Abstract
There is currently limited research available on the secondary metabolites of moulds in workplaces. The aim of this study was to determine the mould contamination in museums (N = 4), composting plants (N = 4) and tanneries (N = 4) and the secondary metabolite profiles of Alternaria, Aspergillus and Penicillium isolates from these workplaces. Alternaria, Aspergillus and Penicillium species were identified using the ITS1/2 sequence of the rDNA region. Mould metabolites were quantitatively analysed on standard laboratory medium and mineral medium containing materials specific to each workplace using liquid chromatography-mass spectrometry. We also examined the cytotoxicity of the moulds using MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assays. Air microbiological contamination analyses showed a number of microorganisms, ranging from 2.4 × 103 CFU m−3 (composting plants) to 6.8 × 104 CFU m−3 (tanneries). We identified high percentages of Alternaria, Aspergillus and Penicillium moulds (air 57–59%, surfaces 10–65%) in all workplaces. The following moulds were the most cytotoxic (>90%): Alternaria alternata, A. limoniasperae, Aspergillus flavus, Penicillium biourgeianum, P. commune and P. spinulosum. The same mould species isolated from different working environments exhibited varying toxigenic and cytotoxic properties. Modifying the culture medium to simulate environmental conditions most often resulted in the inhibition of secondary metabolite production. Moulds isolated from the working environments produced the following mycotoxins (ng g−1): chanoclavines (0.28–204), cyclopiazonic acid (27.1–1045), fumigaclavines (0.33–10,640,000), meleagrin (0.57–13,393), roquefortins (0.01–16,660), rugulovasines (112–220), viridicatin (0.12–957), viridicatol (4.23–2753) and quinocitrinines (0.07–1104), which may have a negative impact on human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Occupational groups that are exposed to moulds include farmers, gardeners, grain elevator workers, food, beer, feed and herb industry workers, cheese producers, forestry workers, carpenters, and individuals who deal with the storage and processing of municipal solid waste and other plant or animal materials contaminated with microorganisms (Schlosser et al. 2009; Oluwafemi et al. 2012). Some data are available on mould exposure and its health implications for workers employed in museums, composting plants and tanneries (Wiszniewska et al. 2009; Persoons et al. 2010; Skóra et al. 2014).
The most widespread moulds in work environments belong to the Aspergillus, Penicillium and Alternaria genera. Aspergillus and Penicillium species can adapt to water activity (a w) values below 0.8 and thus very frequently grow inside buildings (Nielsen 2002). The genus Penicillium contains approximately 100 toxigenic species, and the diversity of mycotoxins is much higher than for other genera. Citrinin, ochratoxin A, patulin, penitrem A, penicillic acid, roquefortine C and viridicatin are the most characteristic extrolites produced by Penicillium species (Frisvad and Samson 2004). Ochratoxin A can also be produced by Aspergillus strains, in addition to aflatoxins, cyclopiazonic acid, fumonisins, tryptoquivaline, trypacidin, sterigmatocystin, gliotoxins and other mycotoxins (Fischer and Dott 2003; Nielsen et al. 2009). The Alternaria genus is a known producer of alternariol, alternariol monomethyl ether, altenuene, altertoxins I, II, and III, tenuazonic acid and other less toxic metabolites, which are present in human food and animal feed (Ostry 2008).
Many working environments contain bioaerosols that have mycotoxins responsible for cytotoxicity. Mycotoxins are present in the air as spores or mycelium fragments as well as dust particles and are rarely emitted in the form of volatile compounds (Sorenson 1999; Halstensen et al. 2006). Inhalation exposure to mycotoxins in work environments is poorly understood. The main reason for this is the inability to clearly identify direct causal relationships between exposure to mycotoxins and disease occurrence (Robbins et al. 2000; Brera et al. 2002; Fischer and Dott 2003). However, it is estimated that the inhalation of mycotoxins can have a tenfold more toxic effect compared to other routes of exposure (dermal, oral and intraperitoneal), as the inhaled toxins can easily penetrate the pulmonary alveoli (Petzinger and Ziegler 2000). Several cases of pulmonary cancers in oil-press workers, probably resulting from aflatoxin B1 inhalation and exposure to contaminated peanut meal, have been described (Hayes et al. 1984). During respiratory exposure, mycotoxins such as ochratoxin A and tremorgenic mycotoxin can pass into the sera to respectively induce systemic effects such as acute renal failure and neurological syndrome (Di Paolo et al. 1993; Gordon et al. 1993). In a previous study, Autrup et al. (1993) showed that the sera of exposed workers in an animal feed production plant had a significantly higher level of aflatoxin albumin adducts compared to a non-exposed control group. This could possibly explain the increased risk of liver cancer in workers from the animal feed processing industry.
