Abstract
The treatment of radioactive liquid waste containing organic compounds was always a cause for concern to radioactive waste management facilities because the processes available are expensive and difficult to manage. Biosorption has been studied as a new process in simulated wastes as an alternative to treating them. Among the potential biomass, the coconut fiber has very attractive features that allow the removal of radionuclides using a low-cost biosorbent. The aim of this study was to evaluate the capacity of coconut fiber to remove uranium, americium, and cesium from real radioactive liquid organic waste. Experiments with the biosorption of these radionuclides in coconut fiber were made including (1) preparation, activation, and characterization of biomass and (2) biosorption assays. The biomass was tested in raw and activated form. Biosorption assays were performed, adding the biomass to real waste solutions. The solutions contain natural uranium, americium-241, and cesium-137. The contact times and the concentrations range were varied. The radioisotopes remaining concentration in the solutions was determined by inductively coupled plasma optical emission spectrometry and gamma spectrometry. The results were evaluated by maximum experimental sorption capacity and isotherm and kinetics ternary models. The highest sorption capacity was observed with the activated coconut fiber, with values of 2 mg/g of U (total), 70E−06 mg/g of Am-241 and 40E−09 mg/g of Cs-137. These results suggest that biosorption with activated coconut fiber can be applied in the treatment of radioactive liquid organic wastes containing uranium, americium-241, and cesium-137.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of radioactive waste is an important stage in management to reduce the volume of the wastes and enhance the safety and/or reduce the costs of further stages. Radioactive liquid waste streams may represent a challenge to treat, in general, as the physical properties and chemical composition vary widely (IAEA 2004).
Several methods have been developed to remove heavy metals from aqueous solutions as precipitation, ion exchange, and electrochemical processes, but all of them have technical and economical limitations and its application depends on physical and chemical properties of these solutions (Eroglu et al. 2009; Witek-Krowiak et al. 2011). For instance, ion exchange, membrane technologies, and activated adsorption processes are expensive as well chemical precipitation and electrochemical treatments are ineffective when metal ion concentration in aqueous solution is lower than 50 mg/L (Das et al. 2008).
Approximately 10% of all radioactive wastes produced in the USA are mixed with hazardous or toxic chemicals and therefore cannot be placed in secure land disposal facilities. Mixed wastes containing hazardous organic chemicals are often incinerated, but volatile radioactive elements are released directly into the biosphere. Some mixed wastes do not currently have any identified disposal option and are stored locally awaiting new developments. Biological treatment has been proposed as a potentially safer alternative to incineration for the treatment of hazardous organic mixed wastes since biological treatment would not release volatile radioisotopes and the residual low-level radioactive waste would no longer be restricted from land disposal (Stringfellow et al. 2004).
Biosorption has been studied as an alternative to conventional methods because it is a low-cost and simple method to remove heavy metals from wastewaters. In addition, it is selective, regenerable, and highly effective in the treatment of dilute effluents (Park et al. 2010). This technique can be defined as the property of biomolecules or biomass to bind metal ions, thereby decreasing its concentration in aqueous solutions (Witek-Krowiak et al. 2011; Yang and Volesky 1999). Microorganisms (Kapoor et al. 1999; Vijayaraghavan and Yun 2008; Wang and Chen 2009), agricultural waste (Bansal et al. 2009; Minamisawa et al. 2004), polysaccharide from biopolymers (Feng and Aldrich 2004; Gok and Aytas 2009; Elwakeel et al. 2014, 2017), and algae (Yang and Volesky 1999) are the most studied biosorbents.
The design and efficient operation of an adsorption process needed efficient equilibrium models. The isotherm models are used for description and prediction of the equilibrium data giving the opportunity to scale up in the adsorption process. However, the adsorption equilibria aspect of the multicomponent system and its modeling still needs to be explored. Yet, a variation of Langmuir equilibrium model is the most extensively used featuring its simple approach in describing multicomponent adsorption equilibrium (Soetaredjo et al. 2013).
Natural sorbents are promising and suitable alternative to traditional adsorbents due to their availability in large quantities, low cost, and their sustainable use. There are a number of articles describing the results obtained with a variety of agricultural by-products in removing metalloids and metals, including actinides and lanthanides (Gadd 2009).
