Abstract
We provide updated diagnoses for the senex-, burtoni- and dimorphus-groups of Camponotus (Myrmobrachys). Dichotomous keys for the C. (Myrmobrachys) groups and species of the dimorphus-group, based on type-specimens are provided. Two new species of the dimorphus-group are described, Camponotus cameloides sp. nov. and Camponotus hyalus sp. nov. We classified C. dolabratus and C. lancifer as members of the dimorphus-group and C. crassicornis, C. subcircularis, and C. championi as members of the senex-group. Scanning Electron Microscopy was used to describe the branched pilosity of C. cameloides and this is the first description of it for adult workers of Camponotini tribe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camponotus Mayr, 1861, is one of the most diverse ant genera in the Formicidae and the most speciose within Formicinae. To date, there are 1087 extant species (Bolton 2024). The genus is widely distributed across the northern temperate zones, as well as tropical and subtropical regions of the world (Fernández 2003; Rasoamanana et al. 2017).
The Camponotus species occupy a wide variety of microhabitats, both in soil and vegetation, forming numerous colonies (Fernandes et al. 2014). They can nest in the ground, in rotten branches or twigs, or in living wood (Bolton 1973). Some species are referred to as Carpenter ants due to their preference for nesting in decaying wood or abandoned termite galleries (Fernández 2003; Yamamoto and Del-Claro 2008; Fernandes et al. 2014; Oliveira et al. 2017). In urban environments, they can be found nesting in various places such as residences, public squares, or urban parks (Bueno and Campos-Farinha 1999; Campos-Farinha et al. 2002; Oliveira et al. 2017).
Grouped in the tribe Camponotini, Camponotus is morphologically characterized by the insertion of the antennae on the head being distant from the posterior edge of the clypeus, mandibles with five to eight teeth, with the third tooth from apex not reduced in size (Bolton 2003; Ward et al. 2016; Fernandez et al. 2019). The taxonomy of species of the genus has been considered challenging. A large number of species, in addition to morphological variation, high level of intraspecific dimorphism/polymorphism, and the variety of shapes and coloration within species have avoiding progress in the Camponotus taxonomy (McArthur and Leys 2006; Oliveira et al. 2017; Rasoamanana et al. 2017).
Camponotus (Myrmobrachys), one of the 43 subgenera of the Camponotus, was designated by Forel in 1912. In this subgenus, Forel (1912) brought together the senex-group, distributed in America, with some groups of species from Madagascar. The subgenus was defined with an ambiguous combination of characters, such as short, robust species, with depressed or sub-depressed thorax, bordered or sub-bordered, sometimes bidentate, often covered with pubescence and generally opaque. Emery (1920) revised Forel's subgenera proposal, removing the Malagasy species groups from C. (Myrmobrachys), leaving it as exclusively Neotropical. Later, he published the definition of subgenera, presenting a more detailed diagnosis for C. (Myrmobrachys), and dividing it into three groups, senex-, dimorphus- and burtoni- (Emery 1925).
Since then, the groups of C. (Myrmobrachys) have not had any taxonomic revision. Recently, Mackay published two works, a taxonomic key for the species distributed in Colombia (Mackay W and Mackay E 2019), and a series of taxonomic keys for the New World, covering the subgenera, some species complexes, and a key to species of the subgenus Camponotus (Mackay 2019). However, the keys do not cover the dimorphus-group at the species level. According to Mackay W & Mackay E (2019), some species of Camponotus (Myrmocladoecus) Wheeler, 1921 are treated as members of C. (Myrmobrachys). In addition, Mackay (2019), does not include C. (Myrmocladoecus) in his key and treats it as a junior synonym of C. (Myrmobrachys).
In this study, we present a contribution to the knowledge of C. (Myrmobrachys) sensu Emery (1925) taxonomy, with emphasis on the dimorphus-group. Specifically, we redefine the subgenus species groups; provide a taxonomic key for the species of the dimorphus-group and describe two new species members of the dimorphus-group.
Material and methods
Material examined
Type specimens were examined through high-resolution images from the MCZbase: The Database of the Zoological Collections at the Museum of Comparative Zoology of Harvard, the Smithsonian’s digital collections, and AntWeb version 8.103.2 (Tab. 1). The acronyms for the collections where the type specimens are deposited, as well as those for the specimens that were examined in person, are listed below:
-
o
BMNH—Natural History Museum, London, England, U.K.
-
o
CELC—Coleção Entomológica do Laboratório de Sistemática e Biologia de Coleoptera da Universidade Federal de Viçosa, Minas Gerais, Brazil.
-
o
DZUP—Coleção Entomológica Padre Jesus Santiago Moure, Curitiba, Paraná, Brazil.
-
o
JLCT—John T. Longino Collection, Utah, USA.
-
o
MCZ—Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA.
-
o
MHNG—Muséum d’Histoire Naturelle, Geneva, Switzerland.
-
o
MPEG—Museu Paraense Emílio Goeldi, Belém, Pará, Brazil.
-
o
MSNG—Museo di Storia Naturale Giacomo Doria, Genova, Italy.
-
o
MZSP—Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil.
-
o
NHMW—Naturhistorisches Museum, Vienna, Austria.
-
o
NMNH—National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
-
o
PSWC—Phil S. Ward Collection, University of California, Davis, CA, USA.
-
o
SDEI—Deutsches Entomologisches Institut Senckenberg, Müncheberg, Germany.
Measurements
Measurements were taken in stereomicroscopes with a millimetric ruler attached to the ocular lens. All measurements are given in mm and the minimum and maximum values for the paratypes. Detailed information is in Supplementary Information 1. Measurements definitions and abbreviations used are listed below and represented in Fig. 1.
-
o
CL (Clypeus Length): Length of clypeus medially in clypeus full-face view, the posterior and anterior margins were at the same focus.
-
o
CW (Clypeus Width): Width between the tentorial pits in clypeus full-face view, the posterior and anterior margins were at the same focus.
-
o
EL (Eye Length): Length of the right eye in full view of the eye from the anteriormost to the posteriormost points of the eye perimeter.
-
o
HL (Head Length): = HMdL + CL.
-
o
HMdL (Head Middle Length): Length from the middle point of epistomal suture to the posterior margin of the head.
-
o
HW: Maximum width of head in full-face view under eyes.
-
o
GL (Gaster Length): The visible anteriormost point in lateral view, to the posteriormost point.
-
o
ML (Mandible Length): Length measured in full face view from the lateral posteriormost point of the outer margin of the mandible to the tip of the apical tooth.