It is well known that the mycotoxins produced by Alternaria, Aspergillus and Penicillium species are strongly cytotoxic. Bünger et al. (2004) found that extracts of Penicillium and Aspergillus moulds were highly toxic to lung, liver and nervous system cell lines.
Based on the above studies, occupational health hazards of workers can be related to the presence of moulds in the workplace and to the ability of these organisms to produce mycotoxins that induce cytotoxicity, allergenicity and mycoses. There is currently limited research on the toxinogenicity and cytotoxicity of moulds isolated from the working environments of museums, composting plants and tanneries. Therefore, our aim was to determine the mould contamination, secondary metabolite profiles and cytotoxicity of Alternaria, Aspergillus and Penicillium strains isolated from these workplaces. In addition, we compared the extrolites produced by the moulds on standard laboratory medium and mineral medium in the presence of extracts from materials from these workplaces.
Materials and methods
Description of the workplaces
Mycological contamination was analysed at four museums, four tanneries and four compost plants (a total of 40 locations) during the 2012–2013 winter season. The temperature and humidity of the air were determined using a PWT-401 hygrometer (Elmetron, Poland). Descriptions of the workplaces are given in Table 1.
Mycological analysis of workplaces
Mycological contamination of the air was determined using an MAS-100 Eco Air Sampler (Merck, Germany). Air samples (50 and 100 L) were collected on MEA medium (Malt Extract Agar, Merck, Germany) with chloramphenicol (0.1%) and DG18 medium (Dichloran 18% Glycerol Agar, Oxoid) to determine the total number of fungi (including hydrophilic and xerophilic strains). Six to eight samples were collected in each room. Samples were also collected from surfaces such as books, furniture, walls, equipment, production machines, wet-blue leather and finished leather at each site. The samples from these surfaces were collected using RODAC Envirocheck® plates with Sabouraud medium (Merck, Germany). For areas with high levels of surface contamination, the traditional swab method was used for the tests. This consisted of collecting samples using swabs containing saline solution (0.85% NaCl) on metal frames with a surface area of 0.0025 m−2 and inoculating them into media (MEA, DG18). Samples were collected from various surfaces in each room, with 6–10 replicates taken for each surface. The samples were incubated at 27 ± 2 °C for 7 days. After incubation, the colonies were counted and the results were expressed in units of CFU m−3 or CFU m−2 after taking into account the volume of air or the surface area, as applicable. The final results were calculated as the arithmetic means of all replicate samples. The percentage and frequency of isolation of the tested species (the percentage of positive samples) were also determined for each working environment.
Identification of moulds
All isolated moulds were identified using taxonomic keys (Klich 2002; Frisvad and Samson 2004; Pitt and Hocking 2009; Bensch et al. 2010; Houbraken et al. 2012; Jurjevic et al. 2012) based on macroscopic and microscopic observations following culture on CYA medium (Czapek Yeast Extract Agar, Difco, USA) and YES medium (yeast extract with supplements according to Samson et al. 1996).
Mould isolates belonging to the Alternaria, Aspergillus and Penicillium genera were confirmed using molecular methods based on a sequence analysis of the ITS1/2 region. Genomic DNA was extracted using the FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH, USA) following the manufacturer’s protocol. Fragments of approximately 500 bp were amplified using the universal primer set ITS1 and ITS4 according to the method reported by White et al. (1990). The PCR mixture contained 40 pmol of each primer, 1.5 U of RedTaq ReadyMix DNA polymerase (Sigma-Aldrich, St. Louis, MO, USA) and 20 ng of template DNA in a final reaction volume of 50 µL. The amplification was performed in the MJ Mini Gradient Thermal Cycler (Bio-Rad, Hercules, CA, USA). The nucleotide sequences of the ITS1/2 region were obtained using the BigDye Terminator Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and were analysed using an Applied Biosystems model 3730 genetic analyser. The nucleotide sequences were assembled and compared with sequences available in The National Center for Biotechnology Information (NCBI) database using the blastn algorithm (BLASTN 2.2.32+) (Zhang et al. 2000).
Cultivation of moulds
The toxinogenicity and cytotoxicity of the moulds were determined using Sabouraud media (Merck, Germany) with 2% agar (as a medium rich in organic compounds) and mineral M0 medium (5 g glucose, MgSO4 × 5 g 7H2O, 3 g (NH4)2SO4, 1 g KH2PO4, 10 g yeast extract, distilled water to 1000 mL, pH 7.0) containing material from the workplaces tested (50 g cellulose, 500 mL compost extract, 50 g fragmented chrome-tanned leather—wet-blue leather shavings). Compost extracts were prepared by suspending 10 g of finished compost in 100 mL of distilled water, then shaking for 30 min, followed by vacuum filtration. The material added was based on the working environment where the strain was isolated (strains isolated from museums were grown on a medium containing cellulose, isolates from tanneries on medium with leather and strains from composting plants on medium with compost extract). Using mineral M0 medium with the above additives allowed us to determine the impact of workplace-specific compounds on the toxigenic and cytotoxic properties of the moulds. The samples were incubated at 27 ± 2 °C for 7 days.
