Abstract
The Pb(II) and Ni(II) biosorption of a fungal biomass isolated from mine drainage of metal-processing industries in Balya (Balıkesir province, Turkey) was optimized using a response surface methodology by altering parameters such as pH, initial metal concentration, contact time and biosorbent dosage. This strain was shown to be highly similar to Penicillium sp. Furthermore, zeta potential measurements and Fourier transform infrared spectroscopy were performed to understand the adsorption mechanism. A Box–Behnken design with 29 experiments was used to evaluate the interactions between independent variables. The results showed that the fungal biomass isolated from the metal mine drainage could have a significant environmental impact through the biosorption of Pb(II) and Ni(II) in waters polluted with heavy metals, particularly in the drainage from metal mines. The maximum removal values were 76 and 47 % at pH 4.5 for both Pb(II) and Ni(II), with 123 and 33 mg/L initial metal concentrations, 65 and 89 min contact times and 0.2 and 1.6 g/L biosorbent, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current active and abandoned mine regions are considered the main sources of metal-contaminated waters. These waters, known as acidic mine drainage (AMD), contain high concentrations of sulfates, heavy metals and metalloids (Johnson and Hallberg 2005). Metal mine drainage (MMD) is one type of AMD. Heavy metals, such as lead, mercury, copper, cadmium, zinc, nickel, and chromium, are among the most common pollutants in MMD that threaten the environment, animals and humans due to their extreme toxicity (Sheoran and Sheoran 2006). Because these environmental conditions destroy aquatic ecosystems, most MMD sites appear lifeless. Furthermore, MMDs choke rivers and lakes with layers of precipitates, such as hematite, schwertmannite and jarosite (Gazea et al. 1996). However, AMDs also harbor several microorganisms, such as bacteria, archaea, fungi, protists, and algae, which tolerate high concentrations of heavy metals (Hallberg and Johnson 2001; Baker et al. 2004; Das et al. 2009).
Microbial eukaryotes may play important roles in AMD communities, but those roles have only been partially determined. Fungal hyphae may contribute to the anchoring of biofilms to pyrite sediments and, in this way, provide surfaces for the attachment of prokaryotes (Baker and Banfield 2003; Ugulu et al. 2012). In addition, fungi surviving in mining facilities were considered to be able to tolerate several heavy metals at higher concentrations due to their habitats (Das et al. 2009; Cerino Córdova et al. 2011). The cell walls of these fungi have high metal-binding capacities and may also show high values of intracellular accumulation (de Rome and Gadd 1987). Heavy metals are toxic and carcinogenic to a variety of aquatic organisms, even at very low concentrations (Akar et al. 2009). Among these metals, nickel is the predominant soluble ion in waste streams (Wang et al. 2007). Lead is also particularly toxic to all organisms, even at low concentrations, and can be harmful to the nervous system, gastrointestinal tract, kidneys and reproductive system, particularly in children (Feng et al. 2004; Akar et al. 2007).
Biosorption can be used for the effective removal and recovery of heavy metal ions from industrial solutions as an alternative to chemical and physical methods, such as precipitation, ion exchange and adsorption (Akar et al. 2006). Although conventional techniques can reduce the levels of metal ions, they do not appear to be very effective due to their limited pH ranges, as well as the associated expense (Wang et al. 2007).
Fungal biomass has been used for the adsorption of heavy metals from aqueous solutions (Gadd and White 1989). Fungal cell walls and their components play major roles in biosorption (Hafez et al. 1997; Kapoor and Viraraghavan 1997). Because fungi can also be easily grown in larger amounts using simple techniques, they can serve as an economical means of treating effluents loaded with toxic metal ions (Volesky and Holan 1995).
Conventional, time-consuming optimization methods require a large number of experiments to determine optimal conditions. All the relevant parameters can be optimized simultaneously by statistical experimental designs, such as response surface methodology (RSM), Taguchi’s design and Plackett–Burman experimental design (Elibol 2002). Among these methods, RSM is a useful method for analyzing the effects of several independent variables on the response (Aslan and Cebeci 2007). In recent years, this method has been widely applied to biotechnology experiments, such as biosorption (Bhunia and Ghangrekar 2008).
