Abstract
Zabrotica Hull, 1958 is revised and six new species are described from Peru: Zabrotica artigasi sp. nov. from Junín, Zabrotica floresi sp. nov. from Huánuco, Zabrotica hockingi sp. nov. from Huánuco and Pasco, Zabrotica hulli sp. nov. from Pasco, Zabrotica maidecita sp. nov. from Cajamarca, and Zabrotica mariae sp. nov. from Apurímac, Cuzco and Puno. Additionally, Aymarasilus Artigas, 1974 syn. nov., is herein proposed as a junior synonym of Zabrotica. A diagnosis for the genus is provided, as well as an identification key to the known species and distribution maps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asilidae, also known as “robber flies”, is the third most diverse Diptera family, with more than 7500 described species, of which over 1500, allocated in 220 genera are recorded in the Neotropical region (Dikow 2021; Papavero 2009; Artigas and Vieira 2014; Vieira and Ayala-Landa 2014; Vieira and Rafael 2014). However, the Neotropical fauna is still poorly known taxonomically. Only a few faunistic inventories of Asilidae were conducted in South America, where many areas have never been sampled for most dipteran groups, evidencing that most taxa are unidentifiable to the species level mainly due to the lack of taxonomic revisionary works (hence many described species may be synonyms) and that an important percentage of species found in surveys represent undescribed taxa (Amorim 2009; Fisher 2009; Vieira et al. 2019).
Zabrotica Hull, 1958 and Aymarasilus Artigas, 1974 are currently two valid names of monotypic genera, both placed within the subfamily Stenopogoninae according to the traditional classification of American genera proposed by Artigas and Papavero (1988, 1991a, b, c), although they were included in different tribes, Tillobromatini and Enigmomorphini, respectively. Zabrotica was erected to allocate Zabrotica clarkei Hull, 1958, a Peruvian species from La Oroya, Junín. Additional records for Peru were made by Lamas (1972). Hull (1958, 1962) suggested a relation with Hypenetes Loew, 1858, in a broad sense, as the Neotropical species formerly included in this genus have been distributed in Alyssomyia Hull, 1962, Creolestes Hull, 1962, Pritchardia Stuardo Ortiz, 1946 and Tillobroma Hull, 1962 (Hull 1962; Carrera and Papavero 1965; Artigas 1970).
On the other hand, Aymarasilus inti Artigas, 1974, the type species of Aymarasilus, was proposed based on material from the Chilean-Bolivian Altiplano, collected near the border with Peru. No additional records of this species have been made since its description and Artigas (1974) also considered it related to Alyssomyia and Creolestes. Thus, the aim of the present work is to taxonomically revise Zabrotica providing descriptions of new species, illustrations, keys for identification, and updated distribution maps for the species.
Material and methods
Specimens included in this work are deposited in the entomological collections of the Natural History Museum, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM), Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Canada (CNC), the National Museum of Natural History, Washington DC, USA (NMNH) and the Museum of Zoology, University of Concepción, Concepción, Chile (MZUC-UCC). This latter institution was visited briefly with the main purpose of examining type material of Zabrotica and other Asilidae genera, consequently, an undescribed species, from Argentina, was examined, but its description had to be postponed for a future visit. Morphological terminology follows Cumming and Wood (2017). Digital images of specimens were taken with a Canon EOS T7 digital camera mounted on a Macro Focusing Rail Slider, and then integrated as single images with Combine ZP software (Hadley 2012).
The treatment of terminalia follows Vieira (2012), with the addition of chlorazol black as staining solution (Carayon 1969) for a better observation of structures of the female genitalia. The structures of the dissected male and female terminalia were photographed with a Leica MC 190 HD digital camera mounted on a Leica SAPO stereoscopic microscope, and then integrated as single images with Zerene stacker software (Zerene Systems LLC). Images and drawings were edited with Photoshop Cs6 and Illustrator Cs6. As the male terminalia in Zabrotica is rotated 180°, in order to avoid confusion with terminology, the structures and images, are described, labeled and presented not rotated (i.e. the epandrium dorsally and the hypandrium ventrally). After examination, the detached parts were stored in polyethylene microvials with glycerin for preservation and pinned with their respective specimens. The specimen data are transcribed in full, forward slashes separate different labels from the same specimen and square brackets enclose complementary or explanatory information not included in labels. The distribution maps were elaborated with SimpleMappr (Shorthouse 2010). The data for this map is based on the information from specimen labels and previously published literature (Hull 1958; Artigas 1974).
Abbreviations
Acanth sp – acanthophorite spine; cerc – cercus; cua – anterior cubital cell; d – discal cell; ej apod – ejaculatory apodeme; epand – epandrium; goncx – gonocoxite; gonst – gonostylus; hypd – hypandrium; m3 – third medial cell; ph – phallus; pped – postpedicel; R4 – upper branch of third branch of radius; R5 – lower branch of third branch of radius; r–m – radial–medial crossvein; sub scl – subepandrial sclerite; spmth – spermatheca; st – sternite; tg – tergite.
Taxonomy
Zabrotica Hull, 1958
Zabrotica Hull, 1958: 253. Type-species, Zabrotica clarkei Hull, 1958, by original designation.
Aymarasilus Artigas, 1974: 227. Type-species, Aymarasilus inti Artigas, 1974, by original designation. Syn. nov.
Diagnosis. Species of Zabrotica can be recognized by the following combination of characters: 1. Antennal postpedicel sessile, subequal to slightly longer in length than scape and pedicel combined and more than three times the length of stylus, the latter composed of one article; 2. Facial gibbosity flattened to gently rounded, occupying about 4/5 of face; 3. Presutural dorsocentral macrosetae on scutum present; 4. Wing relatively long, reaching or surpassing the apex of the abdomen; 5. Wing cell r1 open and m3 closed and stalked; 6. Abdomen more or less laterally compressed; 7. Male terminalia rotated 180°; 8. Phallus single pronged; 9. Hypandrium triangular, not projected apically; 10. Female terminalia with divided tergite 10, bearing six acanthophorite spines; 11. Three oval sclerotized spermathecae.
Distribution. (Fig. 1). Peru, Chile, Bolivia and Argentina (an undescribed species misidentified as Creolestes is deposited in the MZUC-UCCC).