Mould extracts for analyses of the cytotoxicity and secondary metabolites
Three pieces (12 mm in diameter) of Sabouraud and M0 media containing mould (after 5 days of culture; 25 ± 2 °C) were cut and suspended in 5 mL of the extraction solvent (acetonitrile/water/acetic acid 79:20:1, v/v/v). Samples (agarose piece in the extraction buffer) were extracted for 90 min and used for the cytotoxicity and secondary metabolite analyses.
Cytotoxicity analysis
Cell culture and treatment
The LLC-PK1 pig kidney cell line (CLS, Germany, lot no. 607264; from the 36th passage) was used in this study. This cell line is often used as a model for mycotoxin toxicity testing (Gniadek et al. 2010; Nowak et al. 2015). The cells were cultured in T75 Roux flasks (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) as a monolayer in Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F12 (DMEM/Ham’s F12, Sigma-Aldrich, St. Louis, USA) with the addition of 10% heat-inactivated foetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific), 200 mM l-glutamine (Sigma-Aldrich, St. Louis, USA) and 25 mM HEPES (Sigma-Aldrich, St. Louis, USA). The cells were incubated in a CO2 incubator at 37 °C under 5% CO2 for 7–10 days. After reaching confluence, the cells were subcultured every week. The medium was changed every 3–4 days.
LLC-PK1 cells were detached with TrypLE™ Express (Gibco, Thermo Fisher Scientific) for 15–20 min and gently shaken off the plastic flask. The reaction does not need to be terminated with FBS, as this enzyme is of plant origin. After being detached, the cells were suspended in PBS (pH 7.2) and transferred into a 15-mL Falcon tube, centrifuged (182×g, 5 min), decanted and resuspended in fresh DMEM/Ham’s F12 medium. After determining the cell number and viability by trypan blue exclusion (min 90%), the cells were ready to use for experiments.
Cytotoxicity testing using the MTT assay
In the MTT assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole, is reduced to purple formazan in the mitochondria of living cells. The amount of formazan produced is proportional to the amount of MTT in the incubation medium. In the experiments, 5 × 104 LLC-PK1 cells were placed in each well of a 96-well plate (Becton, Dickinson and Co., Franklin Lakes, NJ, USA), and then 100 µL of the complete culture medium was added to each well. The cells were incubated overnight at 37 °C in 5% CO2 to allow them to attach. The following day, the medium was removed and 200 µL of the mycotoxin extract, after evaporation and dilution (composed of 1.7% ethanol, 0.3% DMSO and 98% DMEM/Ham’s F12 medium without FBS), was added to each well, with eight replicate wells for each sample. The control samples consisted of cells without toxic agents. The cells were incubated in a CO2 incubator at 37 °C in 5% CO2 for 48 h. After incubation, the cells were washed with PBS/EDTA, 100 µL of MTT (0.5 mg/mL in PBS; Sigma-Aldrich, St. Louis, MO, USA) was added to each well, and the cells were incubated at 37 °C in 5% CO2 for another 3 h. Following this incubation, the MTT reagent was carefully removed and formazan precipitates were solubilized by the addition of 50 µL of DMSO to each well (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was measured at 550 nm using a microplate reader (ASYS UVM 340, Biogenet). The absorbance of the control sample (untreated cells) was taken to represent 100% cell viability. Cell viability (%) was calculated as follows: [(sample OD (optical density)/control OD)×100%]. Cytotoxicity (%) was calculated as: 100−cell viability (%). The results are presented as the mean ± SD (standard deviation).
Secondary metabolite extraction and analysis
Mould extracts were diluted with the same volume of solvent prior to injection (Sulyok et al. 2006). Centrifugation was not necessary because gravity produced sufficient sedimentation. The extrolites produced by the isolates on laboratory medium were quantitatively analysed using LC–MS/MS, as described by Malachova et al. (2014). Briefly, LC–MS/MS screening of target microbial metabolites was performed with the an QTrap 5500 LC–MS/MS System (Applied Biosystems, Foster City, CA, USA) equipped with a TurboIonSpray electrospray ionization (ESI) source and a 1290 Series HPLC System (Agilent, Waldbronn, Germany). Chromatographic separation was performed at 25 °C on a Gemini® C18-column, 150 × 4.6 mm i.d., 5 μm particle size, equipped with a C18 4 × 3 mm i.d. security guard cartridge (Phenomenex, Torrance, CA, USA).