-
o
LHL (Lateral Head Length): Length from the anteriormost point of the lateral head portion in full-face view, perpendicular to the HW, to the posteriormost point.
-
o
SL (Scape Length): Length of right scape in dorsal view, from anteriormost point, excluding radicle, to the apex.
-
o
PtH (Petiole Height): The tallest vertical measurement from the apex of the node to the sternite, with petiole in profile view.
-
o
PtL (Petiole Length): Distance from the anteriormost visible point of the petiolar node to the upper posteriormost point of articulation with helcium in profile.
-
o
PpH (Propodeum Height): Vertical measurement that goes from the posteriormost lower point of the propodeum in profile, to the perpendicular point of PpL considering the highest point of the propodeal dorsum.
-
o
PpL (Propodeum Length): Horizontal measurement that goes from the anterior margin of the propodeum in profile, to the perpendicular point of PpH considering the posteriormost lower point of the propodeum.
$$\mathbf{T}\mathbf{L}(\text{Total Length}): =\text{ ML }+\text{ HL }+\text{ WL }+\text{ PtL }+\text{ GL}.$$ -
o
WL (Weber’s Length): Diagonal measurement of mesosoma in lateral view, from the anteriormost point of pronotum, excluding the pronotal collar, to the posteriormost lower point of metapleuron.
$$\mathbf{C}\mathbf{I} (\text{Cephalic Index}): =\text{ HW}/\text{HL x }100$$$$\mathbf{C}\mathbf{l}\mathbf{y}\mathbf{I} (\text{Clypeus Index}): =\text{ CW}/\text{CL x }100$$$$\mathbf{P}\mathbf{p}\mathbf{I} (\text{Propodeum Index}): =\text{ PpL}/\text{PpH x }100$$$$\mathbf{S}\mathbf{I} (\text{Scape Index}): =\text{ SL}/\text{HW x }100$$
Descriptions
Morphological terminology follows Delsinne et al. (2019). The notopropodeal sulcus refers to the mesosomal concavity between the mesonotum and propodeum when it is as long, or longer than the metathoracic spiracle. Integument sculpturing follows Harris (1979). Pilosity terms mostly follow Ulysséa and Brandão (2021). Names of new species follow recommendations by Vendetti and Garland (2019) and epithets were chosen using Hedgpeth (1954).
Images
High-resolution images were taken from AntWeb. Additional images for the dichotomous key and of C. hyalus sp. nov. were taken with a Zeiss Discovery V20, coupled with an Axiocam 305 camera, using Zen 2.3 software. Images of C. cameloides sp. nov. were taken with a Leica S8APO with a 2 × auxiliary lens, coupled with a Canon 1100D. Illumination was made with an adaptation of the scalable and modular illumination system presented in Kawada and Buffington (2016). Image stackings were done with Zerene 1.04 Build T2023-06–11-1120. Editing was done in GIMP; some scale bars were added in ImageJ (Schneider et al. 2012) through measurements of the body. For the Scanning Electron Microscopy (SEM), the specimen was fragmented so that the structures could be better explored, then they were covered with gold, attached to aluminum stubs with a double-faced conductive adhesive tape, and fixated on stubs. We obtained SEM images using a Tescan Mira3 FEG (field emission gun) at the Laboratório de Microscopia Eletrônica at MPEG.
Distribution maps
The distribution map of the new species was produced using QGIS 3.28.11- firenze (QGIS Development Team 2023), with geographic coordinates from the specimen labels. The shapefiles of Brazil's administrative boundaries and Brazilian biomes were provided by the Instituto Brasileiro de Geografia e Estatística (IBGE < www.ibge.gov.br >), while the Campo Rupestre shapefile was provided by Silveira et al. (2016).
Results
-
o
Order: Hymenoptera Linnaeus, 1758
-
o
Family: Formicidae Latreille, 1809
-
o
Subfamily: Formicinae Latreille, 1802
-
o
Tribe: Camponotini Forel, 1878
-
o
Genus: Camponotus Mayr, 1861
-
o
Subgenus: Camponotus (Myrmobrachys) Forel 1912
Diagnosis
Minor worker relatively small compared to most Neotropical Camponotus species. Head in full-face view trapezoidal, anterior portion narrower than posterior; lateral margin straight to convex; occipital corner rounded or forming blunt angle. Clypeus wider than long, usually with median portion elevated, forming broad and low, weak longitudinal carina. Eye reaching lateral margin; usually convex. Mesosoma relatively short, about as long as gaster, or slightly more; dorsal profile of mesosoma forming two distinct convexities, separated by well-developed notopropodeal sulcus or relatively deep suture. Pronotum with distinct dorsal and lateral faces, blunt or sharply marginated; pronotal dorsum usually straight to slightly convex. Propodeum without projections. Major worker differing from minor worker by: head shape varies from trapezoid, subrectangular or subquadrate with more convex lateral margin; eye less convex, reaching or not lateral margin, but never surpassing it. Pronotal dorsum in lateral view more convex than in minor worker.
senex-group
Mesonotal dorsal margin in lateral view continuous, propodeum not separated from mesonotum by sulcus or constriction, at most by fine line; pronotum sharply marginate; propodeum usually longer than high. Propodeum in lateral view with distinct dorsal and posterior margins, meeting in an angle; posterior margin deeply concave to straight (except C. championi). Camponotus abscisus, C. arboreus, C. auricomus, C. beebei, C. biolleyi, C. brasiliensis, C. brettesi, C. brevis, C. cameranoi, C. canescens, C. championi new combination, C. cheesmanae, C. conulus, C. crassicornis, C. crassus, C. cuneidorsus, C. excisus, C. formiciformis, C. godmani, C. guayapa, C. kutterianus, C. lindigi, C. mina, C. mus, C. normatus, C. peperi, C. phytophilus, C. piceatus, C. pittieri, C. planatus, C. rubrithorax, C. scipio, C. senex, C. socorroensis, C. sphenoidalis, C. subcircularis, C. textor, C. trapezoideus, C. trepidulus, C. yala, C. zoc.
burtoni-group
Mesosoma higher than long. Dorsal margin of mesonotum in lateral view continuous with propodeum. Propodeum in lateral view higher than long. Unique species, C. burtoni.
dimorphus-group
Dorsal margin of mesonotum in lateral view discontinuous, notopropodeal sulcus as long as or longer than spiracle length. Pronotum and mesonotum forming convex surface, sometimes mesonotum higher than pronotum anteriorly. Propodeum always rounded in lateral view, dorsal and posterior margin continuous sometimes forming blunt obtuse angle; posterior margin convex to slightly concave ventrally. Species list see Table 1.