There are several studies described in the literature involving the use of agricultural residues in biosorption processes (Ferraz et al. 2014; Garg et al. 2008; Gonçalves et al. 2007; Minamisawa et al. 2004; Saka et al. 2012; Thitame and Shukla 2015; Zhu et al. 2009), and among them, coconut fiber (Cocosnucifera L.) (Bhaumik and Mondal 2015; Israel et al. 2010; Parab et al. 2005; Pino et al. 2006). Coconut fiber or coir fiber is an attractive biosorbent because it is generated in a large quantity as a by-product from coconut processing. Specifically, in Brazil almost 2 tons of coconut was produced in 2011, being 85% of this biomass by-product discarded in the environment (Carrijo et al. 2002; Cazetta et al. 2011). This fiber is composed of several constituents including lignin and cellulose which contain different functional groups as phosphate, carboxyl, sulfide, hydroxyl, and amine, responsible for the metal-binding mechanisms and good chemical stability (Volesky 2003; Volesky and Holan 1995). This characteristic is related to its porous structure and the functional groups including carboxylic and phenolic acid groups (Israel et al. 2010). In addition, biosorption capacity presented by these materials can be improved by changing the structure with chemical treatments. Several authors reported a significant improvement in the ability of metal biosorption by chemically modified biomass attributed to the formation of new functional groups (Krishnani et al. 2008; Vilar et al. 2007; Wan Ngah and Hanafiah 2008).
In the nuclear area, there are no studies reporting the application of biomass or biosorption for the treatment of real radioactive waste. But the literature describes works with citrus waste (Saleem and Bhatti 2011), tea leaves (Ding et al. 2012), pine sawdust (Zhou et al. 2014; Zou and Zhao 2011), pollen pini (Wang et al. 2015), giant kelp biomass (Zhou et al. 2016), elodea Canadensis biomass (Yi et al. 2016), rice husk (Yuan and Fa-Cheng 2011), and coconut (Parab et al. 2005) for removal of uranium from aqueous solution, which results indicated the potential application in treating radioactive effluents. Thus, the objective of this study was to evaluate the capacity of coconut fiber to remove uranium, americium, and cesium from real radioactive liquid organic waste stored at the Nuclear and Energy Research Institute (IPEN-CNEN/SP), Sao Paulo, Brazil. This research was done in this institution, during the year of 2015 and 2016.
Materials and methods
The methodology adopted in this study was divided into two steps: i) preparation, activation, and characterization of biomass and ii) biosorption assays.
Preparation of biomass
The biomass used in this work was the coconut fiber provided by West Garden®. The biomass was washed and dried in an oven at 80 °C for 24 h, sterilized by ultraviolet radiation, triturated, and separated by granulometry (sieve Granulotest®). The fraction between 0.297 and 0.500 mm was utilized in the experiments. Part of the biomass obtained from this fraction was separated to perform the activation procedure, by chemical modification.
Activation of biomass
The biomass was activated (chemically modified) according to the procedure described by Rocha et al. (2009). For this treatment, 10 g of triturated biomass was dispersed in 50 mL of HNO3 solution 1.0 mol L−1 and left agitating for 1 h at room temperature. After that, the material was filtered and the solid was washed with deionized water until the removal of the excess of acid. Then, the material was dispersed in 100 mL of a NaOH solution 0.75 mol L−1 and left under agitation for 1 h at the room temperature. The biomass again was filtered and washed exhaustingly with water and then dried at 60 °C.
Physical characterization
The parameters evaluated for physical characterization of biomass were morphological characteristics of coconut fiber, real and apparent density, and surface area.
The morphological characteristics of coconut fiber were evaluated by scanning electron microscopy performed using a Philips model XL30 scanning electron microscope. The samples were coated with a thin, electric conductive gold film.
The real density of biosorbent was determined by helium pycnometry (Micromeritics, Model 1330) (Vilar et al. 2007). The apparent density was determined by filling a measuring beaker with a defined volume of the specimen and weighing it according to the method described in the Brazilian EMBRAPA standard (EMBRAPA 1997).
The surface area of the biosorbent was determined by BET method (Micromeritcs, ASAP 2010 apparatus), based on nitrogen adsorption–desorption isotherms at 77 K (Brunauer et al. 1938; Danish et al. 2012).
Biosorption assays
Biosorption experiments were carried out using 0.2 g of biomass suspended in 10 mL of solutions prepared by dilution of radioactive liquid waste with distilled water. The dilution ranged from 10 to 100%. The flask with a solution and biosorbent was incubated in a shaker (150 rpm) at room temperature for 2 h, filtered, and the concentrations of uranium were determined in the liquid phase.