ESI–MS/MS was performed in the time-scheduled multiple reaction monitoring (MRM) mode in both positive and negative polarities, with two separate chromatographic runs per sample, by scanning two fragmentation reactions per analyte. The MRM detection window of each analyte was set to its expected retention times of ±27 and ±48 s in the positive and negative modes, respectively. The identity of analytes was confirmed by the acquisition of two MRMs per analyte with the exception of moniliformin, which exhibited only one fragment ion. This yielded 4.0 identification points according to European Union Commission decision 2002/657 (EU 2002). The LC retention time and the intensity ratio of the two MRM transitions agreed with the values of a related authentic standard within 0.1 min and 30% rel.
The limits of detection of extrolites were as follows (ng g−1): 3.000—orsellinic acid, verruculogen; 2.400—stemphylperylenol; 1.200—asperfuran; 0.900—cyclopiazonic acid; 0.600—aspterric acid, deoxybrevianamid E, pseurotin A, tenuazonic acid; 0.480—gliocladic acid, helvolic acid, tryptoquivaline F; 0.450—meleagrin; 0.420—pyripyropene A; 0.300—citreoviridin A, penicillic acid, roquefortine C; 0.240—fumonisin B1; 0.180—fumigaclavine C, heptelidic acid, viridicatol; 0.150—chlorocitreorosein, fumitremorgin A, fumitremorgin C; 0.120—cyclopenol, dehydrocyclopeptine, fumiquinazolin A, fumiquinazolin D; 0.090—tryprostatin A, viridicatin; 0.060—3-nitropropionic acid, altertoxin I, bis(dethio)methylthiogliotoxin, brevicompanine B, roquefortine D; 0.048—aurantiamin A, demethylsulochrin, neoechinulin A; 0.042—rugulovasine A; 0.030—alternariol, altersetin, chrysogine, cyclopeptine, neoxaline, oxaline; 0.018—mycophenolic acid, rugulusovine; 0.012—andrastin A, brevianamid F, cyclopenin, nidurufin, O-methylviridicatin; 0.006—chanoclavine, quinocitrinine A, tentoxin; 0.004—emodin; 0.003—averantin; and 0.002—alternariolmethylether, verrucofortine.
Statistical analyses
The results of the assessments of air and surface microbiological contamination, cytotoxicity levels and secondary metabolite concentrations were statistically analysed using STATISTICA 6.0 software (Statsoft, USA). The results were evaluated using one-way analysis of variance (ANOVA) at the 0.05 significance level. When a statistically significant difference was detected (p < 0.05), the means were compared using a post hoc Tukey’s test (microbiological contamination level and secondary metabolite concentrations) and Fisher’s LSD test (cytotoxicity level) at the 0.05 significance level.
Results and discussion
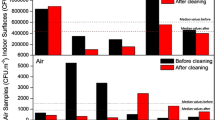
The quantitative analysis of microbiological air contamination showed that the number of airborne microorganisms ranged from 2.4 × 103 CFU m−3 (composting plants) to 6.8 × 104 CFU m−3 (tanneries). The number of microorganisms was significantly different (p < 0.05) in all workplaces tested. The concentration of moulds in the air was lowest in tanneries (7.6 × 102 CFU m−3), higher in composting plants (2.2 × 103 CFU m−3) and highest in museums (3.6 × 103 CFU m−3) (Fig. 1). Surfaces (museum objects, walls, production surfaces, machinery and equipment) were colonized with microorganisms at concentrations between 3.3 × 104 CFU m−2 (tanneries) and 8.9 × 105 CFU m−2 (museums), including the concentration of moulds, which ranged from 1.0 × 104 CFU m−2 to 8.8 × 104 CFU m−2. The total microbial contamination of the surfaces was also significantly different (p < 0.05) depending on the workplace. It is worth highlighting that moulds dominated the microbial community in museums, accounting for 90 and 98% of the microorganisms isolated from the air and surfaces, respectively (Fig. 2).
The total fungal number in all working environments did not exceed the quantitative reference thresholds specified by the Polish Committee for the Highest Permissible Concentrations and Intensities of Noxious Agents in the Workplace (Skowroń and Górny 2012), which is 5.0 × 104 CFU m−3.
We confirmed the results of previous studies carried out in other museums (Gysels et al. 2004; Rojas et al. 2002), which showed microbial contamination, although we detected a higher concentration of moulds. This may be because our study was carried out in the warehouses of museums and not in the exhibition halls, which are more frequently ventilated or dusted.
