Abstract
The ecological role of viruses in aquatic environments is gaining interest due to their abundance and overall diversity. Much focus has been on bacteriophages since they were found to play an important role in the diversification and sustainment at both the micro- and macro-scale. However, the discovery of virophages coexisting with giant viruses in a diverse set of eukaryotic hosts has recently gained attention. Virophages are small double-stranded DNA viruses found parasitizing giant viruses of eukaryotes. Since the discovery of the first virophage (Sputnik) many virophage signatures have been detected from a variety of environmental samples with specific infection cycles. In addition, these parasites display important roles in equilibrating microbial biomass, nutrient cycling and population dynamics. Moreover, virophage-induced evolution between giant viruses and their hosts have also been described. Considering the ongoing discovery of virophages and their dynamics in aquatic ecosystems, this review intends to provide an update of the virophage signatures identified to date, also tending to provide insight on the mechanisms of coinfection as well as their role as agents of biodiversity and nutrient cyclers in water environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In nature, the unforgiving cycle of prey and predator antics is well known to all life forms in every possible environment. Some smaller, more discrete organisms can predate from the inside out such as eukaryotic and prokaryotic viruses (DeBruyn et al. 2004). The invaluable insight gained on the roles of viruses in the medical and environmental fields has shown the imperativeness of viruses in the maintenance of diversity at both the micro- and macroscopic levels. Furthermore, understanding the natural order in how microbial communities regulate chemical and nutrient cycles to maintain a balance is important in reiterating that the importance of viral studies, particularly in water environments cannot be overstated (DeBruyn et al. 2004; Ram et al. 2011).
Considering the remarkable abundance of viruses in nature, it is easy to assume the expansive existence of viral diversity (Yutin et al. 2009). The nucleo-cytoplasmic DNA viruses (NCLDV) were first described in 2001 (Colson et al. 2013) and seems to constitute a monophyletic group of viruses that contained double-stranded genomes (dsDNA) extending from 100 kbp to 1.26 Mbp (Hingamp et al. 2013). Genomic data have shown that many of the hallmark genes found that the NCLDV genomes are complex and contain transferable short element transpovirons (Yutin et al. 2013; Koonin and Dolja 2014). Another unique feature of giant viruses is their infections by virophages (Yutin et al. 2013) that can only replicate within the intracellular viral factory of a large nucleo-cytoplasmic DNA virus that is currently infecting a eukaryotic host of its own (Zhou et al. 2013).
Considering the novelty of virophages and their ultimate role in aquatic ecosystems, the aim of the present work is to pay tribute to the possible modes of virophage coinfection and their impact on virus–host dynamics and to provide an overview of the virophages discovered to date.
The virus of a virus: revelation of the virophage
Virophages are viruses that infect other viruses (Fischer and Suttle 2011; Taylor et al. 2014). These novel viruses are approximately 50–100 nm in size with an icosahedral morphology (Zhou et al. 2013). Three of the most well-characterized virophages have 20- to 25-kbp double-stranded DNA genomes (Yutin et al. 2013). A unique property of the virophage is the way in which replication occurs. Virophages can only replicate within the intracellular viral factory of a large nucleo-cytoplasmic DNA virus (hereafter referred to as giant viruses) that is currently infecting a eukaryotic host of its own (La Scola et al. 2008; Zhou et al. 2013). As a result, virophages replicate exclusively through coinfection (Desnues and Raoult 2010). The virophage is therefore a parasite that flourishes by exploiting the host’s machinery (Desnues and Raoult 2012), resembling in degenerated and anomalous morphologies of the giant virus (Pearson 2008; Sun et al. 2010; Desnues et al. 2012; Katzourakis and Aswad 2014).
The enzymes required for virophage replication are ascertained from the giant virus and not the host organism (Zhou et al. 2013). Giant viruses have similar genomic and physical sizes as small bacteria (Van Etten et al. 2010). Despite their dependency on the host for replication, giant viruses encode their own transcriptional and part of their translational apparatus within the intra-cytoplasmic viral factory (Mutsafi et al. 2010). Since the virophage genome is smaller as compared to that of the giant virus, it does not code for any of the viral factory components (Desnues and Raoult 2010). Several virophage encoding proteins (open reading frames; ORFs) are distantly linked to genes involved in the replication of giant viruses. Other genes seem to stem from mobile genetic elements such as transposons or plasmids (Kristensen et al. 2009).
