Abstract
Multiple sclerosis (MS) is the most common inflammatory disorder of the central nervous system (CNS). Kombucha is produced by the fermentation of sugared tea with a symbiotic culture of bacteria and yeasts. This research was designed to reveal the therapeutic impact and the molecular and cellular processes determining the effect of kombucha on MS alleviation in an experimental autoimmune encephalomyelitis (EAE). The EAE was induced using myelin oligodendrocyte glycoprotein (MOG35–55) peptide emulsified in CFA and injected subcutaneously over two flank areas in C57BL/6 mice. In addition, pertussis toxin was injected intraperitoneally and repeated 48 h later. Treatment groups were received three different doses of kombucha (K1: low dose, K2: medium dose and K3: high dose) to obtain a maximum protection. Clinical scores and other criteria were followed daily for the 25 days. At the end of the course, T-helper-related cytokines (IFN-γ, IL-17, IL-4, and TGF-β) were measured through ELISA. Moreover, nitric oxide (NO) concentration in spinal cord tissue was detected. The severity of disease on the peak of disease in K1, K2, and K3 groups were 3.4 ± 0.18 and 2.6 ± 0.18 and 2 ± 0.14 respectively, compared to the CTRL group with 4.5 ± 0.19 (p < 0.001). Kombucha increased production of interleukin IL-4 (K1 = 95 ± 5, K2 = 110 ± 10, K3 = 115 ± 5 and CTRL = 65 ± 5; p < 0.05) and TGF-β (K1 = 1750 ± 80, K2 = 2050 ± 65, K3 = 2200 ± 75 and CTRL = 850 ± 85; p < 0.001) but concurrently resulted in a remarkable reduction in the production of IFN-γ (K1 = 950 ± 70, K2 = 890 ± 65, K3 = 850 ± 85 and CTRL = 3850 ± 115; p < 0.001) and IL-17 (K1 = 1250 ± 75, K2 = 1050 ± 90, K3 = 970 ± 80 and CTRL = 6450 ± 125; p < 0.001). Moreover, NO concentration in spinal cord tissue in the treatment groups was significantly less than the control group (K1: 35.42 ± 2.1, K2 = 31.21 ± 2.2, K3 = 28.24 ± 2.6 and CTRL = 45.25 ± 2.7; p < 0.05). These results supported that kombucha could reduce the severity of disease in an EAE model through motivating polarization of CD4+ T cells by induction of IL-4 and TGF-β as well as inhibition of IFN-γ and IL-17.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS), the most frequent disabling neurological disorder to afflict young adults, is a complex neurodegenerative autoimmune disease described by the existence of autoantibodies and autoreactive T cells to myelin proteins [1]. The number of people is currently living with this heterogeneous autoimmune disorder worldwide exceeds 2 million, with more prevalence among women rather than men [2]. MS exerts considerable burden of suffering and economic on patients, their families, and society [3] since it affects in adults in the most useful years of their lives [4]. According to the site of neurologic lesions in the central nerves system (CNS), its type, and severity of clinical symptoms are varied significantly between individuals [5]. While no precise cause of MS has yet been described, it is fairly well established that interactions between various genetic and/or non-genetic triggers such as viral infections, and environmental factors have been implicated to an auto-aggressive immune attack on the myelin sheath and other components of CNS axons [6]. During disease progression, activated leukocytes locally secrete inflammatory cytokines, chemokines soluble factors and reactive oxygen species (ROS) get to interruption of the blood–brain barrier (BBB) integrity, finally result in CNS demyelination and axonal degeneration in MS [5, 7].