The aim of this study is to investigate the potential effectiveness of metal biosorption in aqueous solutions using the dried biomass of a fungal strain isolated from MMD that included toxic heavy metals, such as Pb(II) and Ni(II). The optimal conditions for the removal of Pb(II) and Ni(II) were determined using a Box–Behnken experimental design.
The research activities have been conducted in Eskisehir, Turkey. The experiments started in 2011.
Materials and methods
Preparation of biosorbent and metal solutions
A fungus isolated from the drainage of a metal mine in Balya (Balıkesir, Turkey) was used for biosorption studies. Water samples were collected from the mine drainage for fungal isolation. According to these results, the Pb(II) and Ni(II) concentrations were 1.7 and 0.227 mg/L, respectively. The fungal isolation was performed using this water sample. For biosorption studies, the isolated fungus was grown in malt broth medium for 7 days and then washed with distilled water, filtered and dried in an oven overnight (60 °C); then, the sample was finally pulverized to <300 μm and stored at +4 °C for further experiments.
The stock solutions (1 g/L) of Pb(II) and Ni(II) were prepared by dissolving appropriate quantities of Pb(NO3) and NiCl2 salt. All the chemicals were of analytical grade and were obtained from Merck. The stock solution was diluted as necessary to prepare solutions. The initial pH values of the Ni(II) and Pb(II) solutions were adjusted to the required value with HCl and HNO3, respectively, before mixing the biosorbent suspension. Fresh dilutions were prepared for each study.
Optimization studies with the Box–Behnken design
Batch biosorption studies were conducted using metal solutions in 250-mL Erlenmeyer flasks with a 50-mL working volume. The solutions in the flasks were adjusted to different pH values and biosorbent dosages, and the initial metal concentrations and contact times were used to determine the optimal biosorption conditions. The RSM was used to evaluate and determine the optimal conditions. The temperature and agitation rate were held constant at 30 °C and 125 rpm, respectively.
This study used a Box–Behnken design matrix to minimize the number of experiments required and obtain an accurate prediction. According to the Box–Behnken design, 29 experiments were conducted for each metal ion, and the effects of the following independent variables were systematically investigated: pH, initial metal concentration, contact time and biosorbent dosage.
The factor levels used in this study for the Pb(II) and Ni(II) removals were 2.5, 3.5 and 4.5 for pH; 25, 87.5 and 150 mg/L of initial metal concentration, respectively; 5, 47.5 and 90 min of contact time; 0.1, 1.05 and 2 g/L of biosorbent dosage. The design-expert statistical software package was used to analyze the data.
At the end of the experiments, the biosorbent and metal solutions were centrifuged at 7,000 rpm for 10 min, and the supernatants were analyzed to determine the residual metal concentration using atomic absorption spectrometry (Perkin Elmer 3110). These absorbances were then compared with standard curves plotted using different concentrations of the appropriate metal.
Biosorbent characterization
The mechanism of biosorption was investigated by measuring the zeta potential and by performing FTIR spectroscopy. The FTIR spectra of the biosorbent before and after metal adsorption were recorded using a Bruker Tensor 27 spectrophotometer in the region of 400–4,000 cm−1. FTIR spectra were measured on KBr pellets prepared by pressing mixtures of 1-mg dry powdered sample and 100-mg spectrometry-grade KBr under vacuum.
The magnitude of electrostatic interaction between the bacteria cell surfaces and a metal ion is a function of electrokinetic potential expressed here as zeta potential. The net surface charge of the biomass at different pH values was determined from the zeta potential measurements obtained using a Malvern Zetasizer instrument.
Identification of the biosorbent fungus
Many different culture media, such as Czapek solution agar, malt extract agar and potato dextrose agar media, were used to identify the fungal species (Pitt and Hocking 2009; Samson et al. 2002; Watanabe 2002). The micro- and macro-morphologies were assessed, as well as the reverse and surface coloration of the colonies. The fungus was identified to the genus level according to the Barnett and Hunter method (1998).