Taxonomic discussion. The analysis of the type material of Aymarasilus inti Artigas, 1974, syn. nov. (Fig. 2), images of Peruvian specimens of Zabrotica examined by Hull and deposited in CNC, and NMNH, additional specimens deposited at MUSM (Figs. 3, 4, 5, and 6), and original descriptions of both genera and species (Hull 1958, 1962; Artigas 1974) revealed that the material used to describe Aymarasilus belongs to the previously described Zabrotica, and that its type species Aymarasilus inti Artigas, 1974, syn. nov., is conspecific with Zabrotica clarkei Hull, 1958, based mainly on the facial gibbosity apruinose, shiny black, and the gonocoxite long projected, markedly curved backwards and pointed apically. As a consequence, Aymarasilus syn nov. is herein synonymized with Zabrotica.
Type material of Aymarasilus inti Artigas 1974 (MZUC-UCC). a. Male holotype, habitus in lateral view; b. Female allotype, habitus in lateral view; c–d. Labels; e–f. Head, lateral and frontal views; g. Mate terminalia, lateral view; h–i. Phallus, lateral and ventral views. (e–i, modified from Artigas (1974))
Zabrotica clarkei Hull, 1958. a–d. Male determined by Hull (CNC). a. Habitus, lateral view; b. Habitus, dorsal view; c. Head, frontal view; d. Labels; e–f. Female with reddish legs (above) and male with black legs (bellow) in copula (MUSM). g. Male terminalia in lateral view, based on male specimen from Huarochirí (MUSM). Credits. a–d. CNC, ©Her Majesty The King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food, licensed under the Open Government License – Canada, https://www.cnc.agr.gc.ca/taxonomy/TaxonSearch.php, CNC658817, accessed 25 November 2021
Zabrotica clarkei Hull, 1958, male, terminalia based on specimen from Huarochirí. a–b. Lateral and ventral views after treatment with KOH; c. Ventral view showing the apical curvature of gonocoxite in dry specimens; d. Dorsal view; e–f. Gonocoxite and gonostylus, lateral and ventral views; g–h. Phallus, lateral and ventral views; i. Hypandrium; j. Cercus and subepandrial sclerite
Zabrotica clarkei Hull, 1958, female. a–e. Terminalia after treatment with KOH. a–b. Lateral view; c. Dorsal view; d. Spermatheca; e. Dorsal view. f–h. “flavipes” of Hull, nomen nudum, “female holotype”. f. Labels; g. Head in frontal view; h. Habitus in lateral view (NMNH). Credits. f–h. National Museum of Natural History, Smithsonian Institution, https://collections.nmnh.si.edu/, USNMENT01789752, accessed 27 August 2022
Zabrotica clarkei Hull, 1958, a–b. “flavipes” of Hull nomen nudum, “male paratype” (CNC). a. Labels; b. Habitus, lateral view; c–f. “nigra” of Hull, nomen nudum, “male holotype” (NMNH). c. Head, frontal view, d. Labels; e–f. Habitus, lateral and dorsal views. Credits. a–b. CNC, ©Her Majesty The King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food, licensed under the Open Government License – Canada, https://www.cnc.agr.gc.ca/taxonomy/TaxonSearch.php, CNC658812, accessed 25 November 2021. c–f. National Museum of Natural History, Smithsonian Institution, https://collections.nmnh.si.edu/, USNMENT01789755, accessed 27 August 2022
The relation of this genus with Alyssomyia and Creolestes proposed by Artigas (1974) can be interpreted as the same suggested by Hull (1962) with Hypenetes because several of the Neotropical species formerly included in the latter have been transferred to both of the former genera above. However, while Hull (1962) based the relation on external phenotypic resemblances between the species, Artigas discussed the similarities between male terminalia with Alyssomyia, particularly with Alyssomyia brevicornis (Philippi 1865), although he pointed out a closer similarity with the phallus of Creolestes. Regarding this, based on the new species of Zabrotica herein described and the examination of Alyssomyia and Creolestes species deposited at MZUC-UCCC, the male terminalia of Zabrotica shows less variation when compared to the other two genera. In Alyssomyia there is a large interspecific variation in the hypandrium (long projected apically) and less variation in the epandrium (similar to the design of Zabrotica). In contrast, in Creolestes the interspecific variation is more evident in the epandrium, while the hypandrium varies less, and is more similar to that of Zabrotica, which shows a more evident interspecific variation in the gonocoxite, while the phallus is quite similar in all species.
Additionally, from genera with similar habitus in the Tillobromatini or Enigmomorphini tribes sensu Artigas and Papavero (1991a); Zabrotica can be differentiated from those allocated in Tillobromatini due to the cell m3 closed and petiolate; while the presence of presutural macrosetae on scutum separates it from Cylicomera Lynch, Arribálzaga, 1881, Microstylum Macquart, 1838 and Prolepsis Walker, 1851; the facial gibbosity occupying most part of face (about 4/5) and flattened to gently rounded, separates it from Alyssomyia (with face nearly flat or restricted to the lower half), Creolestes and Pritchardia (with facial gibbosity pronounced and occupying 2/3 of face). Finally, the wing relatively long, reaching or surpassing the apex of the abdomen, the abdomen laterally compressed and the hypandrium not projected apically differentiates it from Araujoa Artigas & Papavero, 1991, which has the wing short, not extending beyond abdominal segment 5, the abdomen cylindrical and the hypandrium slightly projected apically.
Remarks. In Peru, the geographic records of Z. clarkei (Fig. 1a) are interestingly distributed across an altitudinal gradient in localities of Central Peru, in the departments of Lima and Junín, including its type locality in the latter. Precisely, the western Andean slopes of Central Peru present a particularly remarkable altitudinal gradient, with temperature and precipitation values lower than expected in these tropical latitudes and thermal inversions between June and October at elevations bellow 1500 m (Castañeda and Gonzales 2022). Thus, the apparent discrepancy in phenology for Z. clarkei among coastal lomas (fog oases) and Andean habitats at higher elevations (Table 1) is probably associated with the differences in climatic seasonality as this species apparently prefers arid to dry habitats (Figs. 7 and 8), but avoiding adverse conditions; in lomas, mainly distributed between 200 and 1200 m, this species is mainly present during the humid season (between June and October) when the humidity favors the plant and animal communities that depend on the incidence of fog water mostly generated by the cold Humboldt current over the Pacific (Gonzales et al. 2023). On the other hand, in higher elevations, dry cloud forests, shrublands and grasslands, this species seems to be more frequent during the dry season (between May and October), becoming rare in the rainy season. This pattern can be also observed for other species of Zabrotica in northern and southern Peru, which so far have been only collected in high elevations almost exclusively during the dry season, although it is possible that species that inhabit localities of the western Andean slopes extend their distribution to lower localities, including coastal lomas. However, more specimens are needed for a better understanding in each case. No specimens of Zabrotica have been collected at mid or low elevations of the eastern Andean slopes, where the precipitation values are considerably higher than similar elevations of the western slopes.