Comments
No type specimens of C. abscisus were studied, but according to the redescription by Forel (1884), C. abscisus has the mesonotum separated from the propodeum by a deep and wide constriction, morphology that would make a member of the dimorphus-group. Still, according to Forel (1884), the propodeum (called by him as metanotum) of C. abscisus is high, as wide as long and short. In the original description of C. elevatus, Forel (1899) argues that C. elevatus has the propodeum higher than the mesonotum, the body color and sculpturing are weaker than in C. abscisus, which presumably does not have the propodeum higher than the mesonotum. One major worker of C. abscisus identified by Mackay in 1997 on AntWeb (CASENT0217599) was observed (AntWeb 2024). This specimen is similar to the type specimens of C. elevatus, but the propodeum is at the same level as the mesonotum, the clypeus is truncate and the anterior head portion is yellowish. These characters justify the identification done by Mackay. Even though we consider the major worker identified by Mackay in 1977 has the typical morphology of C. abscisus, we are not sure if the propodeal height of the workers is a character stable enough to separate C. abscisus from C. elevatus. There are two minors and one major worker at JTLC (respectively CASENT0280101, CASENT0882061 and CASENT0280100) identified as C. abscisus. Looking at the propodeal height in relation to the mesonotum level of these specimens, they seem to be transitional forms between what Mackay in 1997 considered as C. abscisus and the type specimens of C. elevatus. Considering that we did not have access to the type specimen of C. abscisus, which is a gyne, or the specimens on which Forel (1884) based his redescription, we cannot be sure about the morphology of C. abscisus and the diagnostic characters that separate it from C. elevatus. On the other hand, we also cannot synonymize C. elevatus under C. abscisus for the same reasons. To avoid misidentifications, we decided not to include C. abscisus in the key, given its ambiguous species boundaries. We chose to keep C. elevatus in the key because of the availability of type specimen images which reference the morphology of this species.
According to Emery (1925), the senex- and dimorphus- groups can be distinguished mainly by the shape of the mesosoma, which is continuous in senex- and discontinuous in dimorphus-group. Despite this reliable diagnostic characteristic, some species do not conform to it. Camponotus crassicornis, C. subcircularis, and C. championi, according to Emery (1925), are members of the dimorphus-group. Considering that their mesosomata are not discontinuous, and the posterior faces of the propodeum are concave, we reclassify these species into the senex-group. In the case of C. championi, the dorsal and lateral margins of the propodeum, in a lateral view, are continuous and form an obtuse angle, resembling some species of the dimorphus-group. Nevertheless, their mesosomal dorsal margin is continuous. On the other hand, C. dolabratus has a discontinuous dorsal margin, and thus, we reclassified it into the dimorphus-group.
The Camponotus (Myrmosphincta) Forel 1912 species also have a discontinuous mesosomata, but the head is oval to elongate and the mesosoma is usually longer than the gaster (Emery 1925; França pers. obs.). Camponotus lancifer, which is a member of C. (Myrmosphincta), should be considered a member of C. (Myrmobrachys) due to the subtrapezoidal head, wider than long clypeus, and the mesosoma as long as the gaster. Since a well-developed notopropodeal sulcus separates the mesonotum from the propodeum, and then placed in the dimorphus-group.
Key for the species groups of Camponotus (Myrmobrachys) based on minor workers
1—Dorsal profile of mesosoma in lateral view forming a single convexity. Mesonotum continuous with the propodeum or separated from it by a shallow impression (Fig. 2a) …………………………………………………………………………………………… 2.
Camponotus mesosoma in lateral view. A, C. crassus (CASENT0173407, authorship: April Nobile, available at AntWeb.org); B, C. iheringi (CASENT0173425, authorship: April Nobile, available at AntWeb.org); C, C. dimorphus (CASENT0173412, authorship: April Nobile, available at AntWeb.org). Dashed lines highlight the mesosoma shapes
1’ Dorsal profile of mesosoma in lateral view forming two distinct convexities. Mesonotum separated from the propodeum by a well-developed notopropodeal sulcus or a deep suture (Fig. 2b and c) …………………………………………… dimorphus-group.
2 (1)—Mesosoma longer than high, in lateral view (Fig. 3a). Mesonotum as long as the dorsal propodeal margin and separated from it by a well-developed suture ………………………………………………………………………………. senex-group.
2’—Mesosoma higher than long, in lateral view (Fig. 3b). Mesonotum and dorsal propodeal margin narrow, separated each other by vestigial suture ………….. C. burtoni.
Key for the species of the dimorphus-group based on minor workers
1—Mesonotum separated from propodeum by well-developed notopropodeal sulcus in lateral view (Fig. 2c), as long as metathoracic spiracle, or more, in dorsal view ……….. 2
1’—Mesonotum separated from propodeum by deep suture in lateral view; impression shorter than metathoracic spiracle in dorsal view (Fig. 2b) …………………………….. 7
2 (1)—Notopropodeal sulcus as long as the propodeal dorsum (Fig. 4a). Petiolar node in lateral view subquadrate; anterior margin meeting the dorsal margin in a blunt angle; posterior margin distinct and straight …………………………….. C. cameloides sp. nov.
2’ Notopropodeal sulcus shorter than the propodeal dorsum (Fig. 4b). Petiolar node in lateral view varying in shape, but never subquadrate …..…….………………………….. 3
3 (2’)—Lateral face of pronotum coarsely punctate. Petiolar node apically acute in lateral view; anterior and posterior faces separated by sharp edges dorsally (Fig. 4b) ………………………………………………………………………………… C. lancifer.
3’—Lateral face of pronotum smooth and shining, at most with weak and sparse punctures or imbrications. Petiolar node in lateral view of different shapes, never acute; anterior and posterior faces not separated by sharp edges dorsally (Fig. 3a, 5a, and b) ….………………………………………………………………………………………… 4.
4 (3’)—Propodeum distinctly marginate, dorsal and lateral faces separated by blunt edges. Petiolar node not scale-like ……….…………………………………………………….. 5
4’—Propodeum not marginate, dorsal face gradually curving to the lateral face. Petiolar node scale-like ………………………………………………………………………….. 6
5 (4)—Meso and metapleuron smooth and shining. Dorsum of propodeum wider than long in dorsal view. Petiole node in lateral view with anterior and dorsal margins meeting in an obtuse blunt angle (Fig. 5a)..……………………….………………. C. hyalus sp. nov.