Genomic DNA of this filamentous microfungus was extracted using the CTAB protocol. The fungal internal transcribed spacer (ITS1-5, 8S rDNA region) was amplified. The PCR amplification was performed in a 25-μL reaction mixture containing 1X PCR buffer, 1.5 mM MgCI2, 0.4 mM dNTP, 1.25 units Taq DNA polymerase, 0.8 mM primer and 1 μL DNA template. The PCR program consisted of an initial denaturation step at 94 °C for 3 min, followed by 40 cycles of 94 °C for 35 s, 52 °C for 35 s and 72 °C for 45 s, and then a final extension step at 72 °C for 7 min. The PCR products were visualized by gel electrophoresis on a 1 % agarose gel, and then, the ITS region was visualized (White et al. 1990). The PCR products were then purified and subjected to sequence analysis. Sequence similarities were identified using the BLAST tool from the National Center for Biotechnology Information. Phylogenetic trees were constructed with the neighbor-joining method using Mega 4.0 software. The evolutionary distance between organisms is indicated by the horizontal branch length.
Results and discussion
Optimization of biosorption
The Box–Behnken design was used to statistically analyze the experiments and evaluate the effects of process parameters on Pb(II) and Ni(II) removal (%). Table 1 presents the results of the experiments conducted according to the Box–Behnken design to study the effects of the four independent variables.
The sequential model sum of squares, lack of fit tests and model summary statistics were used to determine the adequacy of the model for Pb(II) removal (%). The best-fitting mathematical models of the responses were selected based on the comparisons between several statistical parameters, including the coefficient of determination (R 2), the adjusted coefficient of determination (adj-R 2) and the F value provided by analysis of variance (ANOVA).
The model summary statistics showed that the regression coefficient was best for the quadratic model. Additionally, ANOVA analysis confirmed that the model adequately explained the relationship between the variables and the response. The ANOVA results for Pb(II) and Ni(II) removals are shown in Table 2.
The second-order polynomial model was used to determine the relationship between variables and responses, where A, B, C and D denote pH, initial metal concentration, contact time and biosorbent dosage, respectively. The analysis of variance (Table 2) for Pb(II) removal indicated that the main effects of pH, initial metal concentration, biosorbent dosage and the second-order effects of pH × initial metal concentration, initial metal concentration × biosorbent dosage, pH2 and biosorbent dosage2 were significant model terms. Consequently, the model describing the relation between the independent variables and the response is presented in Eq. (1):
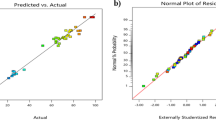
The adjusted R 2 = 0.8936 of the quadratic model in Eq. (1) is in reasonable agreement with the predicted R 2 = 0.8027. The obtained quadratic model is highly statistically significant (p < 0.0001) according to the ANOVA as reported in Table 2. The model F value of 34.606 implies that the model is significant. The ANOVA results confirmed that the simplified quadratic model was satisfactorily adjusted to the experimental data. The normal probability plot of studentized residuals is shown in Fig. 1. The data points on this plot lie reasonably close to the straight line, supporting the significance of the model and confirming that the assumptions of the analysis were satisfied.
The analysis of variance (Table 2) for Ni(II) removal indicated that the main effects of pH, initial metal concentration, contact time and biosorbent dosage and the second-order effects of pH × initial metal concentration, pH2, initial metal concentration2 and contact time2 were significant model terms. By eliminating the insignificant main and interaction terms, the final model for Ni(II) removal (%) in terms of actual factors determined by the design-expert software is shown below:
Using the design-expert software, power transformation with a λ value of 1.85 was found to provide the best fit. The quadratic model with power transformation in Eq. (2) had an adjusted R 2 of 0.952, indicating the fitness of the regression and good agreement with the predicted R 2 of 0.9277. According to the ANOVA presented in Table 2, the high F value (71.156) and the very low probability (p < 0.0001) indicate that the model adequately predicts the experimental results. The ANOVA results confirm that the reduced quadratic model fits the experimental data. The normal probability plot of studentized residuals is shown in Fig. 1. The data points on this plot lie reasonably close to the straight line, supporting the significance of the model and confirming that the assumptions of the analysis were satisfied. The precision value of 33.715 indicates that the design model is sufficiently precise.