Since the discovery of the parasitic virophage and its host, virophages and giant viruses are believed to be highly abundant in aquatic environments, infecting a wide range of hosts (Claverie et al. 2009; Culley 2011). Consequently, virophages may have a role in the host-virus population dynamics (Wodarz 2013). In addition, the impact of the virophage-giant virus dynamic has been shown to influence the rate of carbon cycling in aquatic ecosystems (Culley 2011).
Virophages discovered to date
Currently, four virophages have been isolated from distinct geographical locations (Table 1). The first virophage discovered was coined Sputnik (meaning Russian Satellite) that comprised an 18-kbp double-stranded DNA genome and portrayed an icosahedral morphology (Abrahão et al. 2014). Sputnik was found to be parasitizing a different virus strain, denoted mamavirus (Acanthamoeba castellanii mamavirus, closely related to mimivirus) and was isolated from a cooling tower in Paris in 2008 (Nasir et al. 2012; Parola et al. 2012). The isolation of Sputnik provided two main findings to the current knowledge surrounding virology. The first was the higher rate at which virophage progeny was produced in comparison to Acanthamoeba castellanii mamavirus with the production of defective viral particles, fractional capsid thickening and loss of morphology in mamavirus. The second finding was the encapsidation of the virophage particles into the mamavirus. However, sputnik could not infect the eukaryotic host solely and showed a 70 % declination in mamavirus infectivity and a threefold reduction in its ability to cause lysis when co-inoculated with both sputnik and Acanthamoeba castellanii mamavirus (Ruiz-Saenz and Rodas 2010). The second virophage to be discovered was Mavirus. Mavirus was the first virophage isolated from a marine ecosystem that was found infecting Cafeteria roenbergensis virus (CroV) (Zhou et al. 2013). Thereafter, the Organic Lake Virophage (OLV) isolated from a hypersalinic Antarctic lake associated with an algal host that appears once on an annual basis was isolated. At the same time, Sputnik 2 (associated with mamavirus-like Lentillevirus) was also isolated (Zhou et al. 2013). A third Sputnik strain (Sputnik 3) showing 99 % identity to the other Sputnik strains has been identified (Gaia et al. 2013). Since then, metagenomic sequences have revealed five putative virophage genomes. The nearly complete Ace Lake Mavirus (ALM) genome showed resemblance to Mavirus while the remaining four completed Yellowstone Lake Virophages (YSLV1–4) genomes seem to be related to OLV (Yutin et al. 2013). In addition, the Rio Negro (Campos et al. 2014), Zamilon (Gaia et al. 2014) and three newly discovered Yellowstone Lake virophages (YSLV5–7; (Zhou et al. 2015) were recently identified.
The increasing isolation of virophage genomes associated with phytoplankton-infecting viruses or Mimiviridae-related viruses from diverse environmental samples infer the role of virophages in the regulation of viral populations. In spite of this, the nature of virophages is still debatable (Gaia et al. 2014).
Modes of virophage coinfection
Since virophages can only reproduce within the viral factory of a giant virus, the mechanism in which coinfection occurs is important. Taylor et al. (2014) proposed two different main entry modes (Fig. 1). The first is an independent entry mode (Fig. 1a) where the virus and virophage enter the host independently of each other. The second is referred to as the paired entry mode (Fig. 1b). In this method, co-infection occurs when the virophage and virus are entangled and this entanglement then enters the host. Sputnik strains are assumed to use the paired entry mode (Desnues and Raoult 2010; Taylor et al. 2014). Indirect support of this mode of infection was observed by virophage and virus clustering, suggesting affinity. Furthermore, electron micrographs of virus and virophage present in one phagocytic vacuole after coinfection may further support the paired entry mode of coinfection (Desnues and Raoult 2010). Virus–virophage grouping may also occur as observed by long fibres on the surface of mamavirus (associated with Sputnik strains). These fibres (coated in peptidoglycan) are thought to have a role in phagocytosis, functioning to mimic bacterial prey to the host amoeba (Xiao et al. 2009; Taylor et al. 2014). This was supported by Boyer et al. (2011), where virophages were unable to replicate in a coculture with fibre-deficient mamavirus-like strains. In addition, mushroom-like fibrils coat the capsid of Sputnik (Sun et al. 2010). Although the function of these appendages is unknown, it is thought to promote virophage-virus (Sputnik-mamavirus) entanglement for the paired entry mode of coinfection (Desnues and Raoult 2010; Taylor et al. 2014).