Experimental autoimmune encephalomyelitis (EAE) is a suitable and a well-establish animal model, in which the immune system invades the myelin protein of the CNS as a reaction of an interruption in BBB permeability [8], resulting in oligodendroglial cell death-mediated demyelination, axonal degeneration and consequent breakdown of motor function [9]. Because of its clinical, histopathological, and immunological close similarities to the MS disease, EAE is the most broadly used animal model for investigating the pathogenesis of MS and potential efficacy of therapeutic agents [10]. Imbalance between cells of the immune system such as inflammatory and anti-inflammatory lymphocyte and its related mediators are considered to be crucial in the immunopathogenesis of MS [5, 11]. Generally, immunoinflammatory-mediated demyelination of both EAE and MS has been regarded as a result of differentiation of myelin-specific Th1 and Th17 cells, activation of macrophage or microglial cells, and secretion of inflammatory cytokines in the CNS [12].
Nitric oxide, a diatomic free radical molecule, produced by macrophages and other cell types via nitric oxide synthase (NOS) in response to various inflammatory conditions [13]. Convincing evidence for a potential role of NO in the pathogenesis of EAE and MS resulted in investigations that NO and mRNA of inducible nitric oxide synthase (iNOS) are elevated in the CNS of mice with EAE, and this is associated with the severity of the disease [14]. Increasingly more studies have shown that increased levels of NO and its various oxidative metabolites such as nitrite and nitrate have been detected in the CSF of MS patients linked to the CSF of healthy people [15]. Therefore, modulation of NO is a promising approach for pharmacological intervention in MS.
Given that there is no cure for MS and most of the modern medicines used to treat MS have a variety of adverse effects and only partially effective [16], there has been an explosive growth of research across the world on the potential role of herbal medicinal products and related products in treatment of inflammatory and autoimmunity diseases [17].
Kombucha, a slightly acidic refreshing beverage, is typically made by the fermentation of sugared tea with a symbiotic culture of acetic acid bacteria and yeasts, which has traditionally been used for its health-promoting effects on human health throughout the world especially in Asian countries [18]. Kombucha is composed of various bacteria (Acetobacter, Gluconacetobacter, Lactobacillus, Bifidobacterium etc.), several yeast (Zygosaccharomyces, Dekkera, etc.), and actually kombucha is rich with different probiotics [19]. Besides its use in traditional medicine, kombucha is proven to improve countless disease due to its antimicrobial, antioxidant, hepatic-protective, anticancer properties, modulation of immunity and reduce inflammation [20, 21]. As well, kombucha imparts its therapeutic effects partly by suppression of the nitric oxide production [22]. A recent study demonstrated that kombucha administration suppress immune responses and postpone onset of EAE in C57BL/6 mice [22]. These finding indicate that kombucha may prevent inflammatory responses in both MS and EAE and decreased the clinical symptoms in MOG-induced EAE, however, there was a need for further investigation to understand the mechanisms involved in the effect of kombucha on the treatment of EAE, including the study of more cytokines, the effect of different doses on the treatment process and nitic oxide levels in brain which were evaluated in this study.
Considering these findings, the present study was conducted to determine therapeutic potentials of kombucha on modulation of immunoinflammatory responses in EAE-induced mice. Collectively, these results suggest that kombucha may find as a considerable therapeutic agent for the amelioration of MS/EAE.
Methods
Preparation kombucha tea
Black tea was combined with boiling water and let to infuse for about 5 min. Sucrose 20% was dissolved in filtered infusions, and was allowed to cool. 200 ml of cooled tea was placed in a sterile glass jar and mixed with 3% (w/v) of freshly brewed tea fungus, then cultured for 7 days and fermentation processes were performed. The product was incubated at 25 ± 3 °C for 1 week. Subsequently, the mixture was centrifuged and kept at 4 °C for further work.
Animals
Female C57BL/6 mice (8–10 weeks) were purchased from the Royan Institute for Biotechnology (Isfahan, Iran). The mice were kept in the microbial-free animal house at a temperature of 23 ± 2 °C with a relative humidity of 50 ± 5% and a 12-h light/dark cycle. All mice had free access to food and water. All experimental methods and protocols were performed in accordance with the ethical guidelines approved by Semnan University of Medical Sciences (the approval ID is IR.SEMUMS.REC.1399.112).