The number of microorganisms detected in the work environments of composting plants was in line with previously reported mould numbers for green waste composting plants and composting facilities producing button mushroom substrates (1.3 × 103–6.8 × 104 CFU m−3) (Buczyńska et al. 2008; Persoons et al. 2010).
Microbiological contamination in tanneries has not been well explored in the literature. We found 2.4 × 103 CFU m−3 in the production halls and storage areas of these workplaces.
In all workplaces (composting plants, tanneries and museums), we identified a high percentage of moulds belonging to the Aspergillus, Alternaria and Penicillium genera. These accounted for 57–59% of the total number of microorganisms in the air and 10–65% on surfaces (Fig. 3). Moulds of the Penicillium genus predominated in most samples and were found at a high percentage (35–57%) in the air and on surfaces, except for the surfaces of composting plants where they constituted only 5% of the moulds (Fig. 3). Moulds from the Aspergillus genus were also very common in museums (21–26%) but were found at lower levels in composting plants (3–10%) and tanneries (1%). Strains of the Alternaria genus accounted for 1–3% of all isolated moulds in the tested environments (Fig. 3). A high prevalence of the Penicillium, Aspergillus and Alternaria genera in working environments was also noted in earlier studies (Fischer and Dott 2003; Wiszniewska et al. 2009) (Table 2).
Among the 22 species of moulds isolated in this study, 14 were isolated from the air and 8 from surfaces (Table 3). Their frequency of isolation from the air and surfaces varied from 7 to 92%. The most frequently isolated species were Alternaria alternata (72%), A. limoniasperae (64%), Aspergillus flavus (64%), Penicillium biourgeianum (80%), P. chrysogenum (64%), P. commune (86%), P. echinulatum (92%) and P. spinulosum (50%) (Table 3).
The general cytotoxicity of the mould strains against the LLC-PK1 swine kidney cells was high, with the calculated values ranging from 72 to 99% (Table 3). The following moulds were the most cytotoxic (calculated cytotoxicity > 90%): Alternaria alternata (museum M1), A. limoniasperae (M4), Aspergillus flavus (M4 and tannery T3), Penicillium biourgeianum (T4), P. commune (T3) and P. spinulosum (T3). We found that the same mould species isolated from different working environments exhibited varying but statistically significant (p < 0.05) levels of cytotoxicity (Table 3). Aspergillus flavus had a cytotoxicity in the range of 80–99%; the cytotoxicity of P. biourgeianum (strains from composting plants and tanneries) was 83–95% and that of Penicillium spinulosum was 83–94% (strains from two different tanneries) (Table 3). These results were in agreement with an earlier report (Gutarowska et al. 2014), which showed different levels of cytotoxicity for Penicillium strains depending on the place of isolation. Our study also showed that the cytotoxicity may be different for samples isolated from the air and surfaces (P. commune). The strains isolated from surfaces had higher cytotoxicity. To the best of our knowledge, this has not been previously reported in the literature.
The published data concerning the cytotoxicity of moulds are inconclusive and often depend on the methods used for testing. Schulz et al. (2004) studied the cytotoxicity of spore extracts of various mould species, including Aspergillus fumigatus, A. ochraceus, A. niger, Penicillium expansum, P. chrysogenum, Rhizopus stolonifera and Paecilomyces variotii, using different methods: MTT (methylthiazol tetrazolium), MB (methylene blue) and PTG (pollen tube growth test) assays. They showed that the MTT assay was much more sensitive than the other two methods. Moreover, despite the cytotoxic properties of spores, mycotoxins were only detected in the extracts of A. fumigatus (Schulz et al. 2004). In our study, we used the MTT assay with a swine kidney cell line and found that all mould extracts were cytotoxic.
The profiles of the secondary metabolites were tested for four different mould species from the Alternaria genus. The largest number of secondary metabolites was detected for strains isolated from museums, which included A. alternata and A. limoniasperae (N = 11 and N = 9 compounds, respectively). The number of secondary metabolites detected in isolates from composting plants and tanneries was lower (N = 7) (Table 4). All tested Alternaria strains produced high concentrations of mycotoxins on Sabouraud medium, particularly altertoxin I (5.40–677 ng g−1) and tenuazonic acid (1794–51,100 ng g−1) (Table 4).
We observed a lower concentration of metabolites produced by Alternaria moulds grown on mineral medium containing material from the working environments (cellulose, compost extract and fragmented leather), as a carbon source, than when Sabouraud medium was used for the culture (p < 0.05) The only exception was brevianamid F. Mikušová et al. (2014) also observed variations in the production of secondary metabolites (alternariol, alternariol methylether, altenuete, and tenuazonic acid) for 11 strains of Alternaria depending on the culture medium (CYA and YES).