At the end of the experiment, the biomass was separated by filtration and the concentration of radioisotope remaining in the filtrate was determined by ICP-OES and gamma spectrometry. The following parameters were evaluated: the contact time between the biomass and waste and the concentration of radioisotopes. The contact times adopted were 30 min, 1, 2, and 4 h, and the concentrations tested ranged between 10 and 100%. In this case, solutions were prepared with deionized water and the raw waste maintaining the same pH of the raw waste, adjusted with 0.1 mol L−1 HNO3 when necessary. All experiments were performed in triplicate.
The radioactive liquid waste used in the experiments is a two-phase solution with ethyl acetate (196 ppm) and tributyl phosphate (227 ppm) at pH 2.17. The radioisotopes, their present activity concentrations, as well as the typical concentrations of the radionuclides in the radioactive waste streams stored at the Nuclear and Energy Research Institute (Ferreira et al. 2013) are described in Table 1.
Determination of U (total) by ICP-OES
The uranium (total) in the samples was quantified by inductively coupled plasma optical emission spectrometry (ICP-OES), model 7000DV (PerkinElmer). A calibration curve was prepared using a standard uranium solution (Matthey Johnson Company) to perform the analysis. The wavelength (λ) used in the determination of the uranium was 385.466 nm, and the result is expressed as the average of triplicate measurements.
Determination of Am-241and Cs-137 by gamma spectrometry
Measurement of Cs-137 and Am-241 was done through a gamma spectrometry (Canberra Industries, model GX4510) System (HPGe detector with beryllium window of 0.5 mm thickness). The detector shielding was composed of a lead wall (105 mm thickness), copper wall (2 mm), and lucite wall (4 mm). The activity concentration for Cs-137 and Am-241 was calculated through a specific energy photopeak of 661.66 and 59.54 keV, respectively.
Metal uptake
The metal uptake of species j in biosorbent was determined using Eq. 1:
where C Ij and C fj are the initial and final concentration of metal species j in solution (mg/L), V is the volume of solution (L), and m is the dry mass of biosorbent (g).
Mathematical modeling of the biosorption process
The modeling of equilibrium adsorption in multicomponent systems is more complex of adsorption in the monocomponent system because of the effects of the competition for the metal species by adsorbent sites.
Langmuir and Freundlich monocomponent isotherms are commonly used in biosorption studies, but these approaches are not adequate to model systems with two or more metals because the combined effects of ion mixtures must be considered. In this case, it is necessary to use models that consider the competition of metals by the biosorbent sites, being the most common models multicomponent Langmuir or modified since.
These effects may be too complex and depend on: the number of metal ions that compete for the binding sites, the interactions among the metal ions, and the metal concentrations (Sağ and Kutsal 1996). Biosorption process with more than two metal ions can produce three types of response: synergism, antagonism, and non-interaction. Synergism occurs when the effect of the mixture is greater than the individual effects of the constituents in the mixture. On the other hand, antagonism occurs when the effect of the mixture is less than the sum of individual effects of the constituents in the mixture. When the effect of the mixture is equal to the sum of individual effects, it is called non-interaction.
Adsorption isotherms in multicomponent systems
In this work, models based on Langmuir and Jain and Snoeyink for ternary systems were used in order to assess the biosorption of the metal species in the biomass. A summary of the four models used is shown in Table 2.
Langmuir ternary adsorption model
Most models used to represent the data of adsorption equilibrium in multispecies systems were obtained by modifications of the Langmuir monocomponent isotherm model. The hypotheses assumed for this model are that the molecules are adsorbed in a number of sites well defined, each site adsorbed only one molecule; the surface is energetic homogenous and non-interactions between the species adsorbed (Radhika and Palanivelu 2006).
The Langmuir assumptions for ternary adsorption isotherm are the same for single species. Mathematical expressions representing the Langmuir isotherm model for a ternary mixture can be obtained from the kinetic Eqs. 2, 3, and 4:
where M 1, M 2, and M 3 are the metal species in the fluid phase, B represents the empty sites, and B − M 1, B − M 2, and B − M 3 are metal species adsorbed onto sites of biosorbent.
For a system at equilibrium, from the kinetic equations of the Langmuir model, one can obtain
where \(q_{j}^{{}}\) is the equilibrium concentration of species j in biosorbent (mmol/g), C j is the equilibrium concentration of species j in solution (mmol/L), and q mC, b 1, b 2, and b 3 are parameters of the model.
Langmuir ternary with two sites
This model assumed that the biosorbent has two types of sites, represented by B U and B C. In B U sites, adsorption occurs only with metal species M 1 and, thus, there is no competition for another species to occupy this site. In B c sites, there are competition among metal species (M 2, M 3) to occupy this sites, which are described by the kinetics Eqs. 8, 9, and 10:
For a system at equilibrium, from the kinetic Eqs. 8–10, one can obtain Eqs. 11, 12, and 13:
where q mU, q mC , b 1, b 2, and b3 are parameters of Langmuir two-site model.