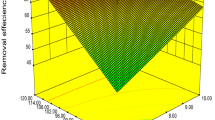
The three-dimensional surface plots are graphical representations of regression equations as functions of two factors, while all other factors are maintained at fixed levels. These plots are useful for understanding both the main and interaction effects of variables. Figures 2a–f and 3a–f show all the possible combinations of 12 response surface plots. These plots can be used to test the interactions between variables.
Figure 2 shows response surface curves for Pb(II) removal and depicts the interactions between independent variables. In Fig. 2a, the analysis of the effects of pH and contact time on Pb(II) removal indicates that Pb(II) removal increases with an increase in pH from 2.5 to 4.5, while other variables are held at their central values. When pH varied from 2.5 to 4.5, the maximum Pb(II) removal was 69.2 % at pH 4.5 and 25 mg/L metal (Fig. 2b). An analysis of Fig. 2c indicates that a minimal biosorbent dosage and a maximum pH lead to high Pb(II) removal. Similarly, Fereidouni and co-workers studied nickel biosorption and found that the Ni(II) removal efficiency of S. cerevisiae increased significantly with an increase in the initial solution pH from 3 to 7 when the biomass dosage was decreased from 4.04 to 0.86 g/L (Fereidouni et al. 2009). Amini and coworkers studied the biosorption of Ni(II) by Aspergillus sp. and found that the optimal Ni(II) uptake of 4.82 mg Ni(II)/L biomass (70.30 %) was achieved at pH 6.25, with a biomass dosage of 2.98 g/L and an initial Ni(II) concentration of 30 mg/L (Amini et al. 2009). Azila et al. used a central composite design (CCD) to optimize the biosorption of Pb(II) ions onto immobilized cells of Pycnoporus sanguineus. Under these conditions, the model predicted a maximum removal of 97.7 % of Pb(II) ions at pH 4, with a 200 mg/L initial Pb(II) concentration and 10 g/L of biosorbent (Azila et al. 2008). According to Fig. 2d, the maximum removal of Pb(II) was observed for 150 mg/L initial metal concentration and 5 min of contact time at pH 3.5 and a biosorbent dosage of 1.05 g/L. The removal of Pb(II) ions increased as the initial metal concentration increased as seen in Fig. 2e. An analysis of the effects of contact time and biosorbent dosage showed that the removal of Pb(II) ions decreased as the biosorbent dosage increased (Fig. 2f). Furthermore, the interaction graph indicates that contact time did not have a significant effect on the response variable. Figure 2d and f showed that contact time did not have a significant effect on the removal of Pb(II) ions.
Figure 3 shows response surface curves for Ni(II) removal and depicts the interactions between independent variables. Figure 3a indicates that when the initial metal concentration and biosorbent dosage are constant at 87.5 mg/L and 1.05 g/L, respectively, Ni(II) removal increases significantly with pH and increases to a lesser degree with contact time. When pH was varied from 2.5 to 4.5, a maximum Ni(II) removal of 42.9 % was observed at pH 4.5 and an initial metal concentration of 25 mg/L as seen in Fig. 3b. However, Kumar et al. observed that the removal of Ni(II) ions increased with increases in pH from 2.0 to 6.0 and Ni(II) concentration from 10 to 60 mg/L, and no decrease was observed after the equilibrium removal of Ni(II) ions was achieved (Kumar et al. 2009). pH had a significant effect on both responses in our study, as indicated by the ANOVA results. The removal of Ni(II) ions was enhanced as the pH was increased. A maximum Ni(II) removal of 41.2 % was observed at pH 4.5 and a biosorbent dosage of 0.10 g (Fig. 3c). Amini et al. also found similar results. They found a maximum Ni(II) uptake of 4.75 mg Ni(II)/g biomass (71.13 %) at an Aspergillus niger dosage of 5.36 g/L and a solution pH of 5.68. They also observed lower Ni(II) uptake above the A. niger dosage of 5.36 g/L and suggested that this effect could be due to a decrease in the rate of uptake due to the attainment of equilibrium between the adsorbent and adsorbate (Amini et al. 2009).