Induction of EAE and clinical assessment
All mice on day 0 injected subcutaneously with 250 µg of myelin oligodendrocyte glycoprotein (MOG35–55) (BioBasic, Canada) emulsified in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO, USA) containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, MI, USA). In addition, 250 ng pertussis toxins (Sigma-Aldrich, St. Louis, MO, USA) were injected intraperitoneally on days 0 and 2 after immunization [23].
The clinical symptoms of EAE and the weight of mice were checked daily for 25 days post-immunization. Clinical evaluation of mice was performed with the following scale: 0, without symptoms; 1 = partial paralysis of the tail; 2 = complete paralysis of the tail; 3 = paralysis of the tail and abnormal gait; 4 = complete paralysis of one leg; 5 = complete paralysis of legs; 6 = paralysis of hands and feet; 7 = injury or death [24]. Mice were given a daily score, and were evaluated for day of onset, the maximum score (on the peak day), the mean score (on the last day) and Cumulative Disease Index (the total score of the disease during the experiment).
Treatment
Mice were divided into five groups: (1) normal group (N; n = 8) (2) control group (CTRL; n = 8), (3) the low-dose kombucha treatment group (K1; n = 8; 2.5 ml per kg kombucha), (4) mdium-dose kombucha treatment group (K2; n = 8; 5 ml per kg kombucha) and (5) high-dose kombucha treatment group (K3; n = 8; 10 ml per kg kombucha). EAE was induced in groups 2 to 5, and therapeutic groups were administered orally (gavage) with three different doses of kombucha to achieve the maximum therapeutic effect. In treatment groups, kombucha should be given daily simultaneous with EAE induction. The control group received sweet tea orally every day.
Detection of nitric oxide (NO) production
To find the concentration of nitric oxide (NO) in the blood and spinal cord tissue, the stable product of nitric oxide conversion was measured the colorimetric method of Griess, which depends on the concentration of endogenous nitrite (NO2−) as a nitric oxide production index. In summary, Griess reagent was produced by dissolving 1 g of sulfanilamide in 100 ml of 5% phosphoric acid mixed with 0.1 g of N-(1-naphthalene)-athylene dihydrochloride (NED) in 100 ml of distilled water. Supernatant from the homogeneous and centrifuged spinal cord was mixed for 10 min with the Griess reagent at room temperature to turn the nitrite into a deep purple compound. The absorption was measured at 540 nm using a microplate reader (Stat Fax 2100 Awarness, Phoenix, Arizona, USA). Nitrite concentrations were determined using the standard curve of 0.1 M sodium nitrite produced in distilled water as a standard.
Cell culture and ELISA for cytokine detection
Spleens and inguinal lymph nodes were removed from C57BL/6 mice on day 25 post-immunization. Red blood cells were lysed using ammonium chloride. Cell suspensions were cultured in RPMI 1640 medium consisting of 10% fetal bovine serum (FBS), 100U/ml penicillin, 100 mg/ml streptomycin (all reagents purchased from Sigma, St. Louis, MO) in round-bottom 24-well plates (2 × 106 cells/well). Cells were cultured with MOG35–55 (20 μg/ml) and incubated for 72 h at 37 °C and 5% CO2. Supernatants were collected after 72 h, and the concentration of cytokines (IL-4, IL-17, IFN-γ, and TGF-β) was assessed by ELISA according to the manufacturer's instructions (R&D Systems, Inc. Minneapolis, MN, USA).
Briefly, the standards and supernatants were incubated at room temperature for 2 h, followed by 1 h of incubation with secondary biotinylated antibodies and then 30 min of incubation with Avidin-HRP. Processes were continued with the use of tetramethyl benzidine (TMB). The reaction was stopped with the stop solution and plates were measured at 450 nm using the microplate reader (Stat Fax 2100 Awarness, Phoenix, AZ, USA). Standard curves were determined based on the analysis of different concentrations of recombinant cytokines. The sensitivity of the kits was 2 pg IL-4/ml and 5.3 pg IFN-γ/ml.
Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey multiple comparison tests was conducted for analysis of clinical signs between groups. Comparison of the effect of daphnetin in the treatment groups (vs. control mice) on the development of clinical signs was conducted via two-way repeated measures ANOVA. The normality of the data was confirmed by Shapiro–Wilk test. Statistical analysis was performed using SPSS software version 21.0 for Windows (SPSS, Chicago, IL, USA). Data were presented as mean ± SEM, and when P < 0.05, a significant difference was considered.
Results
Kombucha treatment suppressed the progress of EAE
Previous studies have shown that kombucha tea has antimicrobial, antioxidant, and anticancer properties. As a result, kombucha tea can be considered as an antioxidant and antimicrobial source in the treatment of MS. The therapeutic effect of kombucha with different doses has not been studied in EAE induced mice. The aim of this study was to demonstrate the optimal doses of kombucha against EAE and possible mechanisms involved in this protection.
The kombucha doses used for this study were low dose (2.5 ml per kg), medium dose (5 ml per kg) and high dose (10 ml per kg). Different doses of kombucha significantly reduced the clinical symptoms of the disease. In all treatment groups, the severity of paralysis and disability decreased compared to the control group.
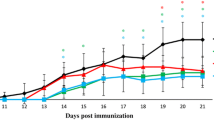
The clinical scores of treatment groups showed a significant decrease in EAE severity. On day 18, when the EAE clinical score peaked, the mean scores of K1, K2, and K3 groups were 3.4 ± 0.18 and 2.6 ± 0.18 and 2 ± 0.14, respectively (p < 0.001), compared to the CTRL group with 4.5 ± 0.19 (Fig. 1a and Table 1). The treatment groups also significantly prevented weight loss in EAE mice. The mean body weight of K1, K2, and K3 groups on day 18 was 18 ± 0.25 and 18.3 ± 0.3 and 18.5 ± 0.2, respectively (p < 0.05), compared to the CTRL group with 17.4 ± 0.25 (Fig. 1b). These results suggest that kombucha reduces the clinical severity of EAE in mice.
Kombucha treatment inhibited the development of in MOG-immunized C57BL/6 mice. Female C57BL/6 mice were treated with 2.5 (K1), 5 (K2) and 10 (K3) ml/Kg Kombucha in treatment groups simultaneous with EAE induction. Mice were monitored for signs of EAE, and the results for all mice, were presented as (a) mean clinical score, and (b) body weight. Results were expressed as mean ± SEM. *p < 0.05, ** p < 0.01, ***p < 0.001, compared with control group
Kombucha inhibited production of NO in the blood and CNS of EAE induced mice
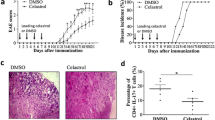
We examined secretion of NO as a pro-inflammatory molecule in the blood and CNS. As shown in (Fig. 2), the content of NO was considerably reduced in the blood and CNS of kombucha-treated groups relative to the control group. Production of NO in low-dose (K1, 35.42 ± 1.8, p < 0.05), middle-dose (K2, 31.21 ± 2.1, p < 0.01) and high-dose (K3, 28.24 ± 1.9, p < 0.01) kombucha-treated mice, obviously inhibited in the CNS in comparison with control group (45.25 ± 2.7).