Ternary Jain and Snoeyink model
Jain and Snoeyink (Jain and Snoeyink 1973) originally proposed isotherm model to predict the behavior of equilibrium sorption on activated carbon in binary systems. This model considers that there are two types of sites: one type is selective and adsorbs only one of the two metallic species and the other type adsorbs both species.
In this work, Jain and Snoeyink’s model was adapted for the ternary model, considering two types of binding sites, represented by B U and B C. In this way, in the B U sites occurs adsorption only with the species M 1 and in the B C sites, all species can be adsorbed. In addition, the binding energy of adsorption between the metal species M 1 onto sites B U and B C is assumed to be equal. Then, considering the metallic species in solution M 1, M 2, and M 3, the kinetic equation for B U sites can be expressed by Eq. 14:
For site B C, which occurs competition, one can obtain the kinetics Eqs. 15, 16, and 17:
For a system at equilibrium, from the kinetic Eqs. 14–17, one can obtain Eqs. 18, 19 and 20:
where q mU, q mC , b 1 , b 1, b 2, and b 3 are parameters of the model.
Jain and Snoeyink ternary model modified
The difference of this model compared to the previously developed is that the energy of binding adsorption between the metal species M 1 and sites B U and B C is distinct. From these considerations, we can write the following mathematical equations:
where q mU, q mC , b U1, b 1, b 2, and b3 are parameters of the model.
For the metal species M 2 and M 3, the equations are identical to previously developed model given by Eqs. 19 and 20.
Estimation of parameters
The parameters of the isotherms models were estimated by using the simplex downhill method proposed by Nelder & Mead (Nelder and Mead 1965) to minimize the objective function, which gives Eq. 22:
where n is the number of experimental data, \(q_{1}^{\text{EXP}}\),\(q_{2}^{\text{EXP}}\), and \(q_{3}^{{}}\) represent, respectively, the experimental concentrations of ions in the biosorbent species M 1, M 2, and M 3; \(q_{1}^{\text{MOD}}\),\(q_{2}^{\text{MOD}}\), and \(q_{3}^{\text{MOD}}\) represent, respectively, the concentrations of ions species M 1, M 2, and M 3 in the biosorbent calculated by the model.
Normalization of the data
With the objective of facilitating the construction and interpretation of the figures, the concentrations were normalized using Eq. 23.
where \(q_{{{\text{norm}}_{j} }}\) is the concentration of the normalized element, \(q_{j}\) is the element concentration, and \(q_{{\max_{j} }}\) is the maximum concentration.
Results and discussion
Physical characterization
Figure 1 presents the micrograph images of coconut fibers before and after activation by scanning electron microscopy. It is possible to note modifications on the surface of the activated form caused by the HNO3/NaOH solutions, exposing clearly its pores.
Real density, apparent density, and surface area
The real and apparent densities of activated fiber (1.6 and 0.2 g/cm3, respectively) were lower than that of raw fiber (1.7 and 0.3 g/cm3, respectively). However, the surface area of the activated fiber (8.6 m2/g) was approximately 28.0% higher than that of a raw one (6.2 m2/g), which difference could be attributed to the changes caused chemical treatment, shown in Fig. 1.
The real densities obtained in this study are higher than those described by the works of (Rozman et al. 2000), (Brígida et al. 2010), and (Ali 2010) and can be justified by the selection of fibers with diameters between 0.297 and 0.500 mm performed this work.
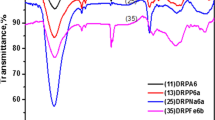
Fourier transform infrared spectroscopy (FTIR)
Functional groups identified by the transmittance spectra obtained by FTIR of biomasses are presented in Table 3. The comparisons of the spectra of biomass, crude and activated, are observed in Fig. 2.
In coconut fiber is observed the occurrence of band 3451 cm−1 that is characteristic of the axial vibration of the OH group. The carbonyl group (COOH) and ester (COO–C) are observed in 1621 and 1442 cm−1, respectively, and the band observed at 1058 cm−1 is related to the stretching vibration of the C–OH group (Ahalya et al. 2010). The activation process did not represent changes in functional groups present in coconut fiber (Fig. 2).
Biosorption assays
Kinetics experiment
Figure 3 shows the results of biosorption as a function of time for uranium (total) (A), Am-241 (B), and Cs-137(C) using the different types of biomass.