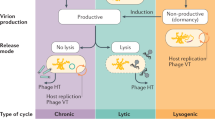
Different stages in the virophage coinfection lifecycle. a Independent entry mode (Taylor et al. 2014): Step 1: free virophage and virus following lysis. Step 2: virophage (free) enters host. Step 3: giant virus enters amoeba. Step 4: virus particles shed capsids. Step 5: virophage genome enters viral factory (viral factory expands). Step 6: virophages leave viral factory and wait for lysis. b Paired entry mode (Taylor et al. 2014), only steps 2 and 3 differ. Step 2: virophage and giant virus entangle. Step 3: the entanglement enters the host as coinfection. c Giant virus and virophage replication the absence of a eukaryotic host (Yau et al. 2011). d Direct contact mechanisms for virophage and giant virus replication (Wodarz 2013)
The paired and independent entry modes modelled by Taylor et al. (2014) included the coexistence of all populations (amoeba host, giant virus and virophage) in the system taking into account the need of coinfection for virophage replication to occur. In comparison, Yau et al. (2011) modelled the virophage–virus relationship in a predator–prey system where virophage growth was theorized as host independent (Fig. 1c). However, the outcome of this event in nature is debatable, since virophage replication has yet to be observed in systems without coinfection with a eukaryotic host present (La Scola et al. 2008). The final model by Wodarz (2013) excluded the outcome of planktonic virophage and virus population within the system. The author posited a direct contact mechanism of virus and virophage transmission among eukaryotic hosts (Fig. 1d). Although direct and indirect transmission models have been observed when degradation occurs, there is currently no experimental evidence suggesting this type of event (Taylor et al. 2014). Recently, a distant CRISPR-Cas-like mechanism, called the MIMIVIRE system, has been unveiled in giant viruses (Levasseur et al. 2016). This mechanism explains the resistance of miniviruses to Zamilon, a unique virophage can infect specific linkages of miniviruses but not others (Gaia et al. 2014). The field of virophage–virus dynamics is still an elusive topic (Slimani et al. 2013). Until more experimental data on the modes of coinfection and virophage dynamics become available, the true nature of virophage replication (specifically entry pathways and interactions) can only be postulated.
The overall replication cycle with the alternative (independent or paired) entry modes of coinfection are illustrated in Fig. 1a, b. Irrespective of the entry mode, once the virophage has entered the eukaryotic host, replication proceeds in the same manner (Taylor et al. 2014). The capsids of the giant virus and virophage are detached from the viral core and genome, respectively. This step has been observed for the giant virus and not the virophage itself (Mutsafi et al. 2010). Thereafter, the virophage genome enters the viral factory of the giant virus. The size of the viral factory expands as virophage genetic material is rapidly produced (Mutsafi et al. 2010; Desnues and Raoult 2012). Fully-fledged capsid enclosed virophages are only expelled from the host cell upon lysis (Taylor et al. 2014).
Virus–host interactions and roles in nutrient cycling
Over recent years, virophages, coexisting with giant viruses and their eukaryotic hosts have been isolated from cooling towers, to contact lens solution to the deepest oceans (Zhou et al. 2013). In aquatic ecosystems, virophages and giant viruses are thought to be highly abundant (Claverie et al. 2009; Culley 2011; Yau et al. 2011; Wodarz 2013). As a result, virophages may regulate population dynamics between giant viruses (Gaia et al. 2014) and protists (Wodarz 2013). In a coinfection of mamavirus and Sputnik virophages (La Scola et al. 2008) demonstrated approximately a 70 % reduction in the production of mamavirus virions and a threefold decrease in amoeba lysis at 24 h. Similarly, coinfection of eukaryotic host Cafeteria roenbergenesis with CroV and Mavirus virophage resulted in increased C. roenbergenesis concomitant with decreased CroV burst size (Fischer and Suttle 2011). These experimental data provided evidence that virophages influences the replication of the giant virus (Slimani et al. 2013). A mathematical and analytical model developed by Taylor et al. (2014) further demonstrated that an increased production of virophage was negatively correlated to the giant virus burst size, acting as a “friend” to the eukaryotic hosts. Furthermore, the relationship between an amoeba–bacterium–virus–virophage system was investigated by Slimani et al. (2013), which revealed that when Acanthamoeba polyphaga was superinfected with bacterium BABL1 and A. polyphaga mimivirus, BABL1 reproduction was stable for 24 h. Thereafter, bacterial growth decreased and mimivirus replication was comparable when alone (amoeba-mimivirus only). However, when Sputnik-infected A. polyphaga mimivirus was superinfected into the bacterium-infected A. polyphaga, the amoeba and bacterium populations were compared to that alone (amoeba and bacterium only) with increased virophage numbers. Taken together, these results indicate the role of virophages in not only eukaryotic and giant virus populations, but also the regulation of bacterial growth in aquatic environments (Slimani et al. 2013).