In all of the metabolite profiles tested, 3-nitropropionic acid (141–38,780 ng g−1) and brevianamid F (4.67–225 ng g−1) from A. flavus strains were present at the highest concentrations (Table 5). It has previously been reported that 3-nitropropionic acid is characteristic of A. flavus species (Hedayati et al. 2007). However, brevianamid F is not specific to this species and has also been described for many others species. In addition, significant amounts of gliocladic acid (425–751 ng g−1) and heptelidic acid (31.5–868 ng g−1) were also detected in our study. These substances were mainly identified from Gliocladium, Chaetomium and Trichoderma moulds in a previous study (Itoh et al. 1980). In the present study, the A. fumigatus strains produced large amounts of fumigaclavine C, fumiquinazolin A, fumiquinazolin CD, fumiquinazolin D and tryptoquivaline F (concentrations ranging from 23,387 to 10,640,000 ng g−1). In addition to the above compounds, the secondary metabolites most commonly produced by A. fumigatus genera include fumagillin, fumitremorgins, helvolic acid and gliotoxin (Boudra and Morgavi 2005). We also detected low concentrations of fumitremorgins and helvolic acid but could not detect fumagillin and gliotoxin in our samples.
We observed statistically significant differences in the quantitative and qualitative production of secondary metabolites by the Aspergillus genus, which depended on the composition of the culture medium. Most compounds (averantin, gliocladic acid, heptelidic acid, nidurufin, chanoclavin, fumigaclavine C, fumigaclavine, fumiquinazolin CD, fumitremorgin C, helvolic acid, methylsulochrin, orsellinic acid, tryprostatin B, tryptoquivaline F and verruculogen) were produced at higher concentrations (p < 0.05) on Sabouraud medium than on the medium simulating the location where the strain was isolated. This dependence did not apply to bis(methylthio)gliotoxin, brevianamid F, cyclopiazonic acid, or 3-nitropropionic acid for A. flavus, or fumiquinazolin A, fumitremorgin B, meleagrin or tryprostatin A for A. fumigatus.
The profiles of the secondary metabolites for 14 strains belonging to the Penicillium genus, isolated from the air and surfaces of composting plants and tanneries, were also tested. All Penicillium isolates produced varying amounts of compounds that are characteristic secondary metabolites for this genus including andrastin A, andrastin D, brevianamid F, cyclopenin, cyclopenol, fumiquinazolin CD, meleagrin, penicillic acid and roquefortine C (Tables 6, 7, 8). These metabolites have been previously described in the literature (Frisvad et al. 2004; Frisvad and Samson 2004; Kozlovsky et al. 2013).
We also found qualitative and quantitative differences in the profiles of secondary metabolites from the same species [P. biourgeianum, P. chrysogenum, P. commune and P. spinulosum (Tables 6, 7, 8)] when they were isolated from different locations. For instance, although there were many common compounds produced by both strains of P. biourgeianum, the strain that was isolated from the composting plant produced orsellinic acid and verrucofortine, while the strain from the tannery produced fumigaclavine and fumonisin B1 (Table 7).
The amounts of secondary metabolites from Penicillium strains also depended on the culture medium. Modifying the culture medium to simulate the environmental conditions most often inhibited secondary metabolite production. The exceptions were brevianamid F, chrysogine, meleagrin, neoxaline, O-methylviridicatin, orsellinic acid, oxaline, pseurotin A, roquefortine C and rugulusovine, which were produced at higher concentrations (p < 0.05) on the medium containing leather and compost than on Sabouraud medium (Tables 6, 7, 8).
All moulds belonging to the Aspergillus, Alternaria and Penicillium genera showed variations in their secondary metabolite profiles depending on the species and culture medium. The production of mycotoxins can be affected by many factors, including the chemical composition of the media, the presence of certain trace elements, temperature, humidity, the presence of accompanying microorganisms and the strain genotype (Jarvis et al. 2000).
We detected various mycotoxins produced by Aspergillus, Penicillium and Alternaria that may have a negative impact on human health. The identified substances affect the central and peripheral nervous systems (rugulovasines, cyclopiazonic acid, fumigaclavines and chanoclavines), can cause neurohumoural and antibiotic activity (roquefortins, meleagrin, quinocitrinines rugulovasines, viridicatin, viridicatol) and are nephrotoxic (cyclopiazonic acid) (Kozlovsky et al. 2013).
