nitrogen adsorption–desorption isotherms of six adsorbents shown in Fig. 10a–f are ascribed as type IV isotherms according to IUPAC, with a wider hysteresis loop at high relative pressure (Sun et al. 2015; Wang et al. 2011b). Meanwhile, N2 isotherms of the modified ACs are lower compared to that of the raw AC, confirming that the surface area of the modified ACs became smaller in comparison with the original AC (Pan et al. 2013). Furthermore, the BJH curves of pore size distribution of samples (Fig. 11a–f) indicate the abundant of pores are in the size of less than 10 nm. The curves also reveal the modification has insignificant effect on the pore size distribution shapes of samples, although the total surface areas decreases.
Adsorption isotherm
Equilibrium isotherm
An equilibrium isotherm indicates the equilibrium relation between the amount of adsorbed cyanide onto the adsorbent and the corresponding cyanide concentration in the liquid phase (Behnamfard and Salarirad 2009). The well-known isotherms including Langmuir, Freundlich, and Redlich–Peterson were investigated and the parameters were determined by nonlinear regression over the experimental data. The Langmuir adsorption model is based on monolayer coverage of adsorbate on the adsorbent (Ali et al. 2018; Lazarević et al. 2012; Papandreou et al. 2011; Saadi et al. 2015). In the Freundlich model, the stronger binding sites are occupied first and then the binding strength decreases with the increasing degree of site occupation (Behnamfard and Salarirad 2009). The three-parameter Redlich–Peterson equation consists of both features of Langmuir and Freundlich models (Green-Pedersen et al. 1997; Vasques et al. 2014). The isotherm equations, corresponding linear expressions, and the method of parameters estimation are demonstrated in Table 1. The Langmuir parameters Q0 (mg/g) and b (L/mg) represent information about the maximum adsorption capacity by the adsorbent and the binding affinity between solid adsorbent and adsorbate molecules respectively. Likewise, in the Freundlich equation parameter kf (L/g) is related to the adsorption capacity, and (1/n) is the heterogeneity factor related to the adsorption intensity (Li et al. 2009). The parameters of A, B and g in Redlich–Peterson model are Redlich–Peterson isotherm constant (L/g), Redlich–Peterson isotherm constant (L/mg)g and Redlich–Peterson constant. Table 4 provides information about the results of isotherms parameters for the cyanide adsorption on the activated carbon and different synthesized adsorbents at pH 10. The pKa of HCN is 9.3; hence, to avoid its liberation into the atmosphere, the adsorption process was performed in a highly alkaline solution. The adsorption behavior of the synthesized adsorbents has been improved compared to that of AC. The maximum adsorption capacity is observed for the ZnO/Fe2O3/AC, which is 101.0 mg/g. The adsorption capacity of TiO2/Fe2O3/AC (96.2 mg/g) is placed in the second level, followed by ZnO/AC (91.7 mg/g) > TiO2/AC (90.9 mg/g) > Fe2O3/AC (86.2 mg/g). The adsorption capacity of the AC achieved 78.1 mg/g is the least adsorption capacity among six adsorbents. The high amount of adsorption capacity for all adsorbents could be attributed to their special surface properties and high surface area. However, it should be pointed out that, except BET, other factors can strongly affect liquid phase adsorption into solids and adsorption capacity (Stavropoulos et al. 2015). These factors include sorbent properties (physical and chemical structure, pHPZC, quantity of active functional groups, the surface charge), sorbate properties (molecular structure and size, solubility, pKa), solution conditions (pH, ionic strength), types of impregnation agents and temperature, which play a vital role in adsorption phenomena, adsorption capacity and investigation of adsorption mechanism (Abussaud et al. 2016) (Ahn et al. 2009; Mondal et al. 2010; Stavropoulos et al. 2015). Impregnation with TiO2, Fe2O3 and ZnO makes some changes in quantum size and specific surface area of AC due to the special physicochemical properties of metal oxides. Although the surface areas of the synthesized samples are negatively charge because the pHPZC for TiO2, Fe2O3, and ZnO are 6.8, 8 and 9, respectively (Arimi et al. 2016; Eskandari et al. 2017; Reed et al. 2000) and therefore the pHPZC of samples is considered in the range of 7–9 which is less than pH of solution (10), the adsorption capacity of all samples are high at pH = 10 due to their high surface densities. These results confirmed the strong role of pH on the cyanide adsorption removal (Stavropoulos et al. 2015). Besides, the deprotonation on the surface of adsorbents at pH of 10 and higher provides functional groups which lead to an ion exchange type of interaction with the cyanide ion (Dash et al. 2009a).