Discovery of the virophage augments thought of its place in the microbial loop (Yau et al. 2011). In an attempt to understand population dynamics on nutrient cycling, mathematical models developed by Yau et al. (2011) demonstrated that the reduction of phycodnaviruses by the OLV virophage and increased frequency of algal blooms affected the carbon flux in the Organic Lake (Vestfold Hills; Antarctica). Through the adaptation of Antarctic lakes to shortened trophic chains and long light–dark cycles, it is probable that reduced phycodnavirus virulence by virophages may play an important role in the stability of microbial food webs in aquatic ecosystems (Cavicchioli 2015).
Virophage signatures found through metagenomic analyses in two tropical lakes (fresh and hypersaline), an ocean upwelling site and an estuary as well as in the Ace Lake (Yau et al. 2011) and other temperate aquatic environments (Zhou et al. 2013) indicate that virophages may regulate carbon flux and play unrecognized roles in aquatic ecosystems (Jassim and Limoges 2013). However, the ecological distribution of virophages in aquatic ecosystems is noteworthy. A shotgun metagenomic analysis conducted by Zhou et al. (2015) of virophages isolated from Yellowstone Lake, depicted that the distribution, diversity and abundance of virophages was concomitant with water temperature and chemistry. Non-hydrothermal ecosystems (preferably temperatures approximately 30 °C) harbour the most amounts of virophages. Therefore, virophage distribution patterns may be linked to their viral and eukaryotic hosts (Zhou et al. 2015).
Virophages as driving forces in microbial evolution
Viruses can drive evolutionary mechanisms of the host in two ways: the first employs direct effect of intrinsic viral activities through host and viral gene transfer between species and speciation (Weitz et al. 2013); regulates competition between dominant organisms (Barros et al. 2010); and induces genotypic and phenotypic changes within the infected hosts with the production of protection against super-infections (Weinbauer and Rassoulzadegan 2004). The second mechanism are indirect effects which include genetic-rich genomes that promote horizontal gene transfer (Martiny et al. 2014); bacterial biomass encouraging microbial composition and diversity (Middelboe et al. 2008); and mechanisms involved in bacterial defence to phage infection (Samson et al. 2013). The broad distribution and the indisputable abundance of viruses allow a variety of transmissible genetic material that promotes evolution and ecological modifications within microbial communities (Sime-Ngando 2014). Some changes may be as bold and successive as to create an entirely new strain or new species (Hartley et al. 2012). However, the alteration of microbial composition and diversity is dependent on host specificity by the virus. It is evident that bacteriophages (viruses infecting bacteria) are highly host-specific and only infect a small portion of bacterial species (Koskella and Meaden 2013). Most host–parasite models have focused on bacterial phage interactions (Avrani et al. 2012). Since the discovery of virophages in nature and its role in nutrient cycling and species dynamics, it is important to evaluate the role of virophages in driving microbial evolution (Wodarz 2013).
There are different types of models that can be used to evaluate aquatic microbial diversity and evolution (Martiny et al. 2014). Antagonistic coevolution is an evolutionary model used to describe the on-going adaptation and counter-adaptation in host–parasite systems. This concept is referred to as an arms race as species continue to adapt to changes imposed on by their counterparts (Avrani et al. 2012). Campos et al. (2014) experimentally demonstrated a higher tolerance of the newly discovered Samba virus to its associated Rio Negro virophage in comparison to Acanthamoeba polyphaga mimivirus. The authors suggest a mechanistic defence developed by the Samba virus to evade the virophage. However, confirmation of this hypothesis has yet to be performed (Campos et al. 2014). The evaluation of the Samba virus–Rio Negro virophage composite over time may allow for the detection of evolutionary traits between the virophage and giant virus populations to be explored.