Zabrotica clarkei Hull, 1958 in the wild. Huarochirí Province, Lima, Peru. a. iNaturalist observation, https://www.inaturalist.org/observations/118397422, accessed 23 Mar 2023, photograph by Brandon Ortiz; b–c. iNaturalist observations, https://www.inaturalist.org/observations/112067217, https://www.inaturalist.org/observations/112067213, accessed 23 Mar 2023, photographs by Oscar Díaz
Zabrotica clarkei Hull, 1958 (Figs. 2, 3, 4, 5, 6, and 7)
Zabrotica clarkei Hull, 1958: 254. Type-locality: Peru, Junín, [La] Oroya. Holotype male, not in NMNH (Lamas 1972) nor in CNC. Probably lost.
Aymarasilus inti Artigas, 1974: 22. Type-locality: Chile, Tarapacá, Putre. Holotype male in MZUC-UCC. Syn. nov. (Fig. 2).
Zabrotica flavipes Hull (manuscript name at NMNH), Nomen nudum. Locality: Peru, Lima, San Cristóbal. (Figs. 5f–h and 6a–b).
Zabrotica nigra Hull (manuscript name at NMNH), Nomen nudum. Locality: Peru, Lima, San Cristóbal. (Fig. 6c–f).
Diagnosis. A black species with wing darkened by microtrichia and bluish reflections on the abdomen depending on the light incidence; facial gibbosity apruinose, shiny black (Figs. 3c, 5g, and 6c); 4–6 apical scutellar macrosetae, mixed white and black; R4+5 bifurcation usually posterior to apex of discal cell; terminalia mostly black setose; epandrium markedly curved downwards (Figs. 3g and 4a); gonocoxite markedly curved and long projected apically, extending well beyond the apex of the hypandrium, usually surpassing the opposite gonocoxite and reaching the apex of the opposite epandrium (Figs. 3g and 4a–c, e–f).
Distribution. (Fig. 1a). Chile. Parinacota (Putre); Peru. Ancash (Huaylas), Arequipa (Atiquipa, Majes), Ayacucho (Puquio), Huánuco (Huacrachucro), Junín (La Oroya, La Unión), Lima (Canta, Lima, Huarochirí), Moquegua (Mariscal Nieto).
Material examined. 88 specimens. Chile: 8 specimens, 5 ♂, 3 ♀. Type material: on flowers of Medicago sativa / col. L. Ruz CHILE / TARAPACA Putre 23-Abr. 1971/ Aymarasilus inti Artigas, 1974 HOLOTYPE MZUC-UCCC N° 40929/ Holotypus Aymarasilus inti Artigas 74; Allotype: Same data as holotype, except: MZUC-UCCC N° 40932/ ZUÑIGA, col. CHILE; Paratypes: Same data as holotype, except: MZUC-UCCC N° 40930, N° 40933 [Montenegro], N° 40934; Aymarasilus inti Artigas Det. J. N. Artigas 74/ PARATYPE/ CHUSMISA ALFALFA col. Perini 19-IV-71/ Aymarasilus inti Artigas, 1974 PARATYPE MZUC-UCCC N° 40931. Additional specimens: TARAPACA Putre 23-Abr. 1971/ Aymarasilus sp. fm y tb rojas [red femora and tibiae]; Belén, Arica, Tarapacá, Chile. 18-VII-1976 coll. H. Vargas C. / Aymarasilus sp. gen.[italia] ♂ diferente de inti (hyp.[hypandrium]) Peru: 80 specimens; Ancash. 3 ♂, 3 ♀. PERU: Dpto. Ancash: Incapamanán (Huaylas) (2,300 mt.) 7.v.84 Pedro Hocking (MUSM). Arequipa. 5 ♂, 3 ♀.Atiquipa [Caravelí, ̴ 15.8°S, 74.3°W] PERU 200 m 11.XII. 51/ Wr / coll. Weyrauch / Zabrotica clarkei Hull Det. J. N. Artigas 85 (♂, MZUC-UCCC); PERU, AR. Caylloma, Lluta, 3772 m, 15°56′21.9″S, 72°5′28.3″O 02.v.2018 E. Quispe (4 ♂, 3 ♀ MUSM); Ayacucho.1 ♀ Puquio (Cap. Lucanas) 3400 m. iv.50 Coll: F. Blancas / Prolepsis n. sp. G. Lamas M. det. 71 (MUSM). Huánuco. 2 ♂, 1 ♀. PERU: HU. Marañón, Huacrachucro, Mamahuaje 8°45′53.7″S, 76°25′29.8″W 1889 x.2017 J. Suárez (MUSM). Junín. 5 ♂, 5 ♀. PERU, JU. [Junín] Tarma, La Unión, Leticia, 3945 m 11°22′3.4″S, 75°48′19.5″O 19.x.2014 M. Cárdenas (MUSM); Lima. 18 ♂, 29 ♀. Atocongo near Lima [ ̴ 12.18S, 76.92°W] PERU 150 m 28.IX.52 leg. Weyrauch/ WKW 6038–4/ Wr/ Zabrotica clarkei Hull Det. J. N. Artigas 85 (♂ MZUC-UCCC); Lima Chosica 8-V-70 col. E. Mendoza/ Zabrotica clarkei Hull G. Lamas M. det. 70 (♀ MZUC-UCCC); Lima L.[Lomas de] Lachay 17-XI-70 col. E. Sánchez/ Zabrotica clarkei Hull G. Lamas M. det. 70 (♀ MZUC-UCCC); Zabrotica clarkei Hull Det. J. N. Artigas / Blancas/ MHN: 4423 Valle Sta. Eulalia (cerca Lima) [Huarochirí], 2180 m. VI. 53 Coll: Blancas (♀ MZUC-UCCC); Lima:Valle Sta. Eulalia (cerca Lima) 2180 m, vi.53 Coll: F. Blancas / MHN 4423 / Zabrotica clarkei Hull G. Lamas M. det. 71 (1 ♂, 3 ♀ MUSM); PERU: Dpto. Lima Bosque Carrión 16.iv.87 (2,400 mt.) Pedro Hocking / Prolepsis sp. Det. A. G. Scarbrough 94’ (4 ♀ MUSM); Surco, [Huarochirí], Lima, Perú 2000 m 25.v.74 G. Lamas (1 ♂, 1 ♀, in copula, pinned together, MUSM); LI: Surco 10-4-76 P. Hocking (♀, MUSM); PERU: Dpto. Lima Surco (1,800 mt.) 2.vi.84 Pedro Hocking / Prolepsis sp. Det. A. G. Scarbrough 94’ (♀); PERU: Dpto. Lima: Cerro de Surco (3,000 mt.) 21.iv.73 (♂, MUSM); PERÚ, LI. Huarochirí, Matucana 11°50′15.14″S, 76°23′38.38″W 3090 m 20–28.vi.2019 M. Lozano (1 ♀); PERU: Li. [Lomas de] Lachay18.xi.63 R. García / Ex Coll. R. García 1977 (1 ♂, 2 ♀); same data as previous, plus na additional label, Prolepsis sp. Det. A. G. Scarbrough 94’ (1 ♀); Lomas [de] Lachay (cerca Chancay) 400 m, 8.xi.51 Coll. Blancas (1 ♀); L. Lachay (Lima) 27.10.88 CR. Ramirez (1 ♂); PERÚ: LI. San Bartolo, Fundo Candelaria 12°22′11.29″S, 76°42′0.49″W 438 m, 21.x.2021 Lomas costeras I. Medina (6 ♂, 7♀) [4 females and 5 males preserved in absolute ethanol and stored at -18°C); PERU, Li. Tabl.[ada de] Lurín 12.x.70 R. García (3♂, 1 ♀, MUSM); Lima, Li. PERU F. Blancas (1 ♂); PERU: Dpto. Lima: Lomas de Atocongo 25.x.86 Pedro Hocking (1 ♀); PERU: LI, Lomas de Amancaes 15–29.xi.2005 [no data of collector] (1 ♂, 3 ♀); PERÚ, LI. Yauyos, Magdalena 12°29′23.56″S, 75°54′23.44″W 2313 m 3.xii.2015 I. Medina (♂, MUSM). Moquegua. 1 ♀ PERU: MO. Mariscal Nieto, Torata, 31.iii – 01.iv.2021 17°9′26.21″S, 70°38′23.85″W 3544 m Manual, L. Ramírez (MUSM).