Moreover, TiO2/Fe2O3/AC and ZnO/Fe2O3/AC have much higher adsorption capacity in comparison with that of TiO2/AC, Fe2O3/AC, and ZnO/AC (Table 4) due to additional surface charge of the cationic Fe3+ on TiO2 or ZnO surface and also interfacial effects between TiO2 or ZnO and Fe2O3, which could have impacts on interionic interaction and cation exchange capacity (Do et al. 2012; Pal et al. 2016), which could enhance the ionic cyanide adsorbed compared to the bare TiO2 or ZnO. Therefore, addition of a second metal ion such as Fe3+ to TiO2 and ZnO during the adsorbent preparation process could synergistically enhance surface effective charge and accordingly increase the adsorption capacity of the AC which is impregnated with two metal ions (Pal et al. 2016). The results also confirm that, the adsorbents surface chemistry and aqueous phase chemistry affect an adsorption mechanism. Meanwhile, the regression results indicate higher correlation coefficients (0.982–0.996) for agreement of experimental equilibrium data with the Langmuir isotherm model compared with the Freundlich model (0.949–0.986). Furthermore, the lower statistical parameters (X2 and ARE) for the Langmuir isotherm represent the model can efficiently describe the cyanide adsorption onto the activated carbon and five synthesized adsorbents. The correlation coefficient and statistical parameters (X2 and ARE) of Langmuir and Redlich–Peterson isotherms are almost the same but Langmuir is much easier to handle and is more beneficial. Therefore, it might be inferred that the cyanide is strongly adsorbed on the surface of AC and five other adsorbents, and the adsorption process obeys a monolayer coverage assumption considered in Langmuir model. The plots of nonlinear Langmuir isotherms along with corresponding experimental data are demonstrated in Fig. 12. The obtained results are in agreement with previously published data which supports suitability of the Langmuir model for AC as an adsorbent (Dwivedi et al. 2016).
The nature of adsorption process evaluated by a dimensionless separation factor (RL) indicating the favorability of the Langmuir model (Saleh et al. 2015; Tan et al. 2009):
where C0 (mg/L) is the initial cyanide concentration and b (L/mg) is the Langmuir constant.
The separation factor for cyanide adsorption on AC and other impregnated AC adsorbents is between 0 and 1 (Table 4) which represents thermodynamically favorable adsorption. Moreover, the constant n in the Freundlich isotherm is greater than unity, indicating that the adsorbents are appropriate and beneficial for cyanide removal (Table 4).