Kombucha suppressed nitric oxide (NO) production in the CNS of EAE-induced mice. NO concentration in brain and spinal cord tissues was measured by the Griess reagent with using the nitrate/nitrite colorimetric assay. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group. Mice were divided into four groups: (1) control group (CTRL), (2) low-dose kombucha treatment (K1), (3) middle-dose kombucha treatment (K2), (4) high-dose kombucha treatment (K3), (5) normal group
Kombucha especially changed the balance of pro-inflammatory and anti-inflammatory cytokines
To understand the role of specific inflammatory mediators such as IL-17 and IFN-γ and anti-inflammatory mediators such as IL-4 and TGF-β, we examined the production of cytokines in the spleen and lymph nodes on day 25 post-immunization. Splenocytes and lymph node cells were removed from EAE-immunized mice, and then restimulated in vitro with immunizing peptide MOG35–55. All kombucha-treated groups produced considerably less IFN-γ and IL-17 and also produced higher IL-4 and TGF-β than in the CTRL group. The IFN-γ levels of K1, K2, and K3 groups were 950 ± 70 and 890 ± 65 and 850 ± 85, respectively (p < 0.001), compared to the CTRL group with 3850 ± 115 (p < 0.001). Also, IL-17 levels in treatment groups, K1 = 1250 ± 75, K2 = 1050 ± 90, K3 = 970 ± 80 in comparison with CTRL = 6450 ± 125 was significantly reduced (p < 0.001). On the other hand, kombucha increased production of interleukin IL-4 (K1 = 95 ± 5, K2 = 110 ± 10, K3 = 115 ± 5 and CTRL = 65 ± 5; p < 0.05) and TGF-β (K1 = 1750 ± 80, K2 = 2050 ± 65, K3 = 2200 ± 75 and CTRL = 850 ± 85; p < 0.001) (Fig. 3).
Kombucha especially changed the balance of pro-inflammatory and anti-inflammatory cytokines. Splenocytes and lymph nodes from immunized mice from all groups were isolated on day 25 post-immunization and restimulated with MOG35–55 for 72 h. Culture supernatants were collected and indicated cytokine levels were measured by ELISA. Cytokine assays were conducted in duplicate wells. (a) IFN-γ and IL-17 ± SEM as a pro-inflammatory cytokines (b) IL-4 and TGF-β ± SEM as an anti-inflammatory cytokines were measured from supernatants of cultures from splenocytes and lymph nodes
These data suggest that kombucha therapy preferably increases the production of Th2 and Treg cytokines and reduces the production of Th1 and Th17 cytokines. In addition, kombucha can change the balance between Th1/Th2 and Th17/Treg cells in the EAE model in favor of Th2 and Treg.
Discussion
The pathogenesis of EAE, as an MS model, is due to recruitment of myelin-reactive encephalitogenic T cells into the CNS, in which levels of anti-inflammatory cytokines are downregulated, while pro-inflammatory cytokines are upregulated in CSF and serum of MS patient [7]. Previous reports have provided supporting evidence that kombucha can be used to treat autoimmune diseases because of its potent anti-inflammatory and antioxidant properties [21, 22]. The aim of this study was to determine the therapeutic capacities of kombucha on the improvement of MOG-induced EAE in C57BL/6 mice and to clarify the relevant mechanisms in three different treatment groups as low, medium, and high doses of kombucha. The results indicated that treatment with kombucha suppressed the development of EAE. The therapeutic doses of kombucha have significantly reduced the incidence of the disease and greatly reduced the clinical manifestations of the disease. According to our findings, a number of recent documents revealed that kombucha ameliorates EAE, as well as reducing the oxidative toxicity caused by oxidative stress [20, 22].
Imbalance of Th1/Th2 and Th17/Treg cytokines might influence the onset and severity of EAE and MS, and many attempts have been spared aiming to restore Th1/Th2 and Th17/Treg balance to treat EAE [25]. Th1 and Th17 were thought originally to be responsible for the inflammatory demyelination in MS and EAE [26]. IFN-γ and IL-17 are the hallmark of the inflammatory process driving neuroinflammation and autoimmunity [27] through inducing T-cell infiltration, activation of APCs, mononuclear phagocytes, and upregulation of cell adhesion molecules on BBB, highlighting a potential target for the treatment of MS [28]. In addition, IFN-γ is able to induce iNOS expression via NF-кB [29].