Additional specimens [Based on images]. S. Cristóbal Lima, Peru 30.9.12 – alt. 850 ft/ CHT Townsend Collector/ Holotype/ Zabrotica flavipes Hull Det. F. M. Hull/ USNMENT 01789752 (♀); Matucana, PERU 10 May 1920 / Cornell Univ. Expedition. Lot 607 Sub 40 / Frank M. Hull Collection C.N.C. 1981 / Zabrotica flavipes Hull Det. F. M. Hull parat. [Paratype]/ CNC 658812 (♂); S. Cristóbal Lima, Peru 30.9.12 – alt. 850 ft/ Holotype/ Zabrotica nigra Hull Det. F. M. Hull/ USNMENT 01789755 (♂); Matucana, [Huarochirí] PERU 19 [?]May 1920 / Cornell Univ. Expedition. Lot 569 / Frank M. Hull Collection C.N.C. 1981 / Zabrotica clarkei Hull Det. F. M. Hull / CNC 658817 (♂).
Taxonomic discussion. Artigas (1974) reported one female with reddish legs, collected alongside with the type series of A. inti, not included in this species until further study. On the other hand, specimens studied by Hull, deposited at CNC and NMNH and labeled as types, indicate that this author intended to describe two additional species of Zabrotica, as “Zabrotica flavipes” Hull (nomen nudum), based on two specimens with reddish yellow legs and reddish male terminalia (Figs. 5f–h and 6a–b), and “Zabrotica nigra” Hull (nomen nudum), based on one specimen with black legs and black terminalia (Fig. 6c–f). Regarding this, from the 41 male specimens identified as Z. clarkei, only one, labeled as paratype of “Z. flavipes”, has reddish yellow legs while the remainder have predominantly black legs. In contrast, from the 47 females examined, only 17 have black legs, suggesting that the reddish coloration is more common, although from the 30 reddish-leg ones, eight have black femora basally, like the one labeled as holotype of “Z. flavipes” (Fig. 5h). Individuals with reddish and black legs usually occur at the same localities as shown in the material studied by Artigas and Hull (the “types” of “Z. flavipes” and “Z. nigra” were collected at the same locality in Lima [the "Cerro San Cristóbal” in the city of Lima, at 850 feet (= ca. 250 m) elevation]). In addition, two specimens, from Surco (near Matucana, where the male “paratype” of “Z. flavipes” was collected), were caught mating, a male with black legs, and a female with reddish legs (Fig. 3e–f). This indicates that the leg coloration highly varies intraspecifically, and that all examined specimens belong to a single taxon broadly distributed, ranging from low elevations, in Peruvian coastal lomas, to higher elevations, in Andean localities at almost 4000 m a.s.l. The male terminalia also varies similarly, from reddish brown to black, being black basally and reddish brown distally in a few specimens.
Moreover, the comparison with the new Zabrotica species described herein shows that Z. clarkei is readily recognized and differentiated from its congeners by the shiny black apruinose facial gibbosity and its gonocoxite long projected, markedly curved and pointed apically. However, four species share morphological similarities with Z. clarkei, although all of them have the facial gibbosity at least partially pruinose. Zabrotica artigasi sp. nov. and Zabrotica hockingi sp. nov. present a similar body and wing coloration (the most similar species at first glance), but they are smaller in size and have the gonocoxite straight and not long projected, while Zabrotica maidecita sp. nov. and Zabrotica mariae sp.nov. are very different in body and wing coloration (especially due to the nearly absence of microtrichia on wing) but they present a gonocoxite long projected and curved, although it is apically blunt in Z. maidecita sp. nov. In the case of Z. mariae sp. nov. the gonocoxite is similar to that of Z. clarkei but this species stands out from all its congeners due to the presence of 5–6 notopleural macrosetae (usually three in other species).
Despite the above, it was difficult to identify some specimens as Z. clarkei, because some male specimens, considered as a dark phenotype of this species, present the gonocoxite not as long and curved to intersect the opposite one. On the other hand, a few specimens were not included in the data provided on the distribution maps because they could not be confidently assigned to this species, and additional material is needed to establish whether they represent an undescribed species very close morphologically to Z. clarkei or a color variant of this species.
Zabrotica artigasi sp. nov. (Figs. 9 and 10)
Zabrotica artigasi sp. nov. a–g. Male terminalia, based on paratype, after treatment with KOH. a. Ventral view; b. Hypandrium; c–d. Gonocoxite and gonostylus, lateral and ventral views; e–f. Phallus, lateral and ventral views; g. Cercus and subepandrial sclerite. h–i. Female. h. Habitus, lateral view; i. Terminalia after treatment with KOH and spermatheca
urn:lsid:zoobank.org:act:42DE56B3-9031-4F63-860C-8CB4BB8F2330
Diagnosis. A black species with wing darkened by microtrichia; facial gibbosity almost entirely covered with pruinosity (Fig. 9d–e); two apical scutellar macrosetae, yellowish; R4+5 bifurcation usually anterior to apex of discal cell; male terminalia mostly yellow setose (Fig. 9f); epandrium gently curved downwards (Fig. 9f–g); gonocoxite not projected apically (Fig. 10c–d), thus it only extends to the apex of hypandrium, the latter with truncate apex (Fig. 10a–b).