Adsorption kinetics
Adsorption kinetic model illustrates the mechanism of interaction between solid solution. The amount of adsorbed cyanide as a function of time for different adsorbents at initial cyanide concentration of 100 mg/L and pH of 10 is shown in Fig. 13. As shown, there is a sharp rise in cyanide removal efficiency during the first stage of hydrolysis attributed to the increased contact time between cyanide ion and adsorbent active sites and high mass transfer driving force between two phases. However, the results indicate after a lapse of time, the active sites gradually occupied with cyanide molecules, leading to a decreased adsorption performance. The removal efficiency almost reached a plateau after 40 h adsorption, which was due to the equilibrium between phases. Among different investigated adsorbents, the ZnO/Fe2O3/AC has the highest cyanide removal efficiency (around 82.5%) at identical contact time. The final removal efficiency by the TiO2/Fe2O3/AC, ZnO/AC, TiO2/AC, and Fe2O3/AC adsorbents were approximately 75.1, 73.5, 72.4, 69.2%, respectively. This should be compared with the removal efficiency by the AC, which was only 66.3%. Generally, impregnation of the AC with metal oxides could successfully enhance both the adsorption capacity and cyanide removal efficiency. The pseudo-first and pseudo-second order kinetic models have been applied to predict the kinetic behavior of cyanide removal using AC and different impregnated adsorbents. The kinetic models, linear forms of equations, and the parameter estimation procedures are summarized in Table 2. The correlation coefficient (Table 5) for the pseudo-first-order model ranged from 0.901 to 0.951, while it is considerably higher in the case of pseudo-second-order model (0.996–0.999). Furthermore, for all investigated adsorbents, the latter model have lower ARE and X2 values, which indicate better agreement with experimental kinetic data. Consequently, the cyanide adsorption followed pseudo-second order confirming chemisorption can be a main mechanism of adsorption (Agarwal et al. 2013; Wang et al. 2011b). Cyanide diffuses into the particles’ interior pores or surface through ion exchange and/or complexation interactions (Ghaedi et al. 2012). The nonlinear form of pseudo-second-order kinetic model along with the experimental data at pH 10, temperature of 25 ◦C and the initial cyanide concentration of 100 mg/L is depicted in Fig. 14. The results were well supported by Singah et al. that modeled cyanide adsorption on raw and impregnated AC and obtained a better agreement with the pseudo-second-order equation (Singh and Balomajumder 2016). The experimental kinetic data are also fitted to the Weber-Morriss equation, which shows a plot of qt (mg/g), vs. the square root of time (t^0.5) and the slope of the straight line equals to k. The Weber–Morriss model compared with experimental data and the results are presented in Fig. 15. As evident, the model plot divided to two distinct areas whereas the first straight step describes the macropore diffusion and the second step represents the micropore diffusion. The first step up to 24 h was very fast which could be attributed to the external surface adsorption and diffusion of the cyanide ions into macropores. However, the rate of mass transfer underwent a rapid decline The Weber-Morriss parameters along with the correlation coefficients (R2) for AC, and all synthesized adsorbents (at pH 10, temperature of 25 °C and the initial cyanide concentration of 100 mg/L) are presented in Table 5.
Regeneration of adsorbents
The reusability and the regeneration of the active sites of adsorbents play a vital role in the decrease of the operational cost and the ecological risk factors. These features also have significant impacts on the feasibility of adsorption process for industrial and large-scale practical applications (Shah et al. 2015). In this work, the adsorption/desorption experiments with deionized water after five cycles of reuse were carried out to investigate the reusability of six adsorbents for cyanide adsorption removal. The results (Fig. 16) show that ZnO/Fe2O3/AC and AC has the highest and the lowest efficiency to adsorb cyanide, respectively, compared to other adsorbents after five cycles of regeneration and reusability. Moreover, the cyanide removal efficiency after five cycles of TiO2/Fe2O3/AC reusing was more than ZnO/AC, TiO2/AC, and Fe2O3/AC. The results also confirmed that the regeneration capacity of the modified adsorbents was far more profound than AC and the amount of the reduction of cyanide removal efficiency by the reused AC was more compared to the synthesized adsorbents. This implies the poorer AC performance and lower regeneration potential of AC than modified AC. Although all of six adsorbents are beneficial for the practical usage in real wastewaters containing cyanide, ZnO/Fe2O3/AC, TiO2/Fe2O3/AC, ZnO/AC, TiO2/AC and Fe2O3/AC have more reusable and stable structure than AC to be used as adsorbents in industrial wastewaters.