Conclusion
A quantitative analysis of microbiological air contamination showed that the number of microorganisms present ranged from 2.4 × 103 CFU m−3 (composting plants) to 6.8 × 104 CFU m−3 (tanneries). In all workplaces, we identified high percentages of Alternaria, Aspergillus and Penicillium moulds (57–59% in the air, 10–65% on the surfaces). The general cytotoxicity of the mould strains against swine kidney cells was high, with the calculated values ranging from 72 to 99%. The highest cytotoxicity (>90%) was found for the following moulds: Alternaria alternata, A. limoniasperae, Aspergillus flavus, Penicillium biourgeianum, P. commune and P. spinulosum. The same mould species isolated from different working environments exhibited varying levels of cytotoxicity. We also found qualitative and quantitative differences in the secondary metabolite profiles of Alternaria, Aspergillus and Penicillium moulds, which depended on the culture medium. The modification of the culture medium to simulate environmental conditions most often inhibited secondary metabolite production.
Aspergillus, Alternaria and Penicillium produced mycotoxins that may have a negative impact on human health. These included chanoclavines, cyclopiazonic acid, fumigaclavines, meleagrin, roquefortins, rugulovasines, viridicatin, viridicatol and quinocitrinines. Due to varying cytotoxicity and toxinogenicity of the isolates, future studies should assess their health hazards to workers.
References
Autrup JL, Schmidt J, Autrup H (1993) Exposure to aflatoxin B1 in animal-feed production plant workers. Environ Health Perspect 99:195–197
Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin HD, Dugan FM, Schroers HJ, Braun U, Crous PW (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud Mycol 67:1–94
Boudra H, Morgavi DP (2005) Mycotoxin risk evaluation in feeds contaminated by Aspergillus fumigatus. Anim Feed Sci Tech 120:113–123
Brera C, Caputi R, Miraglia M, Iavicoli I, Salerno A, Carelli G (2002) Exposure assessment to mycotoxins in workplaces: aflatoxins and ochratoxin A occurrence in airborne dusts and human sera. Microchem J 73(1):167–173
Buczyńska A, Sowiak M, Szadkowska-Stańczyk I (2008) Occupational exposure to mesophilic microorganisms associated with commercial processing of compost for mushroom production. Med Pr 59(5):373–379 (in Polish)
Bünger J, Westphal G, Mönnich A, Hinnendahl B, Hallier E, Müller M (2004) Cytotoxicity of occupationally and environmentally relevant mycotoxins. Toxicology 202(3):199–211
Di Paolo N, Guarnieri A, Loi F, Sacchi G, Mangiarotti AM, di Paolo M (1993) Acute renal failure from inhalation of mycotoxins. Nephron 64:621–625
EU (2002) Commission decision of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC)
Fischer G, Dott W (2003) Relevance of airborne fungi and their secondary metabolites for environmental, occupational and indoor hygiene. Arch Microbiol 179:75–82
Frisvad J, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol 49:1–174
Frisvad JC, Smedsgaard J, Larsen TO, Samson RA (2004) Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol 49:201–241
Gniadek A, Macura AB, Twarużek M, Grajewski J (2010) Cytotoxicity of Aspergillus strains isolated from neonatal intensive care unit environment. Adv Med Sci 55(2):242–249
Gordon KE, Masotti RE, Waddell WR (1993) Tremorgenic encephalopathy: a role of mycotoxins in the production of CNS disease in humans? Can J Neurol Sci 20:237–239
Gutarowska B, Skóra J, Stępień Ł, Twarużek M, Błajet-Kosicka A, Otlewska A, Grajewski J (2014) Estimation of moulds contamination and mycotoxins production at the workplaces in composting plants, tanneries, archives and libraries. World Mycotoxin J 7:345–355
Gysels K, Delalieux F, Deutsch F, Griekena RV, Camuffo D, Bernardi A, Sturaro G, Bussec H-J, Wieser M (2004) Indoor environment and conservation in the royal museum of fine arts Antwerp Belgium. J Cult Herit 5:221–230
Halstensen AS, Nordby KC, Klemsdal SS, Elen O, Clasen PE, Eduard W (2006) Toxigenic Fusarium spp. as determinants of trichothecene mycotoxins in settled grain dust. J Occup Environ Hyg 3:651–659
Hayes RB, van Nieuwenhuize JP, Raatgever JW, Ten Kate FJW (1984) Aflatoxin exposures in the industrial setting: an epidemiological study of mortality. Food Chem Toxicol 22:39–43
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007) Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677–1692
Houbraken J, Frisvad JC, Seifert KA, Overy DP, Tuthill DM, Valdez JG, Samson RA (2012) New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia 29:78–100