5’—Meso and metapleuron coarsely imbricate to costulate. Dorsum of propodeum longer than wide in dorsal view. Petiole node in lateral view with anterior and dorsal margins meeting in an acute blunt angle (Fig. 5b) ………………………..…………………. C. scissus.
6 (5’)—Head and mesosoma surfaces smooth and shining (Fig. 6a). Body color bright yellow to orange …………………………………………………………… C. dimorphus.
6’—Head and mesosoma surfaces punctate or imbricate (Fig. 6b). Body color predominantly dark-brown with anterior portion of head and appendages pale yellow ……………………………………………………………………………… C. dolabratus.
7 (1’)—Propodeum distinctly marginate, lateral faces separated from dorsal and posterior faces by sharp edges (Fig. 7a) …………………………………………………………… 8.
7’—Propodeum not marginate or, at most, with anterior portion marginate, lateral faces continuous with dorsal and posterior faces (Fig. 7b) ………………………………….. 11.
8 (7)—Head in frontal view elongate. Frontal carinae strongly convex posteriorly. Eye globular. (Fig. 8a). Mesosoma smooth and shining …………………………. C. wytsmani.
8’—Head in frontal view not elongate. Frontal carinae parallel. Eye convex. (Fig. 8b). Mesosoma sculptured ……………..….………………………………………………… 9.
9 (8’)—Mesosoma costulate (Fig. 9a). Petiolar node subquadrate in lateral view …….. 10
9’ Mesosoma not costulate (Fig. 9b). Petiolar node subtriangular in lateral view ………………………………………………………………………………… C. iheringi.
(10) Pro and mesonotum at the same level of propodeum in lateral view. Propodeum dorsum convex in lateral view.………………………………………………………… C. striatus
10’ Pro and mesonotum higher than propodeum in lateral view. Propodeum dorsum straight, at most slightly convex, in lateral view …………………………………… C. circularis
11 (7’)—Propodeal dorsum strongly convex anteriorly. Petiolar node scale-like in lateral view, anterior and dorsal margins continuous, forming single convexity (Fig. 10a) ………………. C. elevatus (see dimorphus-group comments section about C. abscisus).
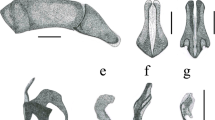
Camponotus propodeum height and petiolar node shapes in lateral view. A, C. elevatus paralectotype (CASENT0910729, authorship: Alexandra Westrich); B, C. caracalla lectotype (CASENT0910725, authorship: Z. Lieberman, available at AntWeb.org); C, C. pachylepis lectotype (CASENT0905520, authorship: Z. Lieberman, available at AntWeb.org). Dashed lines represent petiolar node shapes
11’—Propodeal dorsum slightly convex anteriorly. Petiolar node trapezoidal in lateral view, anterior and dorsal margin forming an angle; node higher posteriorly (Fig. 10b and c)..……………………………………………………………………………………….. 12.
12 (11’)—Petiolar node subtrapezoidal in lateral view, higher posteriorly (Fig. 10b and c) ………………………………………………..……….………………………………….………… 13.
12’—Petiolar node not subtrapezoidal in lateral view, higher medially ………………. 14.
13 (12)—Propodeum dorsum straight to convex in lateral view; posterior margin straight. Petiolar node inclined anteriorly in lateral view (Fig. 10b) …………………. C. caracalla.
13—Propodeum dorsum lower posteriorly in lateral view; posterior margin concave. Petiolar node not inclined anteriorly in lateral view (Fig. 10c) ………………. C. pachylepis.
14 (12’)—Propodeal dorsum only 1/3 descending posteriorly in lateral view. Petiolar node with anterior margin curving near to spiracle (Fig. 11a) ………………. C. propinquellus.
Camponotus propodeal dorsum and petiole anterior margin in lateral view. A, C. propinquellus (CASENT0884449, authorship: Z. Lieberman, available at AntWeb.org); B, C. propinquus lectotype (CASENT0915799, authorship: Harald Bruckner, available at AntWeb.org). Dashed lines representing propodeal dorsum and anterior petiole margin shape
14’—Propodeal dorsum descending 2/3 posteriorly in lateral view. Petiolar node with anterior margin curving distant from the spiracle (Fig. 11b) ……..……… C. propinquus.
Camponotus (Myrmobrachys) cameloides França, Cubillos, Chaul, Prado & Lattke.
Type specimens
Holotype worker. BRAZIL: Minas Gerais: Parque Nacional da Serra do Cipó, Capão dos Palmitos, -19.369074 -43.616814, vii.2014—iii.2015, Fieto, L. col., C4 A2 54P [arboreal pitfall] (DZUP, UFV-LABECOL-009812). Paratype workers. Same data as holotype (11 at CELC: UFV-LABECOL-009224, UFV-LABECOL-009705, UFV-LABECOL-009225, UFV-LABECOL-009235, UFV-LABECOL-009216, UFV-LABECOL-009227, UFV-LABECOL-009232, UFV-LABECOL-009228, UFV-LABECOL-009237, UFV-LABECOL-009229, UFV-LABECOL-009226; one at INPA: UFV-LABECOL-009223; one at DZUP: C1 A11 14S.1 UFV-LABECOL-009231; one at MPEG: UFV-LABECOL-010053; two at MZUSP: UFV-LABECOL-009236, UFV-LABECOL-009670; one at PSWC: ANTWEB1032633).
Etymology
The specific epithet cameloides (from the Latin words camelus, meaning “camel”, + oides suffix, meaning “resembling, having the form of'') is a singular and masculine adjective in the nominative case. It refers to the remarkable mesosomal shape of this species that resembles a camel's back.
Diagnosis
Pronotum not marginate, humeral angles blunt. Mesonotum convex, not separated posteriorly from the notopropodeal sulcus by a suture; in lateral view, not separated from the mesopleuron by a suture. Notopropodeal sulcus almost as long as propodeum in lateral view. Propodeum lower than mesonotal level; convex, shorter and narrower than mesonotum. Petiolar node subquadrate in lateral view, anterior and dorsal margins meeting in a blunt angle.
Minor worker
Measurements and Indexes
Holotype
CL 0.31; CW 0.50; EL 0.28; LHL 0.84; HMdL 0.74; HL 1.05; HW 0.90; GL 1.46; ML 0.40; SL 0.93; PtH 0.34; PtL 0.22; PpH 0.28; PpL 0.25; WL 1.21; TL 4.34. CI 85.71; ClyI 161.29; PpI 89.28; SI 103.33.