Description of the male holotype (Fig. 9a–b). Length: body, 12 mm; wing, 8 mm.
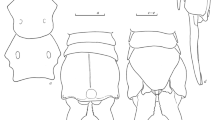
Head (Fig. 9d–e). Black; antenna black, black setose, except dorsally with some yellow setae; scape about 1.2 times as long as pedicel, postpedicel subequal to length of scape and pedicel combined and about four times the length of stylus, the latter plus an apical seta-like sensory element; face yellowish gray pruinose, about a third the width of head at level of antenna, slightly wider bellow; mystacal macrosetae mostly black, except central ones in the lower two thirds, white; proboscis black, yellow setose at ventral base and apically; palpus black, black setose, except for some yellow setae on first segment; frons, vertex and occiput golden pruinose, except on ocellar tubercle and behind it, sparsely pruinose to apruinose; ocellar tubercle black setose, 5 pairs of long setae, about 1.3 times as long as scape and pedicel combined; about 30 postocular macrosetae, more or less in a double row, most of them black, except the uppermost 5–6, yellow. Thorax. Black, golden pruinose, except the dark paramedial and lateral scutal stripes, brown pruinose, and some sparsely pruinose areas on pleura. Chaetotaxy: Antepronotum covered with black macrosetae; postpronotum mixed yellow and black setose, postpronotal lobe black setose; acrostichal and dorsocentral setae black, dorsocentral macrosetae present on most part of scutum, becoming gradually longer and stouter on posterior two thirds; 3 notopleural black macrosetae, 1 supra-alar, also black, and 3 postalar, yellowish; discal scutellar setae absent, two apical scutellar yellowish macrosetae; pleura sparsely setose, some black setae on superoposterior angle of anepisternum, some yellow setae on metanepisternum and katatergite, the latter mixed with black macrosetae. Wing (Fig. 9c). Veins brown; wing membrane densely covered with black microtrichia on anterior margin and distal fourth; crossvein r-m slightly posterior to middle of discal cell; R4+5 bifurcation anterior to apex of discal cell; cell cua narrowly open; halter yellow. Legs. Coxae black, golden pruinose, except posteriorly on fore- and mid-legs, and anteriorly on hind leg, apruinose; femora black, except distal apex, reddish brown; fore- and mid tibiae reddish brown, hind tibia dark brown; tarsi dark brown; claws black, empodia and pulvilli yellowish. Chaetotaxy: Coxae white setose; femora pale yellow setose, with yellow macrosetae; fore femur with 1 posterodorsal, preapical; mid femur with 3 anterior and 1 posterodorsal, preapical; hind femur with 4–5 anterior, 3–4 anteroventral, 2 posteroventral and 1 anterodorsal, preapical; tibia with yellowish setae and macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 3 long ventral macrosetae, mid tibia with 4 anterodorsal, 3 posterodorsal, 4–5 posteroventral, 2 ventral, 1 anteroventral and 2 anterior macrosetae; hind tibia with 3 anteroventral, 4 anterodorsal, 3–4 posterodorsal and 2 posteroventral macrosetae; tarsi with black and yellowish macrosetae. Abdomen. Black, more or less laterally compressed up to segment 5 (i.e. in dorsal view the abdomen looks more or less petiolate) (Fig. 9b); pruinosity golden, almost absent dorsally on tergites 2–5 and abundant on thin posterior margins of tergites 2–3, forming yellow fringes that stand out from the background; setae yellow; tergite 1 with 3–4 lateromarginal yellow macrosetae. Terminalia (Figs. 9f–h and 10a–g). Black, mostly yellow setose, except epandrium and gonocoxite, black setose apically; epandrium gently curved downwards (Fig. 9f–g); hypandrium triangular with truncate apex (Fig. 10a–b); gonocoxite subtriangular, not projected apically, only extending to apex of hypandrium (Figs. 9f–h and 10c); gonostylus with a pincer-like process (Fig. 10d); phallus with single prong, curved downwards (Fig. 10e–f); ejaculatory apodeme wide in lateral view, broader and straight distally; cercus with curved margins posteriorly (Figs. 9h and 10g).
Female (Fig. 10h–i). Similar to male except head golden pruinose, postpronotal lobe yellow setose, 4 notopleural macrosetae, katatergite with yellow macrosetae; all tibiae reddish brown, as tarsi. Terminalia. Three sclerotized spermathecae, oval and similar in size.
Variation. Katatergite entirely or partially with yellow macrosetae; one specimen with R4+5 bifurcation at level of apex of discal cell and apical scutellar macrosetae black instead of yellowish.
Etymology. The specific epithet is a masculine noun in the genitive case. This species is named after Jorge Artigas (1929–2022), entomologist with an exceptional contribution to the knowledge of Neotropical robber flies.
Distribution (Fig. 1b). Peru, department of Junín, Huancayo province. The type locality (Fig. 11), mainly dominated by grasslands and shrublands, is located on the eastern Andean slopes of central Peru. The known specimens were collected in June (Table 1).
Type material. 5 ♂, 1 ♀. Holotype. PERU: JU [Junín]. Huancayo, Pariahuanca, 3355 m, 11°54′41.4″S, 74°56′20.3″O, 23.vi.2019, Matorral E. Medina leg. Pantrap / HOLOTYPE ♂ Zabrotica artigasi Sánchez (MUSM). Paratypes: Same data as Holotype (3 ♂); Huancayo 3217 m. 9.vi.61 col: R. García / Ex Coll. R. García 1977 (1 ♂); Same data as previous, except: Prolepsis sp. Det. A. G. Scarbrough, 1 ♀.
Holotype condition. Good. Detached terminalia (Figs. 9g–h and 10a–g) based on paratype.
Taxonomic discussion. This species resembles Z. clarkei and Z. hockingi sp. nov. in body coloration and the presence of microtrichia on wing. However, Z. clarkei is larger and possess a gonocoxite long projected apically that extends well beyond the apex of hypandrium, while Z. hockingi sp. nov. differs by the color pattern of the mid and hind femora, black anteriorly and reddish posteriorly. Additionally, in Z. artigasi sp. nov. the facial gibbosity is almost entirely pruinose, there are only two apical scutellar macrosetae, yellowish, and the hypandrium is truncate apically.