Comparison of different methods of cyanide removal
There are various cyanide treatment processes from natural methods to highly sophisticated plant applications. These methods include natural degradation in tailings ponds, physical, chemical, biological, advanced oxidation processes (AOPs) and so on (Kuyucak and Akcil 2013). The recent tendency is to develop and apply the best promising technology to degrade cyanide to harmless compounds to be a cost-effective and environmentally friendly method with high degradation efficiency in a short time. Table 6 lists the degradation efficiencies of various types of cyanide removal technologies and their operational conditions to investigate and choose the more effective treatment approaches. The process selection depends on an array of criteria such as the ability to remove high concentration of cyanide, not production of undesirable by-products, capital and operational costs, removal time, ability to be run under ambient conditions and so on(Kuyucak and Akcil 2013). Natural cyanide attenuation method has the advantage of lower capital and operating costs compared to other methods, but due to the prolonged periods of natural treatment process and inability to produce effluents of acceptable quality by natural means, this method is not suggested for the main removal approach and it is applied as an intermediate step for the partial removal of cyanide prior to chemical treatment (Botz et al. 2005, 2016; Kuyucak and Akcil 2013).
Alkaline chloride oxidation as a type of chemical processes is not considered as an appropriate method of cyanide removal from wastewaters because chlorination of cyanide results in highly toxic intermediates of cyanogen chloride (Adhoum and Monser 2002) and the process can be relatively expensive to be operated due to high reagents which are used and the high cost of removal stage of produced hazardous by-products (Botz et al. 2005; Botz et al. 2016).
Biological treatment is a commonly used technique of wastewater treatment owing to its cost-effectiveness and environmental friendliness (Moussavi and Khosravi 2010). However, the slow rate of anaerobic biodegradation process and the susceptibility of microorganisms to toxic cyanide restrict the potential of bioprocesses for cyanide degradation (Do et al. 2012; Moussavi and Khosravi 2010). The comparison of the results of Table 6 also verifies this issue as the study of (Sirianuntapiboon et al. 2008) showed a low concentration of cyanide (2.3 mg/L) was degraded after a prolonged periods of 10 days, while the results of other studies (Table 6) confirm that other removal techniques especially adsorption and photocatalysis had high potential to remove high concentration of cyanide in a short time.
Besides, Table 6 proves that an adsorption process as a simple and versatile method can be highly effective in full-scale applications for the removal of high concentration of cyanide contained in wastewaters. Moreover, the results (Table 6) show activated carbon-based adsorbents have high adsorption capacity and high amount of cyanide can be removed by applying adsorption processes using such great adsorbents. Further, modification of AC with other materials especially TiO2, ZnO, and Fe2O3 significantly improved the adsorption capacity and the cyanide removal yield. Most of these modified activated carbon-based materials not only can be used as a promising adsorbent but also as a high photoactive catalyst (Eskandari et al. 2017, 2019). The research findings also verify that an adsorption process benefits from using low cost and eco-friendly bioadsorbents prepared from agricultural waste materials as the studies of (Dwivedi et al. 2016; Moussavi and Khosravi 2010) showed cyanide (100 mg/L) removal by using 1.5 g/L of pistachio hull waste as an effective bioadsorbent was obtained 99% after 1 h and cyanide was removed by 87 and 82% using 20 g/L of bioadsorbents of PAS and TGL after 2 h, respectively. Therefore, the adsorption processes