Paratypes
CL 0.25–0.27; CW 0.49–0.57; EL 0.28–0.30; LHL 0.84–0.97; HMdL 0.70–0.75; HL 0.97–1.02; HW 0.87–1.00; GL 1.33–1.70; ML 0.43–0.50; SL 0.90–0.98; PtH 0.28–0.37; PtL 0.20–0.24; PpH 0.30–0.38; PpL 0.21–0.28; WL 1.18–1.35; TL 4.13–4.77. CI 85.29–103.09; ClyI 175–220; PpI 61.76–93.33; SI 94.84–103.44.
Head. Sub-trapezoidal in frontal view, anteriorly narrower than posteriorly; lateral margin straight; occipital corner forms single convexity with posterior margin. Clypeus wider than long; anterior margin straight, with series of coarse setae, medial one longest; medially forming broad and low, weak longitudinal carina. Subgenal sulcus in frontal view angular. Frons continuous with frontal lobe. Frontal lobe anteriorly as wide as antennal condyle. Medial frontal suture vestigial. Frontal carinae curved and converging posteriorly, almost parallel. Eye convex; surpassing lateral margin.
Mandible. Outer margin straight at base in frontal view, gradually becoming convex at apex; masticatory margin with three or four teeth; apical tooth about two times bigger than subapical teeth; masticatory teeth gradually decreasing in size.
Antenna. Scape surpassing occipital corner, variable in length (SI 94.84–103.44); basal portion slightly curved; apical portion wider than basal. Pedicel curved at base. Flagellomeres gradually increase in length and width apically; apical flagellomere about two and a half times longer than the anterior.
Mesosoma. Pronotum rounded to marginate; wider than long in dorsal view with rounded lateral margin. Propleuron flat in ventral view. Mesonotum convex and not marginate, continuous with mesopleuron and notopropodeal sulcus; wider than long in dorsal view, dorsal margin higher anteriorly in lateral view. Mesopleuron marginate ventrally by carina. Meso-metapleural suture absent, meso and metapleuron continuous. Notopropodeal sulcus as long as propodeum in dorsal view. Metathoracic spiracle globular. Metapleural-propodeal suture absent, metapleuron continuous to lateral face of propodeum. Bulla of metapleural gland visible; metapleuron posteriorly forming rounded lobe in lateral view. Propodeum not marginate; longer than wide in dorsal view, laterally compressed, dorsal face forming longitudinal blunt keel; dorsal and posterior margins forming single convexity in lateral view. Propodeal spiracle flat; opening circular and directed posteriorly.
Metasoma. Petiolar node with anterior and lateral margins forming single convexity in dorsal view; dorsal face marginate posteriorly, posterior margin straight; subquadrate in lateral view, anterior and dorsal margins meeting in blunt angle; dorsal and posterior margins meeting in straight angle; posterior margin straight. Petiolar spiracle opening circular. Sternum slightly convex becoming straight posteriorly. First gastral tergum with anterior and dorsal margins in lateral view meeting in blunt angle, shorter than second terga.
Color. Body and legs dark brown. Mandible, antenna and tarsi yellowish-brown, scape slightly darker.
Sculpturing. Body imbricate with piliferous punctures; clypeal imbrications weaker than vertex; meso-, metapleural and propodeal imbrications vary from fine to coarse. Mandible dorsal surface smooth and shining with piliferous punctures. Pygidium cataphracted (Fig. 13c and d; Keller, pers. comm.).
Pilosity and pubescence. Thin appressed and relatively short hairs present throughout body. Dorsal surface of mesonotum and metanotum with long suberect branched hairs, branches as long as hair width (Fig. 13a and b). Scape with dense appressed pubescence and short erect hairs on apex.
Comments
Amongst the Camponotus of the dimorphus-group, C. cameloides is unique due to the relatively wide notopropodeal sulcus and the dorsal face of propodeum shorter than the mesonotum. The imbricate sculpturing and petiolar node shape are shared with other species, but the mesosoma shape is enough to recognize it. The imbricate sculpturing on the frontal area and mesosoma varies from weak to coarse. The number of suberect setae on the posterior portion of the head and dorsal face of the mesosoma also varies.
Branched pilosity is an interesting feature of C. cameloides, varying in size and number of branches in the body of the specimens. Branched pilosity are known in larvae of Formicinae and Myrmicinae in general (Wheeler G and Wheeler J 1953; Hölldobler and Wilson 1986; Fox et al. 2007; Ulysséa and Brandão 2021). In the Camponotini larvae, it is less variable (Wheeler G and Wheeler J 1953). In workers of Hylomyrma Forel 1912, as shown in detail by Ulysséa and Brandão (2021), this type of pilosity has a wide range of morphologies. This feature remains to be further investigated comparatively within Camponotus.
Based on the available data on the labels, C. cameloides appears to be a species with arboreal habits, as most samples were collected using methodologies such as arboreal pitfall traps or beating in vegetation. Only a single specimen was collected on the ground through epigeic pitfall trap, suggesting this species may occasionally forage in the epigeic stratum.
Through our search in collections for this species and the examined samples, C. cameloides seems to have a restricted distribution to the Cerrado in Campos Rupestres areas (Fig. 14), besides being a species collected infrequently, given the low number of occurrences during inventories conducted in Brasília (n = 1) and in the Serra do Cipó (n = 3). Additionally, most known records for this species come from conservation areas (i.e., National Park and an Environmental Protection Area), reducing threats to the species. However, in recent years, wildfires in the region have been recorded with greater intensity, representing a concern, particularly considering the vulnerability of ants to anthropogenic actions (Kuchenbecker et al. 2023).
Distribution maps. A, distribution of C. cameloides sp. nov. (black markers) and C. hyalus sp. nov. (red markers). Triangles represent type-localities; colors represent Brazilian biomes: Amazônia (green), Cerrado (yellow), Pantanal (brown), Mata Atlântica (light blue), Caatinga (red), Pampa (dark blue). B, distribution of C. cameloides sp. nov. in Campos Rupestres (green areas). C, image representing the type-locality of C. cameloides sp. nov (by Scarlett Epifânio)
Additional material examined
5 minor workers. BRAZIL: Distrito Federal, Brasília, xii.2017, 15° 46′ 32.31" S 47° 56′ 46.55" W pitfall [epigaeic], Costa, M. B. T. col, (MPEG, 1 worker, MPEG03034550)/ APA Gama Cabeça de Veado, ii-iii.2000, Mireille, P. col., (CELC, 1 worker, UFV-LABECOL-010116). Minas Gerais, Serra do Cipó, vii.2011–1.iii.2015, 960 m, 19°22′3S, 43°37′1W, Cód. C4A254.7, Ribeiro, L. [col.], (DZUP, 1 worker, DZUP591921)/ [same data], Cód. C1781A12 (DZUP, 1 worker, DZUP591923)/Capão dos Palmitos, -19.369074 -43.616814, vii.2014-iii.2015, Fieto, L. col., [arboreal pitfall] (MPEG, 1 worker, UFV-LABECOL-009230).