In this study, we have used the EAE model to examine the modulation ability of kombucha for treatment of MS. In the present study, our ELISA results showed that the production of IFN-γ and IL-17 (Th1 and Th17-related cytokines) was profoundly decreased in spleen and lymph node, suggesting that the suppression of Th1- and Th17-related cytokines is a mechanism of modulation of EAE by kombucha. Our results may be parallel to previous reports that kombucha has inhibitory effects on some inflammatory cytokines. For instance, treatment with kombucha analogues from oak (KAO) significantly reduced levels of pro-inflammatory cytokines IL-6 and TNF-a, which is related to its immune modulatory activity [30]. In another study in the mouse model of multiple sclerosis, it has been shown that treatment with kombucha ameliorates EAE by downregulation of NO and TNF-α serum levels that associated with lower incidence, attenuation in the severity, and also a delay in the onset of disease [22]. There is a positive relation between demyelinating lesions in the CNS in MS and EAE and increased levels of IFN-γ and IL-17, implicating the role of these cytokines in the pathogenesis of MS [31]. On the other side, the pathogenic role of IFN-γ and IL-17 in the development of EAE is supported by an increase in the expression of Th1 and Th17 cytokines during the peak of disease and decreasing during disease remission [32]. However, little research has been conducted to investigate the effect of kombucha on the Th1 and Th17 related cytokines in EAE.
The result of the present study showed that the production of IL-4 and TGF-β was increased in spleen and lymph node. To the best of our knowledge, this is the first investigation that has done the effect of kombucha on the production of Th2- and Treg-related cytokines.
IL-4 and TGF-β, with a potent regulator of immunity, play a neuroprotective role in MS [32]. Th2- and Treg-related cytokines attenuate EAE that can be partly via shifting the immune response from an inflammatory Th1/Th17 to an anti-inflammatory Th2/Treg responses [12]. The role of the Th2-derived cytokine IL-4 in disease pathology was supported further by clinical evidence showing that the level of IL-4 mRNA upregulate during the recovery phases from EAE and IL-4−/− mice exhibited increased susceptibility to EAE, point to a potentially protective role of IL-4 against the incidence and progression of MS [33]. Neutralization or inhibition of IL-4 and TGF-β enhances the EAE disease severity [34].
Another important aspect evaluated in vitro was its possible effects on the production of important inflammatory proteins called nitric oxide. Under abnormal situations, high concentrations of NO released by macrophages and other immune cells exhibit a pro-inflammatory and cytotoxic properties that induces leukocyte migration, and cytokine production [35, 36]. Several lines of evidence suggest that iNOS over expression is associated with demyelinating plaques in the brain of patients with MS [37].
Similarly to pro-inflammatory cytokines, NO is also responsible for axonal injury and subsequent myelin degradation in both MS and EAE [38]. It has also been reported that NO is able to regulate immune responses [39] and mediate microglial toxicity towards oligodendrocytes [40]. Further studies have shown that elevated levels of NO and its various oxidative metabolites, including nitrate and nitrate, have been detected in the CSF of MS patients compared to the CSF of healthy individuals [15]. The influence of inhibitors of NO in ameliorating EAE may be partially related to reductions of inflammatory cytokine or reductions blood–brain barrier disruption [37].
According to our results, the NO concentration in CSF is clearly reduced in all kombucha-treated groups. Our results are consistent with other studies that confirm the effect of kombucha inhibition on NO concentration [22]. It has been suggested that the increase in NO concentration may be due to a decrease in IL-10 production [41]. Mechanistically, kombucha could attenuate NO production due to upregulation of IL-10 production. These findings are, in fact, in good agreement with the results previously reported by Marzban et al. showing that serum levels of NO in kombucha-treated mice were significantly decreased in comparison with control mice [22]. Also recently, in vitro study indicated that downregulation of NO production in kombucha- and KAO-treated macrophages compared with LPS-stimulated macrophages [30]. In another study by Gharib et al. demonstrated that kombucha administration significantly improved trichloroethylene-induced kidney damage by inhibition NO contents and also improved lipid peroxidation and oxidative stress induced nephrotoxicity in rat [42].