are not affected by the toxicity of the target compounds and do not require hazardous chemicals (Dwivedi et al. 2016; Moussavi and Khosravi 2010). The further investigation of the results of Table 6 shows that while some methods of cyanide removal like ozone (O3) injection only degraded cyanide by 30%, the combination of O3 with activated carbon adsorption technology could substantially enhance the degradation efficiency as cyanide was removed completely in a combined treatment system (Sánchez-Castillo et al. 2015). As can be seen in Table 6, the performance of an adsorption process with photolysis (UV) and H2O2 was also compared and the results indicated that using activated carbon or modified activated carbon adsorbents was more beneficial to purify wastewater containing cyanide than using UV, H2O2 or the combination of these two (UV/H2O2) that only degraded cyanide by 12, 8 and 33%, respectively (Eskandari et al. 2017). The comparison of adsorption process by photocatalysis implies that both remediation technologies have strong potential to remove cyanide effectively. The study of (Eskandari et al. 2017) showed that the cyanide removal efficiency by adsorption and photocatalysis process using ZnO/Fe2O3/AC nanoparticles under the optimal conditions of [CN]− = 250 mg/L, pH = 10, Time = 3 h, [ZnO/Fe2O3/AC] = 1.4 g/L were 54 and 80%, respectively. However, in some studies photocatalysis methods were found to be far more effective. The study of (Mishra et al. 2019) confirmed that while the cyanide degradation by TiO2-Pd HAP/UV–vis under the circumstance of [CN] = 100 mg/L, pH = 11, TiO2-Pd HAP = 450 mg, time = 90 min was achieved 52%, the percentage of adsorbed cyanide using TiO2-Pd HAP was insignificant (7%). Although photocatalysis process exhibited higher cyanide degradation efficiency in some cases and have advantages like the lack of sludge production, and high potential for toxicity reduction and decomposition to harmless compounds, this process is much more complex than the adsorption process and needs major investments making photocatalysis process difficult to be applied in large-scale applications and real industrial wastewaters (Solaimany Nazar et al. 2018). By contrast, adsorption methods are taken into account as a cost-effective approach with simple design and using low-cost non-toxic adsorbents and applicable in large-scale industrial wastewaters. In general, the comparison of different methods of cyanide removal verified that adsorption processes are one of the most efficient techniques benefiting from unique properties and simultaneous adopting of this method with AOPs such as O3 or photocatalysis can significantly promote the efficiency of cyanide removal.
Conclusion
Impregnation of the powdered activated carbon with different metal oxides plays a vital role in enhancing the adsorption capacity and the rate of cyanide adsorption. Furthermore, adsorption capacity is dependent on type of metal oxide impregnated on AC, active functional groups, surface area, and the surface charge. The results reveal that higher cyanide removal efficiency could be found by co-impregnation of AC with two metal species (ZnO/Fe2O3/AC 82.5% and TiO2/Fe2O3/AC 75.1%) instead of just one metal oxide (ZnO/AC 73.5%, TiO2/AC 72.4% and Fe2O3/AC 69.2%). The equilibrium experiments show that the Langmuir isotherm could successfully describe the adsorption behavior. Moreover, the kinetic behavior of the adsorption followed the pseudo-second order kinetic model. Moreover, the regeneration study showed that ZnO/Fe2O3/AC and AC have the highest and the lowest efficiency to adsorb cyanide, respectively, compared to other adsorbents after five cycles of regeneration and reusability. The adsorption process for cyanide removal was compared with other methods and adsorption technology is considered as one of the most effective, practical, easy to operate, and economical approaches for water and wastewater treatment.
References