Camponotus hyalus França, Cubillos & Lattke.
Type specimens
Holotype worker. BRAZIL: GO [Goiás]: Anápolis, Campus UEG [Universidade Estadual de Goiás] [-16.381694 -48.946056]—Mata Mesófila, 15.ix.2005, #5 (Lozi, Luciano col.), Coll. Diniz, (DZUP, DZUP591920). Paratype worker. BRAZIL: MG [Minas Gerais]: Varginha, [-21.579944 -45.438167] IX-[19]61, M. Alvarenga [col.], (MZSP, MZSP0106987).
Etymology
The specific epithet hyalus (from the Latin word hyalus = glass) is a Latin singular and masculine in nominative case and refers to the smooth and shining integument of workers, which resembles glass.
Diagnosis
Smooth and shining integument. Notopropodeal sulcus wider than metanotal spiracle. Metanotal spiracle tuberculiform. Propodeum sharply marginated; dorsal margin straight in lateral view. Petiolar node trapezoidal in lateral view, anterior and dorsal margins form blunt obtuse angle; dorsal margin highest posteriorly forming convexity with posterior margin.
Minor worker
Measurements and Indexes
Holotype: CL 0.28; CW 0.40; EL 0.22; LHL 0.80; HMdL 0.77; HL 1.05; HW 0.77; GL 1.33; ML 0.37; SL 0.90; PtH 0.40; PtL 0.18; PpH 0.25; PpL 0.43; WL 1.21; TL 4.14. CI 73.33; ClyI 142.85; PpI 172; SI 116.88.
Paratype
CL 0.28; CW 0.43; EL 0.22; LHL 0.80; HMdL 0.71; HL 0.99; HW 0.84; GL 1.52; ML 0.37; SL 0.96; PtH 0.46; PtL 0.22; PpH 0.34; PpL 0.46; WL 1.24; TL 4.34. CI 84.84; ClyI 153.57; PpI 135.29; SI 114.28.
Head. Sub-trapezoid in frontal view, anterior portion narrower than posterior; lateral margin straight; occipital corner forming single convexity with posterior margin. Clypeus wider than long; anterior margin convex, medially with a series of thin setae; medially forming broad and low, weak longitudinal carina. Subgenal sulcus rounded in frontal view. Frons separated from frontal lobe by weak suture. Frontal lobe anteriorly as wide as antennal condyle. Medial frontal suture vestigial. Frontal carinae parallel posteriorly. Eye convex; surpassing lateral margin.
Mandible. Outer margin convex in frontal view; masticatory margin with five teeth gradually increasing in size apical.
Antenna. Scape surpassing occipital corners, variable in length (SI 114.28–116-88); basal portion slightly curved; apical portion wider than basal. Pedicel curved at base. Flagellomeres gradually decrease in length and increase in width apically; apical flagellomere two times longer than the anterior.
Mesosoma. Pronotum bluntly marginate; wider than long in dorsal view with rounded lateral margin. Propleuron flat in ventral view. Mesonotum bluntly marginate, continuous with notopropodeal sulcus posteriorly; wider than long in dorsal view, dorsal margin anteriorly higher in lateral view. Mesopleuron marginate ventrally by thick lamella. Meso-metapleural suture absent, meso and metapleuron continuous. Notopropodeal sulcus longer than metathoracic spiracle in dorsal view. Metathoracic spiracle shaped as tubercle. Metapleural-propodeal suture absent, metapleuron continuous to lateral face of propodeum. Bulla of metapleural gland visible; metapleural posteriorly forming rounded lobe in lateral view. Propodeum sharply marginated; subtrapezoidal in dorsal view, narrower anteriorly than posteriorly; dorsal margin straight in lateral view, forming blunt angle with posterior margin, both margins subequal in length. Propodeal spiracle globular; opening circular and directed posteriorly.
Metasoma. Petiolar node with dorsal face reduced and narrow in dorsal view; lateral margins convex. Anterior margin with one-third ventral portion vertical and the rest oblique, summit weakly convex, posterior margin slightly convex in lateral view. Petiolar spiracle opening circular. Sternum convex. First gastral tergum with anterior margin gradually forming dorsal margin in lateral view, shorter than second terga.
Color. Head black. Mandible yellowish-brown. Antenna yellowish-brown. Mesosoma black. Legs light-brown, except for dark-brown to almost black procoxa. Petiolar node black; peduncle and sternum light-brown. Gaster dark-brown to almost black.
Sculpturing. Head, mandible dorsal surface, petiole and most of mesosoma smooth and shining with sparse piliferous punctures, inconspicuous imbrications on meso and metapleuron. Gastral terga with inconspicuous imbrications and sparse piliferous punctures.
Pilosity and pubescence. Thin and relatively short hairs present throughout body. Hairs on scape erect and relatively short, becoming longer apically. Long suberect to erect hairs present on dorsal surface of head, mesosoma and petiole; long hairs on gaster limited to anterior face of T1.
Comments
Camponotus hyalus is the only species of the dimorphus-group with the notopropodeal sulcus longer than the metanotal spiracle and the dorsal and lateral faces of propodeum, separated by sharp edges with dorsal margin in lateral view straight. These characteristics are shared with Camponotus raphaelis Forel 1899 and Camponotus hippocrepis Emery 1920, both from Camponotus (Myrmocladoecus) Wheeler, 1921. The propodeal lobes of C. raphaelis and C. hippocrepis vary in proportion but are always present in minor and major workers. The propodeum of C. raphaelis is longer than wide, while in C. hyalus it is as wide as long. The petiolar node is similar to that of C. raphaelis but the propodeum of C. hyalus, lacking lobes or spines, corresponds to the Myrmobrachys dimorphus-group. Considering all these characteristics, the status of C. hyalus as a valid species is corroborated. The pilosity of C. hyalus has a rough aspect, as in C. cameloides, and it is probably branched too. Due to the lack of additional material, we were not able to verify this under SEMt. Camponotus hyalus can be quickly differentiated from C. scissus by the smooth and shining meso and metapleuron, the notopropodeal sulcus longer than the metathoracic spiracle, the propodeum dorsum is wider than long, and the petiolar node not forming acute blunt angle; whereas C. scissus, has coarsely imbricate to costulate meso and metapleuron, the notopropodeal sulcus at most as long as the metathoracic spiracle, the propodeum dorsum is longer than wide, and the petiolar node forming acute blunt angle.