The results of the kinetic experiments of the biosorption processes (Fig. 3) showed that equilibrium was reached approximately after 2 h for all biomasses and radioactive elements. No variation in pH value was observed before and after the biosorption process (around 2–3).
Comparison between the biosorbents in biosorption assays
Table 4 shows the biosorption capacity values at equilibrium obtained in kinetics experiments, observed after 2 h for U (total), Am-241, and Cs-137.
Uranium and americium uptake was higher in activated coconut fiber, unlike the cesium, where no significant difference was observed. Quantities of Am-241 and Cs-137 removed were much lower than that of uranium, because their initial concentrations in radioactive liquid waste are much lower, making it impossible to realize an affinity analysis of the biomass and elements. This is because the intermolecular forces acting on the sorption process are dependent on the concentration (Volesky 2003).
The results show that the activation process for the coconut fiber resulted in an increased sorption capacity, probably due to the increased specific surface area.
The activated form of coconut fiber presented sorption capacity 60% higher for the Am-241. Cs-137 was the least adsorbed element in all tested forms of biomass, with no significant differences being observed in the adsorption of this radionuclide.
It was not found in the literature biosorption studies of a ternary system (Am-241, Cs-137, and U) as described here. There are biosorption studies of single elements in simulated wastes, which do not allow a direct comparison.
For instance, in 2012, (Aly and Luca 2012) published a study on the implementation of Arabica coffee powder residue, after its use in an espresso machine, in removing uranium. In their study, maximum sorption capacity of 34.8 mg/g was achieved, 10% higher than the results obtained in this study. Results with coconut fiber were also lower than those found in the literature, but none used real radioactive waste. The works of (Parab et al. 2005) and (Monteiro and Yamaura 2007) reported 235.27 and 27.00 mg/g removal, respectively, studying the biosorption of uranium by coconut fiber in solutions prepared with deionized water at different pHs.
There are no reports in the literature describing the biosorption of americium by lignocellulosic materials. The biosorbent studied in this work showed a lower removal capacity when compared with biosorbents such as Rhizopusarrihizus (Liao et al. 2004), Candida sp (Luo et al. 2003), and Saccharomyces cerevisiae (Liu et al. 2002), which removed between 0.94 and 237.9 MBq/g [(7.45–1880.0 mg/g) (3.09E−05 to 780E−05 mmol/g)]. However, variations in experimental conditions such as time, temperature, and biosorbent/waste ratio should be tested to make a better comparison between biosorbents and determine the Am-241 removal mechanisms.
The biosorption of Cs-137 observed in this study is similar to that obtained by (Mishra et al. 2007), which observed a removal of 9–4200 Bq/g [(1.77–852.00E−10 mg/g) (1.32–636.00E−13 mmol/g)] of Cs-137 by rice husk. Probably, the low cesium sorption capacity is justified by a high concentration of hydrogen ions in the medium (pH = 4). These cesium ions compete with the binding sites present in the rice husk reducing its adsorption (Mishra et al. 2007).
Sorption isotherms
The models of equilibrium adsorption of a ternary system of Langmuir, Langmuir with two sites, Jain Snoeyink, and modified Jain Snoeyink were used to describe the experimental data of equilibrium. The parameter values obtained in q max1 models (maximum biosorption capacity at site 1), b (rate of adsorption and desorption), q max2 (maximum biosorption capacity at site 2), bb1 (rate of adsorption and desorption of uranium in two sites), ADD (relative error), ADD average (average relative error of the three metals) are presented in Table 5. The indexes 1, 2, and 3 refer, respectively, to the metals uranium (total), Am-241, and Cs-137. Graphical representations of the adjustment to all models are shown in Fig. 4 (raw fiber) and Fig. 5 (activated fiber). The adjustment quality of the ternary equilibrium data is in the same figure, and the concentrations of adsorbed metal were normalized using Eq. 24:
where \(q_{Nj}^{{}}\) is the concentration normalized of species j, \(q_{j}^{{}}\) is the concentration of species j in the biosorbent, \(q_{\hbox{max} j}^{{}}\) is the maximum concentration experimental of species j concentrations in the biosorbent.
Considering the mean relative error values ADD presented in Table 5, the biosorption process using coconut fiber as biosorbent in the raw and activated states is best described by Jain and Snoeyink ternary modified model that assumes the presence of two kinds of sites that have different binding energies of adsorption. The values of q mU and q mC parameter for the (uranium) were close to the experimental values.
The mean relative error values presented in Table 5 for the models of Langmuir ternary and Jain and Snoeyink indicate a good adjust of the experimental data of coconut fiber in the raw state. However, these models do not adjust the experimental data for activated coconut fiber.