Abussaud B, Asmaly HA, Saleh TA, Gupta VK, Atieh MA (2016) Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J Mol Liq 213:351–359
Adhoum N, Monser L (2002) Removal of cyanide from aqueous solution using impregnated activated carbon. Chem Eng Process 41:17–21
Agarwal B, Balomajumder C (2015) Removal of phenol and cyanide in multi-substrate system using copper impregnated activated carbon (Cu-GAC). Environ Prog Sustain Energy 34:1714–1723
Agarwal B, Balomajumder C, Thakur PK (2013) Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon. Chem Eng 228:655–664
Ahn CK, Park D, Woo SH, Park JM (2009) Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J Hazard Mater 164:1130–1136
Ali A, Mannan A, Hussain I, Hussain I, Zia M (2018) Effective removal of metal ions from aquous solution by silver and zinc nanoparticles functionalized cellulose: isotherm, kinetics and statistical supposition of process Environmental Nanotechnology. Monit Manage 9:1–11
Aram M, Farhadian M, Nazar ARS, Tangestaninejad S, Eskandari P, Jeon B-H (2020) Metronidazole and Cephalexin degradation by using of Urea/TiO2/ZnFe2O4/Clinoptiloite catalyst under visible-light irradiation and ozone injection. J Mole Liq 112764
Arimi A, Farhadian M, Nazar ARS, Homayoonfal M (2016) Assessment of operating parameters for photocatalytic degradation of a textile dye by Fe2O3/TiO2/clinoptilolite nanocatalyst using Taguchi experimental design. Res Chem Intermed 42:4021–4040
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: characterisation, kinetic and isotherm studies. J Environ Chem Eng 4:4207–4228
Behnamfard A, Salarirad MM (2009) Equilibrium and kinetic studies on free cyanide adsorption from aqueous solution by activated carbon. J Hazard Mater 170:127–133
Botz M, Mudder T, Akcil A (2005) Cyanide treatment: physical, chemical and biological processes. Adv Gold Ore Process 4528:672–702
Botz M, Mudder T, Akcil A (2016) Cyanide treatment: physical, chemical, and biological processes. In: Gold ore processing. Elsevier, pp 619–645
Dash RR, Balomajumder C, Kumar A (2008) Treatment of metal cyanide bearing wastewater by simultaneous adsorption and biodegradation (SAB). J Hazard Mater 152:387–396. https://doi.org/10.1016/j.jhazmat.2007.07.009
Dash RR, Balomajumder C, Kumar A (2009a) Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J 146:408–413
Dash RR, Gaur A, Balomajumder C (2009b) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163:1–11
Davari N, Farhadian M, Nazar ARS, Homayoonfal M (2017) Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J Environ Chem Eng 5:5707–5720
Deegan A, Shaik B, Nolan K, Urell K, Oelgemöller M, Tobin J, Morrissey A (2011) Treatment options for wastewater effluents from pharmaceutical companies. Int J Environ Sci Technol 8:649–666
Depci T (2012) Comparison of activated carbon and iron impregnated activated carbon derived from Gölbaşı lignite to remove cyanide from water. Chem Eng J 181–182:467–478. https://doi.org/10.1016/j.cej.2011.12.003
Do S-H, Jo Y-H, Park H-D, Kong S-H (2012) Synthesis of iron composites on nano-pore substrates: identification and its application to removal of cyanide. Chemosphere 89:1450–1456
Dwivedi N, Balomajumder C, Mondal P (2016) Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents. Water Resour Ind 15:28–40
Eskandari P, Farhadian M, SolaimanyNazar AR (2017) Performance enhancement and optimization of photocatalytic cyanide degradation in aqueous solution using Zn (II) and Fe(III) oxides as nanostructure supported on activated carbon. J Chem Technol Biotechnol 92:2360–2368
Eskandari P, Farhadian M, Solaimany Nazar AR, Jeon B-H (2019) Adsorption and photodegradation efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/zeolite nanophotocatalysts for the removal of cyanide. Ind Eng Chem Res 58:2099–2112
Federation WE, Association APH (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Ghaedi M, Biyareh MN, Kokhdan SN, Shamsaldini S, Sahraei R, Daneshfar A, Shahriyar S (2012) Comparison of the efficiency of palladium and silver nanoparticles loaded on activated carbon and zinc oxide nanorods loaded on activated carbon as new adsorbents for removal of Congo red from aqueous solution: kinetic and isotherm study. Mater Sci Eng, C 32:725–734