The second type of model involves defence and competitive trade-offs (Winter et al. 2010). In nature, nutrient availability can be used as a tool for the prevention of various stresses such as predation, unfavourable conditions, parasitism and competitive purposes or to promote fitness and increased reproduction. The allocation of resources to either strategy results in a limitation to the other. Trade-offs between viruses and their hosts are poorly understood. However, a report on bacteriophages infecting Escherichia coli showed that a high mortality rate was positively correlated to high replication rates. Thus, a trade-off was made to promote replication and not to sustain the stability of their protective capsids (Winter et al. 2010). By far, the most apparent role of viruses in microbial regulation would be its role in host population mortality. This allows viruses to control microbial diversity in a hypothesis known as “killing the winner (Ktw)”. The Ktw hypothesis is based on negative frequency-dependant selection as bacterial fitness is indirectly related to its frequency in the community (Koskella and Meaden 2013). The general logic of “killing the winner” relies on the fact that infective viruses introduced by migration or mutation provide density dependent regulation of dominant competitive bacteria while allowing less dominant bacterial populations to prevail (Thingstad and Lignell, 1997; Brockhurst et al. 2006). Preferentially, attacking the dominant species provides a self-regulating negative feedback so that the dominant species are down-regulated by the predators while the rest of the populations can recover by granting them a predatory refuge (Vallina et al. 2014a, b). Yau et al. (2011) used the Ktw hypothesis (Lotka–Volterra-type mathematical model) to underline the point of virophages (OLV) in the regulation of population dynamics (Phycodnaviruses and microalgae) with effects on nutrient cycling in the environment. Wodarz (2013) used an Acanthamoeba–mimivirus–Sputnik system to provide a more general explanation of these dynamics with emphasis on the evolutionary dynamics. Since virus–host dynamics can often be viewed as hyperparasitism, this parameter was included in the model. A hyperparasite can be defined as a parasite infecting a different parasite which contributes to a food chain of parasitism (Wodarz 2013). Most hyperparasitic studies have examined population dynamics between insect pests and their biological control using mathematical models (Holt and Hochberg 1998; Wodarz 2013). The overall result produced by all the studies was the reduction of biological control of the pests by the hyperparasite. Since the hyperparasite infects the primary parasite, the host or pest population benefits and can reach increased equilibrium levels (May and Hassell 1981). The model developed by Wodarz (2013) suggests that viral replication rates can impact the stability of the system, which also influences how likely the system is to extinction. If the giant virus can maintain reproductive success through sediment reservoirs (i.e. free-living cells) than through amoeba-induced replication, the stability of the system will be maintained. This ensures the survival of the giant virus in the presence of virophage. Furthermore, the model suggests virophages can select for giant viruses with reduced replicative ratios. Not only would this allow for a direct benefit to the amoeba population, but also lead to an indirect influence on evolution of the giant virus population (Wodarz 2013).
In addition to these models, lateral gene transfer has been observed between amoeba and its parasites (Moreira and Brochier-Armanet 2008) and between virophages and their viral hosts (La Scola et al. 2008). Virophages have also found to be related to eukaryotic DNA transposons referred to as Mavericks (Katzourakis and Aswad 2014). Yutin et al. (2013) found that different mobile elements (Mavericks and transposons) shared certain conserved regions with virophages and other viruses. Although diversity between virophages does exist, the DNA-packaging ATPase and the major capsid protein are conserved among all virophages (Desnues et al. 2012). Therefore, virophages are likely to have a common ancestor, which in turn underwent multiple gene replacements sharing evolutionary connections with giant viruses and mobile genetic elements (Koonin and Yutin 2010).
Concluding remarks
The abundant viral diversity in aquatic ecosystems remains largely undiscovered. Consequently, viral sequences have very limited similarity to sequences found in repositories (López-Bueno et al. 2009). In spite of this, it is distinctly apparent that viruses have a major role in shaping microbial communities, regulating nutrient cycling and driving evolution dynamics in nature. In depth analyses of viruses in aquatic environments is essential to the understanding aquatic ecosystem functioning (Danovaro et al. 2011; Wilkins et al. 2013). The role of virophages in aquatic ecosystems in particular deserves more attention. Global ecological regulation of population dynamics, evolution courses and nutrient cycling by virophages suggests that more virophages have yet to be discovered (Gaia et al. 2014; Durzyńska and Goździcka-Józefiak 2015; Zhou et al. 2015). The findings of several ecological studies of virophages in water bodies do infer some important contribution by these viruses (La Scola et al. 2008; Yau et al. 2011; Campos et al. 2014; Durzyńska and Goździcka-Józefiak 2015). Currently, the existence of virophages and their place in nature is still poorly understood. The concept of coevolution has been linked to the divergence of life forms. Coevolution consists of mutual genetic changes between two or more species in a given community and is an important driver in morphological changes, immune defence, virulence, speciation, drug resistance, and the structuring of communities (Dennehy 2012). Furthermore, the open debate on virophage classification as a parasite on its own (as opposed to being a satellite virus), only enforces the need for more aquatic environmental studies evaluating its role in microbial food web. As a result, metagenomic approaches coupled with extensive population dynamics to identify virophage signatures in the environment is needed.