Itoh Y, Kodama K, Furuya K, Takahashi S, Haneishi T, Takiguchi Y, Arai M (1980) A new sesquiterpene antibiotic, heptelidic acid producing organisms, fermentation, isolation and characterization. J Antibiot (Tokyo) 33(5):468–473
Jarvis BB, Hinkley SF, Nielsen KF (2000) Stachybotrys: an unusual mold associated with water-damaged buildings. Mycotoxin Res 16A:105–108
Jurjevic Z, Peterson SW, Horn BW (2012) Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 3(1):59–79
Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures Utrecht, The Netherlands
Kozlovsky AG, Zhelifonova VP, Antipova TV (2013) Biologically active metabolites of Penicillium fungi. Signpost Open Access J Org Biomol Chem 1:11–21
Malachova A, Sulyok M, Beltran E, Berthiller F, Krska R (2014) Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all relevant mycotoxins in four model food matrices. J Chromatogr A 1362:145–156
Mikušová P, Sulyok M, Šrobárová A (2014) Alternaria mycotoxins associated with grape berries in vitro and in situ. Biologia 69(2):173–177
Nielsen KF (2002) Mould growth on building materials. Secondary metabolites, mycotoxins and biomarkers. Biocentrum-DTU Technical University of Denmark, Lyngby
Nielsen KF, Mogensen JM, Johansen M, Larsen TO, Frisvad JC (2009) Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal Bioanal Chem 395:1225–1242
Nowak A, Śliżewska K, Gajęcka M, Piotrowska M, Żakowska Z, Zielonka Ł, Gajęcki M (2015) The genotoxicity of cecal water from gilts following experimentally induced Fusarium mycotoxicosis. Vet Med-Czech 60(3):133–140
Oluwafemi F, Odebiyi T, Kolapo A (2012) Occupational aflatoxin exposure among feed mill workers in Nigeria. World Mycotoxin J 5(4):385–389
Ostry V (2008) Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J 1(2):175–188
Persoons RS, Parat M, Stoklov Perdrix A, Maitre A (2010) Critical working tasks and determinants of exposure to bioaerosols and MVOC at composting facilities. Int J Hyg Environ Health 213:338–347
Petzinger E, Ziegler K (2000) Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther 23:91–98
Pitt JI, Hocking AD (2009) Fungi and Food Spoilage. Springer, New York
Robbins CA, Swenson LJ, Nealley ML, Gots RE, Kelman BJ (2000) Health effects of mycotoxins in indoor air: a critical review. Appl Occup Environ Hyg 15:773–784
Rojas TI, Martinez E, Gomez Y, Alvarado Y (2002) Airborne spores of Aspergillus species in cultural institutions at Havana University. Grana 41:190–193
Samson RA, Hoekstra ES, Frisvad JC (1996) Introduction to foodborne fungi, 5th edn. Centraalbureau voor Schimmenuturees, Baarn
Schlosser O, Huyard A, Cartnick K, Yanez A, Catalan V, Quang Z (2009) Bioaerosol in composting facilities: occupational health risk assessment. Water Environ Res 81:866–877
Schulz T, Senkpiel K, Ohgke H (2004) Comparison of the toxicity of the reference mycotoxins and spore extracts of common indoor moulds. Int J Hyg Environ Health 207:267–277
Skóra J, Gutarowska B, Stępień Ł, Otlewska A, Pielech-Przybylska K (2014) The evaluation of microbial contamination in the working environment of tanneries. Med Pr 65(1):15–32
Skowroń J, Górny R (2012) Harmful biological agents. In: Augustyńska D, Pośniak M (eds) The interdepartmental commission for maximum admissible concentrations and intensities for agents harmful to health in the working environment: limit values 2012. Centralny Instytut Ochrony Pracy–Państwowy Instytut Badawczy, Warszawa
Sorenson WG (1999) Fungal spores: hazardous to health. Environ Health Perspect 107(Supl.3):469–472
Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20:2649–2665
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetic. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wiszniewska M, Walusiak-Skorupa J, Pannenko I, Draniak M, Pałczyński C (2009) Occupational exposure and sensitization to fungi among museum workers. Occup Med 59:237–242
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7(1–2):203–214
Acknowledgements
Studies in libraries were realized within the project of the Polish National Center for Research and Development coordinated by Central Institute for Labour Protection National Research Institute, Grant number III.B.03. Development of principles for evaluation and prevention of hazards caused by biological agents in the working environment using indicators of microbial contamination.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Xu Han.
Rights and permissions
About this article
Cite this article
Skóra, J., Sulyok, M., Nowak, A. et al. Toxinogenicity and cytotoxicity of Alternaria, Aspergillus and Penicillium moulds isolated from working environments. Int. J. Environ. Sci. Technol. 14, 595–608 (2017). https://doi.org/10.1007/s13762-016-1172-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1172-3