Ghasemi R, Sayahi T, Tourani S, Kavianimehr M (2016) Modified magnetite nanoparticles for hexavalent chromium removal from water. J Dispersion Sci Technol 37:1303–1314
Gicheva G, Yordanov G (2013) Removal of citrate-coated silver nanoparticles from aqueous dispersions by using activated carbon. Colloids Surf A 431:51–59
Green-Pedersen H, Jensen B, Pind N (1997) Nickel adsorption on MnO2, Fe (OH) 3, montmorillonite, humic acid and calcite: a comparative study. Environ Technol 18:807–815
Guo Y, Zhao C, Li C (2015) CO2 Adsorption Kinetics of K2CO3/Activated Carbon for Low-Concentration CO2 removal from confined spaces. Chem Eng Technol 38:891–899
Halet F, Yeddou AR, Chergui A, Chergui S, Nadjemi B, Ould-Dris A (2015) Removal of cyanide from aqueous solutions by adsorption on activated carbon prepared from lignocellulosic by-products. J Dispersion Sci Technol 36:1736–1741
Hernández A, Maya L, Sánchez-Mora E, Sánchez EM (2007) Sol-gel synthesis, characterization and photocatalytic activity of mixed oxide ZnO-Fe2O3. J Sol-Gel Sci Technol 42:71–78
Hijosa-Valsero M, Molina R, Schikora H, Müller M, Bayona JM (2013) Removal of cyanide from water by means of plasma discharge technology. Water Res 47:1701–1707
Islam MA, Khan MM, Mozumder MS (2004) Adsorption equilibrium and adsorption kinetics: a unified approach. Chem Eng Technol Ind Chem Plant Equip Process Eng Biotechnol 27:1095–1098
Kim SH, Lee SW, Lee GM, Lee B-T, Yun S-T, Kim S-O (2016) Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 143:106–114
Kim T-K, Kim T, Jo A, Park S, Choi K, Zoh K-D (2018) Degradation mechanism of cyanide in water using a UV-LED/H2O2/Cu2+ system. Chemosphere 208:441–449
Komkiene J, Baltrenaite E (2016) Biochar as adsorbent for removal of heavy metal ions [Cadmium (II), Copper (II), Lead (II), Zinc (II)] from aqueous phase. Int J Environ Sci Technol 13:471–482
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50:13–29
Lazarević S, Janković-Častvan I, Potkonjak B, Janaćković D, Petrović R (2012) Removal of Co 2 + ions from aqueous solutions using iron-functionalized sepiolite. Chem Eng Process 55:40–47
Li K, Zheng Z, Huang X, Zhao G, Feng J, Zhang J (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. J Hazard Mater 166:213–220
Maulana I, Takahashi F (2018) Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J Water Process Eng 22:80–86
Maya-Treviño M, Guzmán-Mar J, Hinojosa-Reyes L, Ramos-Delgado N, Maldonado MI, Hernández-Ramírez A (2014) Activity of the ZnO–Fe2O3 catalyst on the degradation of Dicamba and 2, 4-D herbicides using simulated solar light. Ceram Int 40:8701–8708
Mishra J, Pattanayak DS, Das AA, Mishra DK, Rath D, Sahoo NK (2019) Enhanced photocatalytic degradation of cyanide employing Fe-porphyrin sensitizer with hydroxyapatite palladium doped TiO2nano-composite system. J Mol Liq 287:110821
Mondal P, Mohanty B, Balomajumder C (2010) Treatment of arsenic contaminated groundwater using calcium impregnated granular activated carbon in a batch reactor: optimization of process parameters CLEAN–Soil. Air, Water 38:129–139
Mor S, Chhoden K, Negi P, Ravindra K (2017) Utilization of nano-alumina and activated charcoal for phosphate removal from wastewater Environmental Nanotechnology. Monit Manage 7:15–23
Moussavi G, Khosravi R (2010) Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: parametric experiments, kinetics and equilibrium analysis. J Hazard Mater 183:724–730
Ouzzine M, Romero-Anaya AJ, Lillo-Ródenas MA, Linares-Solano A (2014) Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon 67:104–118
Pal B, Kaur R, Grover IS (2016) Superior adsorption and photodegradation of eriochrome black-T dye by Fe 3 + and Pt 4 + impregnated TiO 2 nanostructures of different shapes. J Ind Eng Chem 33:178–184