We found one specimen in DZUP from Panamá (DZUP591922) which likely represents a new species similar to C. hyalus. It also has smooth and shining tegument and tubercle-like metathoracic spiracles, but the propodeum is not marginate, resembling more the propodeum in C. caracalla. This specimen has a broken gaster and due to the lack of more specimens, we decided to not describe it.
Discussion
Camponotus is a taxonomically challenging genus due to the myriad of species and available names. Most of the species of C. (Myrmobrachys) have high-quality images of type specimens available online. It was possible to examine 15 type specimens from the 20 available names for the dimorphus-group (Tab. 1), due to the unavailable images of the type specimens of C. abscisus, C. bajulus, C. propinquus baretoi, C. alfaroi and C. granulatus. In this case, the images were enough to recognize diagnostic characters useful for the dichotomous key. In particular, for C. abscisus, it is necessary to locate the type specimen, considering that it is currently a valid species. In Bolton (2024), the status of the type depository is unknown. The type material of Roger is principally in DEIB (Deutsches Entomologisches Institut, Berlin, Germany), MNHN (Muséum Nationale d’Histoire Naturelle, Paris, France), MNHU (Museum für Naturkunde der Humboldt-Universität Berlin, Germany), and ZSBS (Zoologisches Sammlunge des Bayerischen Staates, München, Germany). We were not able to find the type of C. abscisus in the digital repositories of these collections.
The identification key for the Neotropical Camponotus subgenera (Mackay 2019) and the Colombian key for species and complexes (Mackay W and Mackay E 2019), allows the users to easily get to the subgenus C. (Myrmobrachys), despite lacking some of its constituent species and being extensive keys. Additionally, the lack of colored, high-quality images gives these keys extra difficulty, leading to misinterpretations of the characters. With better images and highlighted characters, we hope to reduce ambiguity for the users. Furthermore, by proposing a key only to a subset of the C. (Myrmobrachys) species (those of the dimorphus-group), we considerably reduce the size of the key, reducing the chances of users getting lost. According to Mackay (2019), major workers are essential for the species identification of Camponotus. In the dimorphus-group, though, we found enough diagnostic characters in the minor. As the minor workers are more frequent in collections, we chose to rule out major characters from the key and species diagnosis.
According to Mackay (Mackay 2019, Mackay W & Mackay E (2019)), the subgenus C. (Myrmocladoecus) would be a synonym of C. (Myrmobrachys), since the author includes in both keys C. (Myrmocladoecus) species among the C. (Myrmobrachys) species. The synonym was not published and then, C. (Myrmocladoecus) was kept as a valid subgenus. Considering the diagnostic features proposed by Emey (1925), we consider both subgenera as distinct. The only species that might be confused with C. (Myrmocladoecus) is C. hyalus since it is similar to C. raphaelis and C. hippocrepis. Even though, C. hyalus does not have propodeal projections and the petiole node mucronate, which are diagnostic characters for C. (Myrmobrachys) (Emery 1925).
Revisions of local fauna, subgenera or groups of species can be beneficial to solve taxonomic issues of hugely diverse and difficult genera such as Camponotus. In the case of C. (Myrmobrachys), it is still necessary to review the senex-group, which is the richest in species and the one having a great number of specimens in the ant collections.
Conclusions
Amongst the Camponotus subgenera, the C. (Myrmobrachys) dimorphus-group is easily recognizable due to the discontinuous mesosoma. The availability of high-quality images of type specimens was an important factor that allowed this study. Notwithstanding, the status of C. abscisus and C. elevatus as valid species needs attention, and it is necessary to review more specimens, especially associated minor and major workers.
Even a hundred years later, the diagnosis for the Camponotus subgenera of Emery (1925) remains useful, but specific cases need attention and updated diagnosis are necessary. Identification keys are important to help myrmecologists recognize species, groups or subgenera and hence contribute to phylogenetics, ecology and natural history knowledge. We recommend taxonomic revisions for subgenera or species groups with relatively low numbers of species.
References
AntWeb (2024) AntWeb Versão 8.91.2. California Academy of Science. https://www.antweb.org. Accessed 20 January 2024
Bolton B (1973) The ant genera of West Africa: a synonymic synopsis with keys (Hymenoptera: Formicidae). Bulletin of the British Museum (Natural History). Entomology 27:317–368
Bolton B (2003) Synopsis and classification of Formicidae. Mem Am Entomol Instit 71:1–370
Bolton B (2024) An online catalog of the ants of the world. https://antcat.org. Accessed 20 January 2024
Bueno O, Campos-Farinha AEC (1999) As formigas domésticas. In: Mariconi FAM (Coord) Insetos: e outros invasores de residências. Piracicaba: FEALQ, cap.6, p 135–180
Campos-Farinha AEC, Bueno OC, Campos MCB, Kato LM (2002) As formigas urbanas no Brasil: retrospecto. O Biológico 64(2):129–133
Delsinne T, Serna FJ, Leponce M, Boudinot BE (2019) Glosario de morfología. Capítulo 13. In: Fernández F, Guerrero RJ, Delsinne T (eds) Hormigas de Colombia, 1st edn. Universidad Nacional de Colombia, Bogotá, pp 387–457
Emery C (1920) Le genre Camponotus Mayr. Nouvel essai de la subdivision en sous-genres. Revue Zoologique Africaine (brussels) 8:229–260
Emery C (1925) Hymenoptera. Fam Formicidae Subfam Formicinae Genera Insectorum 183:1–302
Fernandes EF, Castro MM, Barbosa BC, Prezoto F (2014) Variation in Nesting Behavior of the Arboreal Ant Camponotus sericeiventris (Hymenoptera: Formicidae). Florida Entomol 97:1237–1239
Fernández F (2003) Subfamilia Formicinae. In: Fernández F (ed) Introducción a las Hormigas de la región Neotropical, Inst. Invest. Recursos Biológicos Alexander von Humboldt, Bogotá, Colombia, pp 299 – 306
Forel A (1884) Études myrmécologiques en 1884 avec une description des organes sensoriels des antennes. Bulletin De La Société Vaudoise Des Sci Naturelles 20:316–380