In conclusion, kombucha could mitigate EAE in mice at least partly mediated through their ability to induce the production of anti-inflammatory cytokines (IL-4 and TGF-β) and inhibit production of inflammation-promoting cytokines such as IFN-γ and IL-17. Therefore, these results highlight a potential role for kombucha in modulating of the immune system and suggest its use in the treatment of MS and other autoimmune diseases is mediated by Th1/Th17 cells. Further investigation is needed to confirm these possibilities.
References
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558
Namjooyan F, Ghanavati R, Majdinasab N, Jokari S, Janbozorgi M (2014) Uses of complementary and alternative medicine in multiple sclerosis. J Tradit Complement Med 4(3):145–152
Ransohoff RM (2012) Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci 15:1074
Petermann F, Korn T (2011) Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett 585(23):3747–3757
Yadav V, Shinto L, Bourdette D (2010) Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol 6(3):381–395
Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13(1):25–36
Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Khodadadi B, Jazayeri S et al (2015) Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutritional neuroscience. 18(4):169–176
Ljubisavljevic S, Stojanovic I, Pavlovic D, Milojkovic M, Vojinovic S, Sokolovic D et al (2012) Correlation of nitric oxide levels in the cerebellum and spinal cord of experimental autoimmune encephalomyelitis rats with clinical symptoms. Acta Neurobiol Exp (Wars) 72(1):33–39
Bando Y (2015) Myelin morphology and axon pathology in demyelination during experimental autoimmune encephalomyelitis. Neural Regener Res 10(10):1584–1585
Muili KA, Gopalakrishnan S, Meyer SL, Eells JT, Lyons JA (2012) Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS ONE 7(1):e30655
Høglund RA, Maghazachi AA (2014) Multiple sclerosis and the role of immune cells. World J Exp Med 4(3):27–37
Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM et al (2017) Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother. 95:1535–1548
Lan M, Tang X, Zhang J, Yao Z (2018) Insights in pathogenesis of multiple sclerosis: nitric oxide may induce mitochondrial dysfunction of oligodendrocytes. Rev Neurosci 29(1):39–53
O’Brien NC, Charlton B, Cowden WB, Willenborg DO (1999) Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol 163(12):6841–6847
Letourneau S, Hernandez L, Faris AN, Spence DM (2010) Evaluating the effects of estradiol on endothelial nitric oxide stimulated by erythrocyte-derived ATP using a microfluidic approach. Anal Bioanal Chem 397(8):3369–3375
Theoharides TC (2009) Luteolin as a therapeutic option for multiple sclerosis. J Neuroinflamm 6:29
Venkatesha SH, Astry B, Nanjundaiah SM, Kim HR, Rajaiah R, Yang Y et al (2016) Control of autoimmune arthritis by herbal extracts and their bioactive components. Asian J Pharm Sci 11(2):301–307
Dufresne C, Farnworth E (2000) Tea, kombucha, and health: a review. Food Res Int 33:409–421
Marsh AJ, O'Sullivan O, Hill C, Ross RP, Cotter PD (2014) Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol 38:171–178
Gharib OA (2009) Effects of Kombucha on oxidative stress induced nephrotoxicity in rats. Chin Med 4:23
Jayabalan R, Malbaša R, Lončar E, Vitas J, Sathishkumar M (2014) A review on kombucha tea—microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Saf 13:538–550
Marzban F, Azizi G, Afraei S, Sedaghat R, Seyedzadeh MH, Razavi A et al (2015) Kombucha tea ameliorates experimental autoimmune encephalomyelitis in mouse model of multiple sclerosis. Food Agric Immunol 26(6):782–793
Haghmorad D, Mahmoudi MB, Mahmoudi M, Rab SZ, Rastin M, Shegarfi H et al (2014) Calcium intervention ameliorates experimental model of multiple sclerosis. Oman Med J 29(3):185–189