References
Abrahão JS, Dornas FP, Silva LCF, Almeida GM, Boratto PVM, Colson P, La Scola B, Kroon EG (2014) Acanthamoeba polyphaga mimivirus and other giant viruses: an open field to outstanding discoveries. Virol J 11:120
Avrani S, Schwartz DA, Lindell D (2012) Virus–host swinging party in the oceans. Mob Genet Elem 2:88–95
Barros N, Farjalla VF, Soares MC, Melo RCN, Roland F (2010) Virus–bacterium coupling driven by both turbidity and hydrodynamics in an Amazonian Floodplain Lake. Appl Environ Microbiol 76:7194–7201
Boyer M, Azza S, Barrassi L, Klose T, Campocasso A, Pagnier I, Fournous G, Borg A, Robert C, Zhang X, Desnues C, Henrissat B, Rossmann MG, La Scola B, Raoult D (2011) Mimivirus shows dramatic genome reduction after intraamoebal culture. PNAS 108:10296–10301
Brockhurst MA, Fenton A, Roulston B, Rainey PB (2006) The impact of phages on interspecific competition in experimental populations of bacteria. BMC Ecol 6:19
Campos RK, Boratto PV, Assis FL, Aguiar ER, Silva LC, Albarnaz JD, Dornas FP, Trindade GS, Ferreira PP, Marques JT, Robert C, Raoult D, Kroon EG, La Scola B, Abrahão JS (2014) Samba virus: a novel mimivirus from a giant rain forest, the Brazilian Amazon. Virol J 11:95
Cavicchioli R (2015) Microbial ecology of Antarctic aquatic systems. Nat Rev Microbiol 13:691–706
Claverie JM, Grzela R, Lartigue A, Bernadac A, Nitsche S, Vacelet J, Ogata H, Abergel C (2009) Mimivirus and Mimiviridae: giant viruses with an increasing number of potential hosts, including corals and sponges. J Invertebr Pathol 101:172–180
Colson P, De Lamballerie X, Yutin N, Asgari S, Bigot Y, Bideshi DK, Cheng XW, Federici BA, Van Etten JL, Koonin EV, La Scola B, Raoult D (2013) “Megavirales”, a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses. Arch Virol 158:2517–2521
Culley AI (2011) Virophages to viromes: a report from the frontier of viral oceanography. Curr Opin Virol 1:52–57
Danovaro R, Corinaldesi C, Dell’Anno A, Fuhrman JA, Middelburg JJ, Noble RT, Suttle CA (2011) Marine viruses and global climate change. FEMS Microbiol Rev 35:993–1034
DeBruyn JM, Leigh-Bell JA, Mckay RML, Bourbonniere RA, Wilhelm SW (2004) Microbial distributions and the impact of phosphorus on bacterial activity in Lake Erie. J Great Lakes Res 30:166–183
Dennehy JJ (2012) What can phages tell us about host-pathogen coevolution? Int J Evol Biol. doi:10.1155/2012/396165
Desnues C, Raoult D (2010) Inside the lifestyle of the virophage. Intervirology 53:293–303
Desnues C, Raoult D (2012) Virophages question the existence of satellites. Nat Rev Microbiol 10:234
Desnues C, La Scola B, Yutin N, Fournous G, Robert C, Azza S, Jardot P, Monteil S, Campocasso A, Koonin EV, Raoult D (2012) Provirophages and transpovirons as the diverse mobilome of giant viruses. PNAS 109:18078–18083
Durzyńska J, Goździcka-Józefiak A (2015) Viruses and cells intertwined since the dawn of evolution. Virol J 12:169
Fischer MG, Suttle CA (2011) A virophage at the origin of large DNA transposons. Science 332:231–234
Gaia M, Pagnier I, Campocasso A, Fournous G, Raoult D, La Scola B (2013) Broad spectrum of Mimiviridae virophage allows its isolation using a mimivirus reporter. PLoS ONE 8:e61912
Gaia M, Benamar S, Boughalmi M, Pagnier I, Croce O, Colson P, Raoult D, La Scola B (2014) Zamilon, a novel virophage with Mimiviridae host specificity. PLoS ONE 9:e94923
Hartley M-A, Ronet C, Fasel N (2012) Backseat drivers: the hidden influence of microbial viruses on disease. Curr Opin Microbiol 15:1–8