Both Ni(II) and Pb(II) competed with other cations for the limited number of negatively charged active sites on the cell walls; because the cell surface charge was positive at highly acidic pHs, the metal uptake at lower pH values was lower (Dursun 2006; Amini et al. 2008). In this study, however, as demonstrated by many other authors, biosorption efficiency also increased when pH was increased to the pH at which the metal precipitates. Many functional groups, such as carboxylic and phosphate groups, carry negative charges, and these negatively charged sites could interact with positively charged metal ions via electrostatic interactions (Qu et al. 2011).
Figure 3d shows the interaction effect of initial metal concentration and contact time on Ni(II) uptake in a 3D plot. According to the figure, maximum Ni(II) uptake was obtained with an increase in the initial metal concentration and contact time. In Fig. 3e, the removal of Ni(II) ions increased as the biosorbent dosage and initial metal concentration increased. According to the observations of Amini et al., the quantity of Ni(II) absorbed per unit mass of biosorbent increased when the initial Ni(II) concentration increased (Kumar et al. 2009). When the biosorbent dosage was varied from 0.1 to 2 g/L, a maximum of 38.4 % Ni(II) removal was observed at a contact time of 90 min (Fig. 3f).
A confirmatory experiment was used to validate the accuracy of the model. The aim of this experiment was to validate the model and determine whether the conclusions drawn from the analyses were correct. A useful confirmation is to determine whether the new observation falls within the predicted response interval at that point (Montgomery 2005). The optimal conditions for the removal of Ni(II) and Pb(II) are shown in Table 3. For the optimal combinations of responses, additional experiments were performed under the same conditions as the original experiment to assess the validity of the regression models. The responses were measured, and the results of the confirmatory trials are also shown in Table 3. The validation was performed by predicting the response at the optimum point using the developed model, calculating the prediction interval, and then conducting a confirmatory experiment. The results measured in these confirmatory experiments were found to be reasonably close to the predicted results, and they felt inside the prediction interval, confirming the validity and adequacy of the predicted models.
Biosorbent identification and characterization
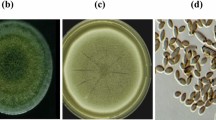
The high acidity of MMD and the high levels of dissolved heavy metals, such as copper, nickel, lead, zinc, manganese, iron and arsenic, make this acidic water extremely toxic to most aquatic organisms (Penreath 1994). Studies have reported a lower diversity of fungal species in soils contaminated with heavy metals. This study isolated a fungal strain from MMD and used it for biosorption studies. The biosorbent in the present study was identified by the classical method as Penicillium sp. Molecular identification also identified the same genus. According to BLAST analysis of the ITS sequence, this strain exhibited 95 % similarity to Penicillium janthinellum. Figure 4 shows the phylogenetic tree related to the sequence analyses of the biosorbent in this study. AMD-2 indicates the isolate used as biosorbent in this study. This genus has been reported to inhabit acidic, metal-rich environments (Baker et al. 2004), and this strain has exhibited metal tolerance (Kumar et al. 2012; Iskandar et al. 2011).
To determine the main functional groups of the biomass involved in Ni(II) and Pb(II) biosorption, infrared spectroscopy was performed before and after biosorption. A strong, broad band for O–H stretching in carboxylic groups was observed at 3,444 cm−1, along with a C–H symmetric stretching vibration band of CH2 in the region of 2,927 cm−1. After Ni(II) and Pb(II) treatment, the spectra indicated some changes in the functional groups on the biomass (Fig. 5). The absorption bands of the –CH symmetric stretching, –CH bending, –SO3 stretching, –S=O stretching and –C–O stretching were shifted after Ni(II) treatment. The peak at 1,242 cm−1 shifted to 1,236 and 1,234 cm−1 following Ni(II) and Pb(II) treatment, respectively, while the peak at 1,149 cm−1 shifted to 1,135 and 1,155 cm−1 following treatment with Ni(II) and Pb(II), respectively (Limin et al. 2009). These observed shifts in the FTIR analysis demonstrated that these metals bind to active functional groups, possibly carboxylic acid (1,000–1,260 cm−1) and phosphate groups. These results indicate that chemical interactions occur between the Ni(II) and Pb(II) ions and the hydrogen atoms of the carboxyl and phosphate groups (Qu et al. 2011). The absorption bands of the –C=O stretching and the amide-I band did not change. The bands at 1,076 cm−1 (–C–O stretching and carboxylic acid) and 1,406 cm−1 (stretching vibration band of C–O of COOH in uronic acid) observed for the untreated biomass did not appear in the Ni(II)- and Pb(II)-treated biomass samples (Fereidouni et al. 2009). The FTIR spectrum of Ni(II) loaded biomass indicates the presence of –C–H bending vibrations (aromatic) and –N–H bending, but these bands were not observed in the unloaded biomass (Table 4).