Pan H, Tian M, Zhang H, Zhang Y, Lin Q (2013) Adsorption and desorption performance of dichloromethane over activated carbons modified by metal ions. J Chem Eng Data 58:2449–2454
Papandreou A, Stournaras C, Panias D, Paspaliaris I (2011) Adsorption of Pb(II), Zn (II) and Cr(III) on coal fly ash porous pellets. Miner Eng 24:1495–1501
Reed BE, Vaughan R, Jiang L (2000) As (III), As (V), Hg, and Pb removal by Fe-oxide impregnated activated carbon. J Environ Eng 126:869–873
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32:787–799
Saleh TA, Alhooshani KR, Abdelbassit MS (2015) Evaluation of AC/ZnO composite for sorption of dichloromethane, trichloromethane and carbon tetrachloride: kinetics and isotherms. Journal of the Taiwan Institute of Chemical Engineers 55:159–169
Sánchez-Castillo MA, Carrillo-Pedroza FR, Fraga-Tovar F, Soria-Aguilar MdJ (2015) Ozonation of cyanide catalyzed by activated carbon. Ozone Sci Eng 37:240–251
Shah I, Adnan R, Ngah WSW, Mohamed N (2015) Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: regeneration and kinetics studies. PloS one 10
Shen H-X, Yao J-L, Gu R-A (2009) Fabrication and characteristics of spindle Fe2O3@ Au core/shell particles. Trans Nonferr Metals Soc China 19:652–656
Singh N, Balomajumder C (2016) Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated carbon prepared from coconut shell Journal of Water. Process Eng 9:233–245. https://doi.org/10.1016/j.jwpe.2016.01.008
Sirianuntapiboon S, Chairattanawan K, Rarunroeng M (2008) Biological removal of cyanide compounds from electroplating wastewater (EPWW) by sequencing batch reactor (SBR) system. J Hazard Mater 154:526–534
Solaimany Nazar AR, Jokar Baloochi S, Goshadrou A (2018) 2, 4-dichlorophenoxyacetic acid adsorption from contaminated water through activated carbon reclaimed with zero-valent iron and titanium dioxide. Scientiairanica 25:1395–1411
Stavropoulos G, Skodras G, Papadimitriou K (2015) Effect of solution chemistry on cyanide adsorption in activated carbon. Appl Therm Eng 74:182–185
Sun Q, Hu X, Zheng S, Sun Z, Liu S, Li H (2015) Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol 274:88–97b
Tan IAW, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164:473–482. https://doi.org/10.1016/j.jhazmat.2008.08.025
Valizadeh S, Younesi H, Bahramifar N (2016) Highly mesoporous K2CO3 and KOH/activated carbon for SDBS removal from water samples: batch and fixed-bed column adsorption process Environmental Nanotechnology. Monit Manage 6:1–13
Vasques ÉDC, Carpiné D, Dagostin JLA, Canteli AMD, Igarashi-Mafra L, Mafra MR, Scheer AdP (2014) Modelling studies by adsorption for the removal of sunset yellow azo dye present in effluent from a soft drink plant. Environ Technol 35:1532–1540
Wang C, Shi H, Li Y (2011a) Synthesis and characteristics of natural zeolite supported Fe3 + -TiO2 photocatalysts. Appl Surf Sci 257:6873–6877
Wang L, Zhang J, Zhao R, Zhang C, Li C, Li Y (2011b) Adsorption of 2, 4-dichlorophenol on Mn-modified activated carbon prepared from Polygonum orientale Linn. Desalination 266:175–181
Yeddou AR, Nadjemi B, Halet F, Ould-Dris A, Capart R (2010) Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of activated carbon prepared from olive stones. Miner Eng 23:32–39
Yeddou AR et al (2011) Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated activated carbon. Miner Eng 24:788–793
Yu X-Z (2015) Uptake, assimilation and toxicity of cyanogenic compounds in plants: facts and fiction. Int J Environ Sci Technol 12:763–774
Acknowledgment
The authors would like to thank the Environmental Research Institute—University of Isfahan and Iranian National Nanotechnology Initiative for scientific assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Fatih ŞEN.
Rights and permissions
About this article
Cite this article
Eskandari, P., Farhadian, M., Solaimany Nazar, A.R. et al. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: a comparative study. Int. J. Environ. Sci. Technol. 18, 297–316 (2021). https://doi.org/10.1007/s13762-020-02791-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02791-0