Hingamp P, Grimsley N, Acinas SG, Clerissi C, Subirana L, Poulain J, Ferrera I et al (2013) Exploring nucleo-cytoplasmic large DNA viruses in Tara Oceans microbial metagenomes. Int Soc Microbiol Ecol J 7:1678–1695
Holt RD, Hochberg ME (1998) The coexistence of competing parasites. Part II—hyperparasitism and food chain dynamics. J Theor Biol 193:485–495
Jassim SAA, Limoges RG (2013) Impact of external forces on cyanophage–host interactions in aquatic ecosystems. World J Microbiol Biotechnol 29:1751–1762
Katzourakis A, Aswad A (2014) The origins of giant viruses, virophages and their relatives in host genomes. BMC Biol 12:51
Koonin EV, Dolja VV (2014) Virus world as an evolutionary network of viruses and capsidless selfish elements. Mol Biol Rep 78:278–303
Koonin EV, Yutin Y (2010) Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses. Intervirology 53:284–292
Koskella B, Meaden S (2013) Understanding bacteriophage specificity in natural microbial communities. Viruses 5:806–823
Kristensen DM, Mushegian AR, Dolja VV, Koonin EV (2009) New dimensions of the virus world discovered through metagenomics. Trends Microbiol 18:11–19
La Scola B, Desnues C, Pagnier I, Robert C, Barrassi L, Fournous G, Merchat M, Suzan-Monti M, Forterre P, Koonin E, Raoult D (2008) The virophage as a unique parasite of the giant mimivirus. Nature 455:100–105
Levasseur A, Bekliz M, Chabrière E, Pontarotti P, La Scola B, Raoult D (2016) MIMIVIRE is a defence system in mimivirus that confers resistance to virophage. Nature. doi:10.1038/nature17146
López-Bueno A, Tamames J, Velázquez D, Moya A, Quesada A, Alcamí A (2009) High diversity of the viral community from an Antarctic Lake. Science 326:858–861
Martiny JBH, Riemann L, Marston MF, Middelboe M (2014) Antagonistic coevolution of marine planktonic viruses and their hosts. Annu Rev Mar Sci 6:393–414
May RM, Hassell MP (1981) The dynamics of multiparasitoid–host interactions. American Naturalist 117:234–261
Middelboe M, Jacquet S, Weinbauer M (2008) Viruses in freshwater ecosystems: an introduction to the exploration of viruses in new aquatic habitats. Freshw Biol 53:1069–1075
Moreira D, Brochier-Armanet C (2008) Giant viruses, giant chimeras: the multiple evolutionary histories of mimivirus genes. BMC Evol Biol 8:12
Mutsafi Y, Zauberman N, Sabanay I, Minsky A (2010) Vaccinia-like cytoplasmic replication of the giant mimivirus. PNAS 107:5978–5982
Nasir A, Kim KM, Caetano-Anolles G (2012) Giant viruses coexisted with the cellular ancestors and represent a distinct supergroup along with superkingdoms Archaea, Bacteria and Eukarya. BMC Evol Biol 12:156
Parola P, Renvoisé A, Botelho-Nevers E, La Scola B, Desnues C, Raoult D (2012) Acanthamoeba polyphaga mimivirus virophage seroconversion in travelers returning from Laos. Emerg Infect Dis 18(9):1500–1502
Pearson H (2008) “Virophage” suggests viruses are alive. Nature 454:677
Ram ASP, Rasconi S, Jobard M, Palesse S, Colombet J, Sime-Ngando T (2011) Study of prokaryotic viruses in freshwater lakes reveals high lytic infection rates but low abundances in a humic lake (Vassiviere, Massif Central, France). Appl Environ Microbiol 77:5610–5618
Ruiz-Saenz J, Rodas JD (2010) Viruses, virophages, and their living nature. Acta Virol 54:85–90
Samson JE, Magadán AH, Sabri M, Moineau S (2013) Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11:675–687
Sime-Ngando T (2014) Environmental bacteriophages: viruses of microbes in aquatic ecosystems. Front Microbiol 5:355