Figure 5 presents the surface charge of the biomass at different pHs. The zeta potential of the biomass was measured as positive at pH 1.5, 2.5 and 3.5. However, the surface of the biomass was negatively charged at pH values between 4.5 and 5.5. The minimum negative zeta potential value (zP = −2.98 mV) was observed at pH 4.5, corresponding to the maximum biosorption efficiency of Ni(II) and Pb(II) (Cabuk et al. 2007).
Conclusion
This study used RSM to optimize the biosorption of Ni(II) and Pb(II) ions by adjusting the process variables. The quadratic models developed using the Box–Behnken design validated the good agreement between the observed and predicted values. Analysis of variance results showed a high coefficient of determination. The optimum conditions that maximized the removal efficiency of metal ions were determined using numerical optimization.
The habitats of biomasses are significant for their biosorbent ability, and habitats such as AMD that have low pHs, and high metal concentrations are thought to harbor organisms with the potential to adsorb heavy metals. These results are comparable to previously reported results in the literature.
References
Akar T, Cabuk A, Tunali S, Yamac M (2006) Biosorption potential of the macrofungus Ganoderma carnosum for removal of lead(II) ions from aqueous solutions. J Environ Sci Heal A 41:2587–2606
Akar T, Tunali S, Çabuk A (2007) Study on the characterization of lead(II) biosorption by fungus Aspergillus parasiticus. Appl Biochem Biotechnol 136:389–485
Akar ST, Akar T, Kaynak Z, Anilan B, Cabuk A, Tabak Ö, Demir TA, Gedikbey T (2009) Removal of copper(II) ions from synthetic solution and real wastewater by the combined action of dried Trametes versicolor cells and montmorillonite. Hydrometallurgy 97:98–104
Amini M, Younesi H, Bahramifar N, Lorestani AAZ, Ghorbani F, Daneshi A, Sharifzadeh M (2008) Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J Hazard Mater 154:694–702
Amini M, Younesi H, Bahramifar N (2009) Biosorption of nickel(II) from aqueous solution by Aspergillus niger: response surface methodology and isotherm study. Chemosphere 75:1483–1491
Aslan N, Cebeci Y (2007) Application of Box–Behnken design and response surface methodology for modeling of some Turkish coals. Fuel 86:90–97
Azila YY, Mashitah MD, Bhatia S (2008) Process optimization studies of lead (Pb(II)) biosorption onto immobilized cells of Pycnoporus sanguineus using response surface methodology. Bioresour Technol 99:8549–8552
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF (2004) Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl Environ Microbiol 70:6264–6271
Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi, 4th edn. APS Press, St. Paul, MN, p 218
Bhunia P, Ghangrekar MM (2008) Statistical modeling and optimization of biomass granulation and COD removal in UASB reactors treating low strength wastewaters. Bioresour Technol 99:4229–4238
Cabuk A, Akar T, Tunali S, Gedikli S (2007) Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomass of Pinus nigra: equilibrium and mechanism analysis. Chem Eng J 131:293–300
Cerino Córdova FJ, García León AM, Garcia Reyes RB, Garza González MT, Soto Regalado E, Sánchez González MN, Quezada Lopez I (2011) Response surface methodology for lead biosorption on Aspergillus terreus. Int J Environ Sci Tech 8(4):695–704
Das BK, Roy A, Koschorreck M, Mandal SM, Wendt-Potthof K, Bhattacharya J (2009) Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res 43:883–894
De Rome L, Gadd GM (1987) Copper adsorption by Rhizopus arrhizus, Cladosporium resinae and Penicillium italicum. Appl Microbiol Biotechnol 26:84–90
Dursun AY (2006) A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. Biochem Eng J 28:187–195
Elibol M (2002) Response surface methodological approach for inclusion of perflurocarbon in actinorhodin fermentation medium. Process Biochem 38:6767–6773