The parameter b of isotherm models is connected with the metal affinity for sites of the adsorbent material. The higher values of these parameters as compared to the raw and activated biomass are different, showing that the chemical treatment modified adsorbent properties and hence influenced its adsorption capacity. For all forms and models evaluated, the values of the parameters b showed the same affinity sequence, or b 2 (Am-241) > b 1(U) > b3 (Cs-137). This behavior shows that the models are consistent with evaluation of experimental data.
Conclusion
The results obtained in the experiments allow us to conclude that:
The activation resulted in an increased sorption capacity of coconut fiber. The activation process has changed the density of biomass, causing its reduction. For the coconut fiber, activation increased specific surface area. The kinetic and isotherm ternary models have been effective to assess the simultaneous biosorption of uranium, cesium, and americium in organic liquid radioactive waste. All tested biosorbents presented biosorption capacity. However, activated coconut fiber proved the most suitable for the treatment of liquid waste containing Am-241, Cs-137, and uranium because they have greater sorption capacity.
References
Ahalya N, Kanamadi RD, Ramachandra TV (2010) Removal of hexavalent chromium using coffee husk. Int J Environ Pollut 43:106–116. doi:10.1504/IJEP.2010.035917
Ali M (2010) Coconut fibre—a versatile material and its applications in engineering. In: Main Vol. 3. Presented at the 2nd international conference on sustainable construction materials and technologies, pp 1441–1451
Aly Z, Luca V (2012) Uranium extraction from aqueous solution using dried and pyrolyzed tea and coffee wastes. J Radioanal Nucl Chem 295:889–900. doi:10.1007/s10967-012-1851-6
Bansal M, Garg U, Singh D, Garg VK (2009) Removal of Cr(VI) from aqueous solutions using pre-consumer processing agricultural waste: a case study of rice husk. J Hazard Mater 162:312–320. doi:10.1016/j.jhazmat.2008.05.037
Bhaumik R, Mondal NK (2015) Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: thermally and chemically activated coconut fibre dust. Clean Technol Environ Policy 17:2157–2172. doi:10.1007/s10098-015-0937-6
Brígida AIS, Calado VMA, Gonçalves LRB, Coelho MAZ (2010) Effect of chemical treatments on properties of green coconut fiber. Carbohydr Polym 79:832–838. doi:10.1016/j.carbpol.2009.10.005
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. doi:10.1021/ja01269a023
Carrijo OA, de Liz RS, Makishima N (2002) Fibra da casca do coco verde como substrato agrícola. Hortic Bras 20:533–535
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, Silva TL, Moraes JCG, Almeida VC (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125. doi:10.1016/j.cej.2011.08.058
Danish M, Hashim R, Ibrahim MNM, Rafatullah M, Sulaiman O (2012) Surface characterization and comparative adsorption properties of Cr(VI) on pyrolysed adsorbents of Acacia mangium wood and Phoenix dactylifera L. stone carbon. J Anal Appl Pyrolysis 97:19–28. doi:10.1016/j.jaap.2012.06.001
Das N, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7:159–169
Ding D-X, Liu X-T, Hu N, Li G-Y, Wang Y-D (2012) Removal and recovery of uranium from aqueous solution by tea waste. J Radioanal Nucl Chem 293:735–741. doi:10.1007/s10967-012-1866-z
Elwakeel KZ, Atia AA, Guibal E (2014) Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour Technol 160:107–114. doi:10.1016/j.biortech.2014.01.037
Elwakeel KZ, Daher AM, Abd El-Fatah AIL, Abd El Monem H, Khalil MMH (2017) Biosorption of lanthanum from aqueous solutions using magnetic alginate beads. J Dispers Sci Technol 38:145–151. doi:10.1080/01932691.2016.1146617
EMBRAPA (1997) Soil analysis method manual. Centro Nacional de Pesquisa de Solos, Empresa Brasileira de Pesquisa Agropecuária, Rio De Janeiro
Eroglu H, Yapici S, Nuhoglu C, Varoglu E (2009) Biosorption of Ga-67 radionuclides from aqueous solutions onto waste pomace of an olive oil factory. J Hazard Mater 172:729–738. doi:10.1016/j.jhazmat.2009.07.054