Slimani M, Pagnier I, Raoult D, La Scola B (2013) Amoebae as battlefields for bacteria, giant viruses, and virophages. J Virol 87:4783–4785
Sun S, La Scola B, Bowman VD, Ryan CM, Whitelegge JP, Raoult D, Rossmann MG (2010) Structural studies of the Sputnik virophage. J Virol 84:894–897
Taylor BP, Cortez MH, Weitz JS (2014) The virus of my virus is my friend: ecological effects of virophage with alternative modes of coinfection. J Theor Biol 354:124–136
Thingstad TF, Lignell R (1997) Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol 13:19–27
Vallina SM, Follows MJ, Dutkiewicz S, Montoya JM, Cermeno P, Loreau M (2014a) Global relationship between phytoplankton diversity and productivity in the ocean. Nat Comm 5:4299
Vallina SM, Word BA, Dutkiewicz S, Follows MJ (2014b) Maximal feeding with active prey-switching: a kill-the-winner functional response and its effect on global diversity and biogeography. Prog Oceanogr 120:93–109
Van Etten JL, Lane LC, Dunigan DD (2010) DNA viruses: the really big ones (giruses). Annu Rev Microbiol 64:83–99
Weinbauer MG, Rassoulzadegan F (2004) Are viruses driving microbial diversification and diversity? Environ Microbiol 6:1–11
Weitz JS, Poisot T, Meyer JR, Flores CO, Valverde S, Sullivan MB, Hochberg ME (2013) Phage–bacteria infection networks. Trends Microbiol 21:82–91
Wilkins D, Yau S, Williams TJ, Allen MA, Brown MV, DeMaere MZ, Lauro FM, Cavicchioli R (2013) Key microbial drivers in antarctic aquatic environments. FEMS Microbiol Rev 37:303–335
Winter C, Bouvier T, Weinbauer MG, Thingstad TF (2010) Trade-offs between competition and defence specialists among unicellular planktonic organisms: the ‘killing the winner’ hypothesis revisited. Microbiol Mol Biol Rev 74:42–57
Wodarz D (2013) Evolutionary dynamics of giant viruses and their virophages. Ecol Evol 3:2103–2115
Xiao C, Kuznetsov YG, Sun S, Hafenstein SL, Kostyuchenko VA, Chipman PR, Suzan-Monti M, Raoult D, McPherson A, Rossmann MG (2009) Structural studies of the giant mimivirus. PLoS Biol 7:958–966
Yau S, Lauro FM, DeMaere MZ, Brown MV, Thomas T, Raftery MJ, Andrews-Pfannkoch C, Lewis M, Hoffman JM, Gibson JA, Cavicchioli R (2011) Virophage control of antarctic algal host–virus dynamics. PNAS 108:6163–6168
Yutin N, Wolf YI, Raoult D, Koonin EV (2009) Eukaryotic large nucleo-cytoplasmic DNA viruses: clusters of orthologous genes and reconstruction of viral genome evolution. Virol J 6:223. doi:10.1186/1743-422X-6-223
Yutin N, Raoult D, Koonin EV (2013) Virophages, polintons, and transpovirons: a complex evolutionary network of diverse selfish genetic elements with different reproduction strategies. Virol J 10:158
Zhou J, Zhang W, Yan S, Xiao J, Zhang Y, Li B, Pan Y, Wang Y (2013) Diversity of virophages in metagenomic data sets. J Virol 87:4225–4236
Zhou J, Sun D, Childers A, McDermott TR, Wang Y, Liles MR (2015) Three novel virophage genomes discovered from yellowstone lake metagenomes. J Virol 89:1278–1285
Acknowledgments
The authors would like to thank the National Research Foundation, South Africa for providing the Masters scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Marie, V., Lin, J. Cannibalistic viruses in the aquatic environment: role of virophages in manipulating microbial communities. Int. J. Environ. Sci. Technol. 13, 2097–2104 (2016). https://doi.org/10.1007/s13762-016-1027-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1027-y