Zabrotica floresi sp. nov. (Figs. 12 and 13)
urn:lsid:zoobank.org:act:065A3E49-235D-4622-8396-BA001E1649EF
Diagnosis. A black species with wing darkened by microtrichia; facial gibbosity apruinose, shiny black, with mystacal macrosetae almost entirely black (Fig. 12c–d); postpedicel reddish; two apical scutellar macrosetae, yellowish; R4+5 bifurcation slightly posterior to apex of discal cell; abdomen almost entirely covered with brown and golden pruinosity; male terminalia mostly black setose (Fig. 12e–g); gonocoxite not projected apically, barely extending to the apex of hypandrium (Fig. 12e, h).
Description of the male holotype (Fig. 12a–b). Length: body, 12 mm; wing, 10.5 mm.
Head (Fig. 12c–d). Black; scape an pedicel black, the former about 1.3 times as long as the latter, black setose, except dorsally with some yellow setae; postpedicel reddish, 1.2 times as long as scape and pedicel combined and about five times the length of stylus, the latter plus an apical seta-like sensory element; face about a third the width of head at level of antenna, slightly wider bellow, golden pruinose, except on facial gibbosity and partially above it, apruinose; mystachal macrosetae almost entirely black, except very few white macrosetae in the lower third; proboscis black, black setose at ventral base and yellow setose apically; palpus black, black setose; frons, vertex and occiput golden pruinose, except on ocellar tubercle and behind it, apruinose; ocellar tubercle black setose, 6 pairs of long setae, about 1.3 times as long as scape and pedicel combined; about 20 postocular macrosetae, more or less in a double row, most of them black, except the uppermost 2–3, reddish. Thorax. Black, golden pruinose, pruinosity sparse on scutum and pleura (paramedial and lateral scutal stripes apparently undefined). Chaetotaxy: Antepronotum covered with black macrosetae, postpronotum black setose, postpronotal lobe black setose; acrostichal and dorsocentral setae black, dorsocentral macrosetae present on most part of scutum, becoming gradually longer and stouter on posterior two thirds; 3 notopleural reddish macrosetae,1 supra-alar, also reddish, and 3 postalar, yellowish; discal scutellar setae absent, two apical scutellar macrosetae, yellowish; pleura sparsely setose, some black setae on superoposterior angle of anepisternum and some yellow setae on metanepisternum; katatergite with black macrosetae. Wing (Fig. 12b). Veins reddish brown; wing membrane densely covered with brown microtrichia on anterior half and distal fourth; crossvein r-m posterior to middle of discal cell; R4+5 bifurcation slightly posterior to level of apex of discal cell; cell cua narrowly open; halter yellow. Legs. Coxae black, with sparse golden pruinosity; femora black, except distal apex, reddish brown; tibiae and tarsi reddish brown with dark apex; claws black, empodia and pulvilli yellowish. Chaetotaxy: Coxae yellow setose; femora yellow setose, with reddish macrosetae; fore femur with 1 posterodorsal, preapical; mid femur with 2 anterior and 1 posterodorsal, preapical; hind femur with 4 anterior, 1 anteroventral, and 2 posteroventral; tibia with yellow setae and reddish macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 3 long ventral macrosetae; mid tibia with 5 anterodorsal, 5 posterodorsal, 5 posteroventral, 3 ventral, and 2 anteroventral macrosetae; hind tibia with 2 anteroventral, 2 anterodorsal, 3 posterodorsal and 1 posteroventral macrosetae; tarsi with black and reddish macrosetae. Abdomen. Black, more or less laterally compressed up to segment 5 (i.e. in dorsal view the abdomen looks petiolate) (Fig. 12b); pruinosity mostly brown, sparse golden pruinosity also present, abundant on posterior margins of tergites 2–5, forming yellow fringes that stand out strongly from the background; setae yellow, except ventrally from sternite 5, black setose; lateromarginal setae longer on tergites 1–3; tergite 1 with 4 lateromarginal yellow macrosetae. Terminalia (Fig. 12e–h and 13a–g). Black, black setose, except some apical reddish setae on hypandrium and gonocoxite; epandrium gently curved downwards (Fig. 12e, h); hypandrium triangular with acute apex (Fig. 13a–b); gonocoxite subtriangular, not projected apically, barely extending to apex of hypandrium (Figs. 12e, h and 13c); gonostylus with a lobe-shaped process (Fig. 13d); phallus with single prong, curved downwards (Fig. 13e–f), ejaculatory apodeme wide in lateral view, broader and rounded distally; cercus with oblique margins posteriorly (Figs. 12f and 13g).
Female. Unknown.
Etymology. The specific epithet is a masculine noun in the genitive case. This species is named after my beloved grandfather, Justino Flores.
Distribution (Fig. 1b). Peru, department of Huánuco, Pachitea province. The type locality (Fig. 14a), mainly dominated by grasslands and shrublands, is located on the eastern Andean slopes of central Peru. The holotype was collected in June and one specimen was photographed in Bolivia (Sud Yungas, 16.45°S, 67.83° W, observation in iNaturalist) in the month of July (See Fig. 14b and Table 1).
a. Type locality of Zabrotica floresi sp. nov. Panao, Huánuco, Peru. Photograph by Anthony Almeida; b. Z. floresi sp. nov. in the wild, Sud Yungas, La Paz, Bolivia, iNaturalist observation, https://www.inaturalist.org/observations/128538007, accessed 23 Mar 2023, photograph by Beto Espinoza
Type material. 1 ♂. Holotype: PERU: HU [Huánuco] Pachitea, Panao, 16.vi.2019, 10°7′15.80″S, 75°50′6.35″W, 3704 m. Pitfall trap, C. Rossi / HOLOTYPE ♂ Zabrotica floresi Sánchez.
Holotype condition. Detached terminalia stored in glycerin and pinned along with specimen; fore right tarsus missing from second tarsomere.
Taxonomic discussion. This species is quite different from other of Zabrotica due to its reddish brown postpedicel, its facial gibbosity shiny black, the mystacal macrosetae almost entirely black and the scutum only covered with circumambient pruinosity and mostly with reddish macrosetae. Furthermore, the abdomen is almost fully covered with pruinosity but the yellow fringes on the posterior margins of tergites 2–5 still stand out strongly from the background. This species was collected in sympatry with Z. hockingi sp. nov. that is morphologically more similar to Z. clarkei, but smaller.