Feng Q, Lin Q, Gong F, Sugita S, Shoya M (2004) Adsorption of lead and mercury by rice husk ash. J Colloid Interface Sci 278:1–8
Fereidouni M, Daneshi A, Younesi H (2009) Biosorption equilibria of binary Cd(II) and Ni(II) systems onto Saccharomyces cerevisiae and Ralstonia eutropha cells: application of response surface methodology. J Hazard Mater 168:1437–1448
Gadd GM, White C (1989) Removal of thorium from simulated acid process steams by fungal biomass. Biotechnol Bioeng 33:592–597
Gazea B, Adam K, Kontopoulos A (1996) A review of passive systems for the treatment of acid mine drainage. Mineral Eng 9:23–42
Hafez N, Abdel-Razek AS, Hafez MB (1997) Accumulation of some heavy metals on Aspergillus flavus. J Chem Technol Biotechnol 68:19–22
Hallberg KB, Johnson DB (2001) Biodiversity of acidophilic microorganisms. Adv Appl Microbiol 49:37–84
Iskandar NL, Zainudin NAIM, Tan SG (2011) Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci 23:824–830
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Biores Technol 61:221–227
Kumar R, Singh R, Kumar N, Bishnoi K, Bishnoi NR (2009) Response surface methodology approach for optimization of biosorption process for removal of Cr(VI), Ni(II) and Zn(II) ions by immobilized bacterial biomass sp. Bacillus brevis. Chem Eng J 146:401–407
Kumar R, Bhatia D, Singh R, Bishnoi NR (2012) Metal tolerance and sequestration of Ni(II), Zn(II) and Cr(VI) ions from simulated and electroplating wastewater in batch process: kinetics and equilibrium study. Int Biodeter Biodegr 66:82–90
Limin D, Juan D, Xin B, Naili Y, Chunhui F, Ying Z (2009) Mechanism of Pb(II) biosorption by Saccharomyces cerevisiae. Int Conf Environ Sci Inf Appl Technol 1:712–715
Montgomery DC (2005) Design and analysis of experiments, vol 425, 6th edn. Wiley, New York, pp 394–395
Penreath RJ (1994) The discharge of waters from active and abandoned mines. In: Hester RE, Harrison RM (eds) Mining and its environmental impact. Issues in environmental science and technology no. 1. Royal Society of Chemistry, Herts, UK, pp 121–132
Pitt JI, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, Dordrecht
Qu Y, Li H, Li A, Ma F, Zhou J (2011) Identification and characterization of Leucobacter sp. N-4 for Ni(II) biosorption by response surface methodology. J Hazard Mater 190:869–875
Samson R, Hoekstra E, Frisvad J, Filtenborg O (2002) Introduction to food- and airborne fungi. The Netherlands, Central Bureau Voor Schimmelcultures, Utrecht
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116
Ugulu I, Dogan Y, Baslar S, Varol O (2012) Biomonitoring of trace element accumulation in plants growing at Murat Mountain. Int J Environ Sci Tech 9(3):527–534
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Wang XS, Huang J, Hu HQ, Wang J, Qin Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of nickel(II) from aqueous solutions by Na-mordenite. J Hazard Mater 142:467–468
Watanabe T (2002) Trichoderma harzianum: pictorial atlas of soil and seed fungi morphologies of cultured fungi and key to species, 2nd edn. CRC Press, New York
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Acknowledgments
The authors are grateful to the Forest, Soil and Ecology Research Institute of the Ministry of the Environment for metal analyses. We thank Dr. Semra İlhan and Ercin Kocabiyik for their support for the molecular identification of the fungal isolate used in this study. We thank Molecular Synthesize and FT-IR Spectroscopy Research Laboratory at Eskisehir Osmangazi University. The abstract of this study was sent to The 15th European Congress on Biotechnology and the only abstract was published in New Biotechnology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aytar, P., Gedikli, S., Buruk, Y. et al. Lead and nickel biosorption with a fungal biomass isolated from metal mine drainage: Box–Behnken experimental design. Int. J. Environ. Sci. Technol. 11, 1631–1640 (2014). https://doi.org/10.1007/s13762-013-0354-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0354-5