Feng D, Aldrich C (2004) Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy 73:1–10. doi:10.1016/S0304-386X(03)00138-5
Ferraz AI, Amorim C, Tavares T, Teixeira JA (2014) Chromium(III) biosorption onto spent grains residual from brewing industry: equilibrium, kinetics and column studies. Int J Environ Sci Technol 12:1591–1602. doi:10.1007/s13762-014-0539-6
Ferreira EGA, Ferreira RVP, Araujo LG, Taddei MHT, Dellamano JC Marumo JT (2013) Chemical analysis of radioactive mixed liquid wastes by alpha/gamma spectrometry, ICP-OES and Arsenazo III. Presented at the international nuclear atlantic conference—INAC 2013, Recife, PE, Brazil
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. doi:10.1002/jctb.1999
Garg U, Kaur MP, Jawa GK, Sud D, Garg VK (2008) Removal of cadmium (II) from aqueous solutions by adsorption on agricultural waste biomass. J Hazard Mater 154:1149–1157. doi:10.1016/j.jhazmat.2007.11.040
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375. doi:10.1016/j.jhazmat.2009.02.063
Gonçalves MMM, de Mello LAO, da Costa ACA (2007) The use of seaweed and sugarcane bagasse for the biological treatment of metal-contaminated waters under sulfate-reducing conditions. Appl Biochem Biotechnol 147:97–105. doi:10.1007/s12010-007-8091-1
IAEA (2004) Predisposal management of organic radioactive waste, Technical reports series/International Atomic Energy Agency. International Atomic Energy Agency, Vienna
Israel A, Ogali R, Akaranta O, Obot IB (2010) Removal of Cu(II) from aqueous solution using coconut (Cocosnucifera L.) coir dust. Pharma Chem 2:60–75
Jain JS, Snoeyink VL (1973) Adsorption from bisolute systems on active carbon. J Water Pollut Control Fed 45:2463–2479
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104. doi:10.1016/S0960-8524(98)00192-8
Krishnani KK, Meng X, Boddu VM (2008) Fixation of heavy metals onto lignocellulosic sorbent prepared from paddy straw. Water Environ Res 80:2165–2174. doi:10.2175/106143008X304785
Liao J, Yang Y, Luo S, Liu N, Jin J, Zhang T, Zhao P (2004) Biosorption of americium-241 by immobilized Rhizopus arrihizus. Appl Radiat Isot 60:1–5. doi:10.1016/j.apradiso.2003.10.001
Liu N, Luo S, Yang Y, Zhang T, Jin J, Liao J (2002) Biosorption of americium-241 by Saccharomyces cerevisiae. J Radioanal Nucl Chem 252:187–191. doi:10.1023/A:1015276813386
Luo S, Liu N, Yang Y, Zhang T, Jin J, Liao J (2003) Biosorption of americium-241 by Candida sp. Radiochim Acta. doi:10.1524/ract.91.6.315.20024
Minamisawa M, Minamisawa H, Yoshida S, Takai N (2004) Adsorption behavior of heavy metals on biomaterials. J Agric Food Chem 52:5606–5611. doi:10.1021/jf0496402
Mishra SP, Prasad SK, Dubey RS, Mishra M, Tiwari D, Lee S-M (2007) Biosorptive behaviour of rice hulls for Cs-134 from aqueous solutions: a radiotracer study. Appl Radiat Isot 65:280–286. doi:10.1016/j.apradiso.2006.09.007
Monteiro RA, Yamaura M (2007) Coir pith of the green coconut in the decontamination of radioactive aqueous effluent. Presented at the international nuclear atlantic conference—INAC 2007, Santos
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308–313. doi:10.1093/comjnl/7.4.308
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96:1241–1248. doi:10.1016/j.biortech.2004.10.016
Park D, Yun Y-S, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102. doi:10.1007/s12257-009-0199-4
Pino GH, Souza de Mesquita LM, Torem ML, Saavedra Pinto GA (2006) Biosorption of cadmium by green coconut shell powder. In: Miner. Eng., Selected papers from processing and disposal of minerals industry wastes’05 19, pp 380–387. doi:10.1016/j.mineng.2005.12.003
Radhika M, Palanivelu K (2006) Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent—Kinetics and isotherm analysis. J Hazard Mater 138:116–124. doi:10.1016/j.jhazmat.2006.05.045
Rocha CG, Zaia DAM, da Alfaya RVS, da Alfaya AAS (2009) Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J Hazard Mater 166:383–388. doi:10.1016/j.jhazmat.2008.11.074
Rozman HD, Tan KW, Kumar RN, Abubakar A, Ishak ZAM, Ismail H (2000) The effect of lignin as a compatibilizer on the physical properties of coconut fiber–polypropylene composites. Eur Polym J 36:1483–1494. doi:10.1016/S0014-3057(99)00200-1