Forel A (1899) Formicidae. [concl.]. Biologia Centrali-Americana Hym 3:137–160
Forel A (1912) Formicides néotropiques. Part VI. 5me sous-famille Camponotinae Forel. Mémoires De La Société Entomologique De Belgique 20:59–92
Fox EGP, Solis DR, De Jesus CM, Bueno OC, Yabuki AT, Rossi ML (2007) On the immature stages of the crazy ant Paratrechina longicornis (Latreille 1802) (Hymenoptera: Formicidae). Zootaxa 1503(1):1–11
Harris RA (1979) A glossary of surface sculpturing. Occasional papers of the Bureau of Entomology of the California Department of Agriculture, 28, 1–31
Hedgpeth JW (1954) Composition of Scientific Words. A manual for the methods and a lexicon of materials for the practice of logotechnies. Roland Wilbur Brown. Published by the author, US National Museum, Washington Science, 121(3146), 550–551
Hölldobler B, Wilson EO (1986) Soil-binding pilosity and camouflage in ants of the tribes Basicerotini and Stegomyrmecini (Hymenoptera, Formicidae). Zoomorphology 106(1):12–20
IBGE (Inst.Brasileiro de Geografia e Estatística) (2004) Mapa de biomas do Brasil primeira aproximação. Rio de Janeiro, Brasil. https://www.ibge.gov.br. Accessed 08 February 2024
Smithsonian Institution (2024) NMNH Data. https://collections.nmnh.si.edu/search/ento/. Accessed 22 January 2024
Kawada R, Buffington ML (2016) A scalable and modular dome illumination system for scientific microphotography on a budget. PLoS ONE 11(5):e0153426
Kuchenbecker J, Camarota F, da Silva PG, Perillo LN, do Vale Beirão M, de Castro FS, Fernandes GW, do Espírito-Santo MM, Santos NC, Cardoso IGS, Neves FdS, (2023) Differential response of fire on the community dynamics of five insect taxa in a tropical mountaintop forest archipelago. Ecol Evol 13:e10806. https://doi.org/10.1002/ece3.10806
Mackay WP, MacKay EE (2019) Género Camponotus. Capítulo 26. In: Fernández F, Guerrero RJ, Delsinne T (eds) Hormigas de Colombia, 1st edn. Universidad Nacional de Colombia, Bogotá, pp 743–790
Mackay WP (2019) New World carpenter ants of the hyperdiverse genus Camponotus (Hymenoptera: Formicidae), Volume 1: introduction, subgenera, species complexes, and subgenus Camponotus. Mauritius: LAP LAMBERT Academic Publishing, USA
McArthur AJ, Leys R (2006) A morphological and molecular study of some species in the Camponotus maculatus group (Hymenoptera: Formicidae) in Australia and Africa, with a description of a new Australian species. Myrmecologische Nachrichten 8:99–110
Museum of Comparative Zoology Harvard (2024) MCZBASE: The Database of the Zoological Collections. https://mczbase.mcz.harvard.edu/Specimens.cfm. Accessed 22 January 2024
Oliveira AA, Campos AEC, Harakava R (2017) Genetic Diversity of Urban Camponotus Mayr (Hymenoptera: Formicidae) Ants Revealed by Capture of Alates and DNA Sequencing. Neotropic Entomol (impresso) 1:1–8
QGIS Development team (2023) GISA free and open SourceGeographic information system. Open Source Geospatial Foundation Project. https://qgis.org
Rasoamanana N, Csősz S, Fisher BL (2017) Taxonomic revision of imitating carpenter ants, Camponotus subgenus Myrmopytia (Hymenoptera, Formicidae) of Madagascar, using morphometry and qualitative traits. ZooKeys 681:119–152
Schneider C, Rasband W, Eliceiri K (2012) ImageJ. Nat Methods 9:671–675
Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL, Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403:129–152
Ulysséa MA, Brando CRF (2021) Taxonomic revision of the Neotropical ant genus Hylomyrma Forel, 1912 (Hymenoptera: Formicidae: Myrmicinae), with the description of fourteen new species. Zootaxa 5055(1):1–137
Vendetti JE, Garland R (2019) Species name formation for zoologists: a pragmatic approach. J Nat Hist 53(47–48):2999–3018
Ward PS, Blaimer BB, Fisher BL (2016) A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa 4072:343–357. https://doi.org/10.11646/zootaxa.4072.3.4
Wheeler GC, Wheeler J (1953) The ant larvae of the subfamily Formicinae. Ann Entomol Soc Am 46(1):126–171
Yamamoto M, Del-Claro K (2008) Natural history and foraging behavior of the carpenter ant Camponotus sericeiventris Guérin, 1838 (Formicinae, Campotonini) in the Brazilian tropical savanna. Acta Ethol 11:55–65
Acknowledgements
Dr. Amanda Nunes, for logistic support with the image acquisition hardware and software, Mdc. Frederico Marcineiro for image stacking, Dr. Rodrigo Feitosa, for allowing access to the DZUP collection and general support, Dr. Hilton Costi and the Laboratório de Microscopia Eletrônica (MPEG) for the SEM images, Dr. W. Mackay and Dr. P. Ward for comments.
Funding
ECBF (Finance Code 130862/2020–7) was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); LPP (Finance Code 001), JCMC (Finance Code 001), and DCCC (Finance Code 88887.910734/2023–00) were funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES-DM).
Author information
Authors and Affiliations
Contributions
Zoobank Registration Numbers: Camponotus cameloides França, Cubillos, Chaul, Prado & Lattke. http://zoobank.org/urn:lsid:zoobank.org:act:733FFAD6-041F-4FD8-B7F0-27F4D2EF-1CE8. Camponotus hyalus França, Cubillos & Lattke. http://zoobank.org/urn:lsid:zoobank.org:act:4AE9A47A-C802-405B-843E-B15C-44B7087A.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Eder Cleyton Barbosa França and Daniela Carolina Cubillos Cañizares. The first draft of the manuscript was written by both authors and all authors collaborated with data collection, reviewing and editing to previous versions of the manuscript. All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by José A Marin Fernandes
Supplementary Information
Below is the link to the electronic supplementary material.
13744_2024_1193_MOESM1_ESM.xlsx
Supplementary file1 Detailed measurements of C. cameloides sp. nov. and C. hyalus sp. nov. Measurements are given in mm. (XLSX 70 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
França, E.C.B., Cubillos, D., Chaul, J.C.M. et al. Taxonomic Additions to the Neotropical Subgenus Camponotus (Myrmobrachys) Forel 1912 (Hymenoptera: Formicidae) with Emphasis on the dimorphus-Group. Neotrop Entomol (2024). https://doi.org/10.1007/s13744-024-01193-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13744-024-01193-y