Zabrotica hockingi sp. nov. (Figs. 15 and 16)
urn:lsid:zoobank.org:act:B6E53F74-1B10-494A-8CB3-B0EDC3A89EF7
Diagnosis. A black species with wing darkened by microtrichia; facial gibbosity apruinose in the upper half (Fig. 15c–d); fore femur almost entirely black, while the mid and hind femora are black anteriorly and reddish posteriorly, a pattern also found in all tibiae and tarsi; abdomen almost entirely apruinose; male terminalia mostly yellow setose; epandrium gently curved downwards (Fig. 15e–f); gonocoxite short projected apically, although it only extends to the apex of the hypandrium (Figs. 15f and 16b).
Description of the male holotype. (Fig. 15a–b). Length: body, 11 mm; wing, 8 mm.
Head (Fig. 15c–d). Black; antenna black, black setose; scape about 1.5 times as long as pedicel; postpedicel subequal to length of scape and pedicel combined and about four times the length of stylus, the latter plus an apical seta-like sensory article; face about a third the width of head at level of antenna, slightly wider bellow, yellowish gray pruinose, except on the upper half of facial gibbosity, apruinose; mystacal macrosetae mostly black, except central ones in the lower two thirds, white; proboscis black, except tip, brown, yellow setose at ventral base and apically; palpus black, yellow setose on the first segment and black setose on the second; frons, vertex and occiput yellowish gray pruinose, except on ocellar tubercle and behind it, apruinose; ocellar tubercle black setose, 5 pairs of long setae, about 1.3 times as long as scape and pedicel combined; about 25 postocular black macrosetae. Thorax. Black, yellowish gray pruinose, except the dark paramedial and lateral scutal stripes. Chaetotaxy: Antepronotum covered with black macrosetae, postpronotum yellow setose, postpronotal lobe mixed black and yellow setose; acrostichal and dorsocentral setae black, dorsocentral macrosetae present on most part of scutum, becoming longer and stouter on posterior three quarters; 2–3 notopleural black macrosetae,1 supra-alar, also black and 3 postalar, 2 yellowish, 1 black; discal scutellar setae absent, four apical scutellar macrosetae, black; pleura sparsely setose, some yellow setae on metanepisternum and katatergite; katatergite with black and yellow macrosetae. Wing (Fig. 15b). Veins brown; wing membrane densely covered with brown microtrichia on anterior margin, distal fourth, and narrowly on posterior margin; crossvein r-m posterior to middle of discal cell; R4+5 bifurcation slightly posterior to apex of discal cell; cell cua narrowly open; halter pale yellow. Legs. Coxae black, yellowish gray pruinose, except posteriorly on fore- and mid femora and anteriorly on hind femur, apruinose; fore femur black, except distal apex and narrow base ventrally, reddish brown, mid and hind femora black anteriorly and reddish brown posteriorly; all tibiae and tarsi also black anteriorly and reddish posteriorly; claws black, empodia and pulvilli yellowish. Chaetotaxy: Coxae white setose; femora white setose, with yellowish macrosetae; fore femur with 1 posterodorsal, preapical; mid femur with 3 anterior and 1 posterodorsal, preapical; hind femur with 4 anterior, 2 anteroventral, and 3 posteroventral; tibia mostly with yellowish setae and macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 2 long ventral macrosetae; mid tibia with 3 anterodorsal, 5 posterodorsal, 3 posteroventral, 3 ventral, and 2 anteroventral macrosetae; hind tibia with 3 anteroventral, 5 anterodorsal, 4 posterodorsal and 1 posteroventral macrosetae; tarsi with black and yellowish macrosetae.
Abdomen. Black, more or less laterally compressed; apruinose, except on posterior margins of tergites 2–4, with abundant yellowish gray pruinosity that forms yellowish fringes that stand out from the background; setae yellow, lateromarginal ones longer on tergites 1–3; tergite 1 with 4 lateromarginal yellow macrosetae. Terminalia (Figs. 15e–h and 16a–f). Reddish brown, except base of epandrium and posterior 4/5 of hypandrium, dark brown; setae mostly yellow, except some black setae posteriorly on hypandrium and ventrally on gonocoxite; epandrium gently curved downwards (Fig. 15e–f); hypandrium triangular with pointed apex (Figs. 15h and 16a); gonocoxite subtriangular, short projected and pointed apically, only extending to apex of hypandrium (Figs. 15f and 16b); gonostylus with a pincer-like process (Fig. 16c); phallus with single prong, curved downwards, ejaculatory apodeme wide in lateral view, broader and rounded distally (Fig. 16d–e); cercus with oblique margins posteriorly (Figs. 15g and 16f).
Female. Unknown.
Variation. The only paratype bears only two apical scutellar macrosetae alongside with one seta.
Etymology. The specific epithet is a masculine noun in the genitive case. This species is named after Pedro Hocking (1938–2022), a passionate naturalist who collected many specimens deposited in the MUSM.
Distribution (Fig. 1b). Peru, departments of Pasco and Huánuco. The type locality, Santa Bárbara, Huancabamba, in Oxapampa, Pasco (Fig. 17) harbors an elfin forest located on the eastern Andean slopes of central Peru. Z. hockingi sp. nov. extends its distribution to the department of Huánuco, in the Pachitea Province, as one specimen was collected inhabiting Andean grasslands, in sympatry with Z. floresi sp. nov. The known specimens were collected in June and July (Table 1).
Type material. 2 ♂. Holotype: PERU: PA [Pasco]. Oxapampa, Huancabamba, [Santa Bárbara] 3433 m, 10°22′21.68"S, 75°39′18.94"W, 02.vii.2019, Yellow pan trap, N. Zenteno leg./ HOLOTYPE ♂ Zabrotica hockingi Sánchez (MUSM). Paratype: PERU: HU [Huánuco], Panao [Pachitea], 16.vi.2019, 10°7′15.80″S, 75°50′6.35″W, 3704 m. Sweeping, C. Rossi (♂)
Holotype condition. Good. Detached terminalia stored in glycerin and pinned along with specimen.