Nosratabadi R, Rastin M, Sankian M, Haghmorad D, Tabasi N, Zamani S et al (2016) St. John's wort and its component hyperforin alleviate experimental autoimmune encephalomyelitis through expansion of regulatory T-cells. J Immunotoxicol. 13(3):364–374
Ip FCF, Ng YP, Or TCT, Sun P, Fu G, Li JYH et al (2017) Anemoside A3 ameliorates experimental autoimmune encephalomyelitis by modulating T helper 17 cell response. PLoS ONE 12(7):e0182069
Nosratabadi R, Rastin M, Sankian M, Haghmorad D, Mahmoudi M (2016) Hyperforin-loaded gold nanoparticle alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Nanomed Nanotechnol Biol Med 12(7):1961–1971
Ottum PA, Arellano G, Reyes LI, Iruretagoyena M, Naves R (2015) Opposing roles of interferon-gamma on cells of the central nervous system in autoimmune neuroinflammation. Front Immunol 6:539
Sanvito L, Constantinescu C, Gran B, Hart B (2010) The multifaceted role of interferon-γ in central nervous system autoimmune demyelination. Open Autoimmunity J. 2:151–159
Jolivalt CG, Howard RB, Chen LS, Mizisin AP, Lai C-S (2003) A novel nitric oxide scavenger in combination with cyclosporine A ameliorates experimental autoimmune encephalomyelitis progression in mice. J Neuroimmunol 138(1):56–64
Vazquez-Cabral BD, Larrosa-Perez M, Gallegos-Infante JA, Moreno-Jimenez MR, Gonzalez-Laredo RF, Rutiaga-Quinones JG et al (2017) Oak kombucha protects against oxidative stress and inflammatory processes. Chem Biol Interact 272:1–9
Arellano G, Ottum PA, Reyes LI, Burgos PI, Naves R (2015) Stage-specific role of interferon-gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol 6:492
Imitola J, Chitnis T, Khoury SJ (2005) Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther 106(2):163–177
Fernando V, Omura S, Sato F, Kawai E, Martinez NE, Elliott SF et al (2014) Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci 15(2):1700–1718
Speck S, Lim J, Shelake S, Matka M, Stoddard J, Farr A et al (2014) TGF-beta signaling initiated in dendritic cells instructs suppressive effects on Th17 differentiation at the site of neuroinflammation. PLoS ONE 9(7):e102390
Cardia GFE, Silva-Filho SE, Silva EL, Uchida NS, Cavalcante HAO, Cassarotti LL et al (2018) Effect of Lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid Based Complement Altern Med 2018:10
Lehmann HC, Köhne A, Meyer zu Hörste G, Dehmel T, Kiehl O, Hartung H-P et al (2007) Role of nitric oxide as mediator of nerve injury in inflammatory neuropathies. J Neuropathol Exp Neurol 66(4):305–312
Pozza M, Bettelli C, Aloe L, Giardino L, Calza L (2000) Further evidence for a role of nitric oxide in experimental allergic encephalomyelitis: aminoguanidine treatment modifies its clinical evolution. Brain Res 855(1):39–46
Dias DS, Fontes LB, Crotti AE, Aarestrup BJ, Aarestrup FM, da Silva Filho AA et al (2014) Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules (Basel, Switzerland) 19(8):12814–12826
Ibiza S, Serrador JM (2008) The role of nitric oxide in the regulation of adaptive immune responses. Inmunología 27(3):103–117
Stanislaus R, Gilg AG, Singh AK, Singh I (2002) Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neurosci Lett 333(3):167–170
La Flamme AC, Patton EA, Bauman B, Pearce EJ (2001) IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol 166(3):1903–1911
Gharib OA (2009) Effects of kombucha on oxidative stress induced nephrotoxicity in rats. Chin Med 4:23
Funding
The authors would like to thank the authorities in research council of Semnan University of Medical Sciences for their financial support (Grant Numbers 671).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghmorad, D., Yazdanpanah, E., Sadighimoghaddam, B. et al. Kombucha ameliorates experimental autoimmune encephalomyelitis through activation of Treg and Th2 cells. Acta Neurol Belg 121, 1685–1692 (2021). https://doi.org/10.1007/s13760-020-01475-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-020-01475-3