Sağ Y, Kutsal T (1996) The selective biosorption of chromium(VI) and copper(II) ions from binary metal mixtures by R. arrhizus. Process Biochem 31:561–572. doi:10.1016/S0032-9592(95)00100-X
Saka C, Şahin Ö, Küçük MM (2012) Applications on agricultural and forest waste adsorbents for the removal of lead (II) from contaminated waters. Int J Environ Sci Technol 9:379–394. doi:10.1007/s13762-012-0041-y
Saleem N, Bhatti HN (2011) Adsorptive removal and recovery of U(VI) by citrus waste biomass. BioResources 6:2522–2538. doi:10.15376/biores.6.3.2522-2538
Soetaredjo FE, Kurniawan A, Ki OL, Ismadji S (2013) Incorporation of selectivity factor in modeling binary component adsorption isotherms for heavy metals-biomass system. Chem Eng J 219:137–148. doi:10.1016/j.cej.2012.12.077
Stringfellow WT, Komada T, Chang L-Y (2004) Biological treatment of concentrated hazardous, toxic, and radionuclide mixed wastes without dilution. Lawrence Berkeley Natl, Lab
Thitame PV, Shukla SR (2015) Adsorptive removal of reactive dyes from aqueous solution using activated carbon synthesized from waste biomass materials. Int J Environ Sci Technol 13:561–570. doi:10.1007/s13762-015-0901-3
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. doi:10.1016/j.biotechadv.2008.02.002
Vilar VJP, Botelho CMS, Boaventura RAR (2007) Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behaviour. J Hazard Mater 147:120–132. doi:10.1016/j.jhazmat.2006.12.055
Volesky B (2003) Biosorption process simulation tools. Hydrometall Biohydrometall Fundam Technol Sustain Dev 71:179–190. doi:10.1016/S0304-386X(03)00155-5
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250. doi:10.1021/bp00033a001
Wan Ngah WS, Hanafiah MAKM (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99:3935–3948. doi:10.1016/j.biortech.2007.06.011
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. doi:10.1016/j.biotechadv.2008.11.002
Wang F, Tan L, Liu Q, Li R, Li Z, Zhang H, Hu S, Liu L, Wang J (2015) Biosorption characteristics of Uranium (VI) from aqueous solution by pollen pini. J Environ Radioact 150:93–98. doi:10.1016/j.jenvrad.2015.07.002
Witek-Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265:126–134. doi:10.1016/j.desal.2010.07.042
Yang J, Volesky B (1999) Biosorption of uranium on Sargassum biomass. Water Res 33:3357–3363. doi:10.1016/S0043-1354(99)00043-3
Yi Z, Yao J, Zhu M, Chen H, Wang F, Yuan Z, Liu X (2016) Batch study of uranium biosorption by Elodea canadensis biomass. J Radioanal Nucl Chem. doi:10.1007/s10967-016-4839-9
Yuan FENG, Fa-Cheng YI (2011) Adsorptive property of rice husk for uranium. At Energy Sci Technol 45:161–167
Zhou L, Huang Z, Luo T, Jia Y, Liu Z, Adesina AA (2014) Biosorption of uranium(VI) from aqueous solution using phosphate-modified pine wood sawdust. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3725-6
Zhou L, Wang Y, Zou H, Liang X, Zeng K, Liu Z, Adesina AA (2016) Biosorption characteristics of uranium(VI) and thorium(IV) ions from aqueous solution using CaCl2-modified Giant Kelp biomass. J Radioanal Nucl Chem 307:635–644. doi:10.1007/s10967-015-4166-6
Zhu C-S, Wang L-P, Chen W (2009) Removal of Cu(II) from aqueous solution by agricultural by-product: peanut hull. J Hazard Mater 168:739–746. doi:10.1016/j.jhazmat.2009.02.085
Zou W, Zhao L (2011) Removal of uranium(VI) from aqueous solution using citric acid modified pine sawdust: batch and column studies. J Radioanal Nucl Chem 292:585–595. doi:10.1007/s10967-011-1452-9
Acknowledgements
This research was supported by the Nuclear and Energy Research Institute, The National Nuclear Energy Commission and the National Council of Technological and Scientific Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Abhishek RoyChowdhury.
Rights and permissions
About this article
Cite this article
Ferreira, R.V.P., Silva, E.A., Canevesi, R.L.S. et al. Application of the coconut fiber in radioactive liquid waste treatment. Int. J. Environ. Sci. Technol. 15, 1629–1640 (2018). https://doi.org/10.1007/s13762-017-1541-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1541-6