Taxonomic discussion. This species is similar in appearance to Z. clarkei, but smaller. It also resembles Z. artigasi sp. nov., which is similar in size. However, Z. hockingi sp. nov. can be easily distinguished from these two species by the characteristic pattern of legs coloration, which are black anteriorly and red posteriorly, except the fore femur. A similar pattern is found in Z. hulli sp. nov., Z. maidecita sp. nov. and Z. mariae sp. nov. although they have all legs black anteriorly, and red posteriorly. These species are very different from Z. hockingi sp. nov. in coloration (especially in the nearly absence of microtrichia on wing). About this, the singular and contrasting coloration observed in the legs of some Zabrotica species can possibly be explained as a response to the environmental conditions in Andean localities. It is known that color is a major trait to understand how species respond to climate, and that solar radiation and temperature can vary across altitudinal gradients, and dark bodies warm faster than lighter ones (Dufour et al. 2018). Thus, it is possible that this color pattern, where the outer side of the legs is black and the inner is red, is influenced by environmental factors. Furthermore, this could also explain the case of Z. clarkei, where darker phenotypes are usually found at higher elevations, whereas females with reddish legs are more frequent in mid and low elevations. Additional aspects, such as oviposition and camouflage, may also be involved.
Zabrotica hulli sp. nov. (Figs. 18 and 19)
Zabrotica hulli sp. nov. a–f. Male terminalia, based on paratype, after treatment with KOH. a. Hypandrium; b–c. Gonocoxite and gonostylus, lateral and ventral views; d–e. Phallus, lateral and ventral views; f. Cercus and subepandrial sclerite; g–h. Female. g. Habitus, lateral view; h. Terminalia after treatment with KOH and spermathecae
urn:lsid:zoobank.org:act:2BA9B962-1A91-4BE7-9C4F-1EC0EE8CF585
Diagnosis. A dark brown species with microtrichia nearly absent on wing; facial gibbosity almost entirely covered with pruinosity (Fig. 18c–d); usually two apical scutellar macrosetae, black; R4+5 slightly posterior to apex of discal cell; femora black anteriorly and red posteriorly, a pattern that extends to the tibiae and tarsi; mid femur with only one anterior macroseta, preapical; male terminalia white setose; epandrium gently curved downwards (Fig. 18e–f); gonocoxite subtriangular and not projected apically, barely extending to the apex of the hypandrium (Figs. 18e–f and 19b).
Description of the male holotype (Fig. 18a–b). Length: body, 14 mm; wing, 11 mm.
Head (Fig. 18c–d). Dark brown; antenna black, black setose, except some yellow setae dorsally; scape slightly longer than pedicel; postpedicel 1.2 times as long as scape and pedicel combined and about five times the length of stylus, the latter plus an apical seta-like sensory article; face about a fourth the width of head at level of antenna, slightly wider bellow, yellowish gray pruinose; mystacal macrosetae mostly white, except the lateral and the uppermost ones, black; proboscis black, white setose at ventral base and yellow setose apically; palpus black, white setose on the first segment and mainly black setose on the second one; frons, vertex and occiput golden pruinose, except on ocellar tubercle, apruinose; ocellar tubercle black setose, 5 pairs of long setae, about 1.3 times as long as scape and pedicel combined; about 30 postocular yellow macrosetae. Thorax. Dark brown, golden pruinose, except the dark paramedial and lateral scutal stripes. Chaetotaxy: Antepronotum covered with yellow macrosetae, postpronotum yellow setose, as postpronotal lobe, the latter with some macrosetae, shorter than scutal and scutellar ones; acrostichal and dorsocentral setae black, dorsocentral macrosetae present on most part of scutum, as long and stout as the notopleural and the postalar ones; scutal macrosetae mixed black and yellow, 4 notopleural, 3 in a row and 1 in front of these, thinner, 2 supra-alar and 3 postalar; discal scutellar setae absent, 3 apical scutellar macrosetae, black; pleura sparsely setose, some yellow setae on superoposterior angle of anepimeron; katatergite with yellow macrosetae. Wing. Veins brown; wing membrane with microtrichia absent; crossvein r-m posterior to middle of discal cell; R4+5 bifurcation slightly posterior to apex of discal cell; cell cua narrowly open; halter yellow. Legs. Coxae black, golden pruinose, except some apruinose areas; femora, tibiae and tarsi black anteriorly and reddish brown posteriorly, although in the fore femur the black color extends a little posterodorsally; claws and empodia black, pulvilli yellowish. Chaetotaxy: Coxae white setose; femora white setose, with pale yellow macrosetae; fore femur with 1 posterodorsal, preapical; mid femur with 1 anterior and 1 posterodorsal, both preapical; hind femur with 4 anterior, 3–4 anteroventral, and 2–3 posteroventral; tibiae with white setae and pale yellow macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 2 long ventral macrosetae, mid tibia with 4 anterodorsal, 4 posterodorsal, 4 posteroventral, 2 ventral, and 2 anteroventral macrosetae; hind tibia with 3 anteroventral, 4 anterodorsal, 4 posterodorsal and 1–2 posteroventral macrosetae; tarsi with black and pale yellow macrosetae. Abdomen. Dark brown, more or less laterally compressed; covered with golden pruinosity, more abundant on posterior margins of tergites 2–4, forming yellowish fringes that do not stand out from the background; setae white; tergite 1 with 4 lateromarginal pale yellow macrosetae. Terminalia (Figs. 18e–g and 19a–f). Reddish brown, except epandrium and hypandrium, black distally; setae white; epandrium gently curved downwards (Fig. 18e–f); hypandrium triangular with pointed apex (Fig. 19a); gonocoxite subtriangular, not projected apically, barely extending to apex of hypandrium (Figs. 18e–f and 19b); gonostylus with a pincer-like process (Fig. 19c); phallus with single prong, curved downwards, ejaculatory apodeme wide in lateral view, broader and rounded posteriorly (Fig. 19d–e); cercus with curved margins posteriorly (Figs. 18g and 19f).
Female (Fig. 19g–h). Similar to male except terminalia; three sclerotized oval spermathecae, the central one slightly bigger.
Variation. Some specimens with a few black postocular macrosetae, usually the uppermost ones; most paratypes have 2 apical scutellar macrosetae, except one female that has 4, one of them weakened.
Etymology. The specific epithet is a masculine noun in the genitive case. This species is named after Frank M. Hull, well known for his work on the taxonomy of robber flies of the world.
Distribution (Fig. 1b). Peru, department of Pasco, Daniel Alcides Carrión province. The type locality (Fig. 20d), dominated by shrublands and grasslands, is located on the eastern Andean slopes of central Peru. The known specimens were collected in September (Table 1).
Type material. 3 ♂, 5 ♀. Holotype: PERU: PA. Daniel Alcides Carrión, San Pedro de Pillao, Tinka, 10°26′6.85″S, 76°30′56.48″W, 4085 m, 22-23.ix.2021, N. Zenteno/ HOLOTYPE ♂ Zabrotica hulli Sánchez (MUSM). Paratypes: Same label data as holotype (1 ♂, 3 ♀); same label data as holotype, except 10°26′11.53″S, 76°30′51.30″W, 4024 m, 24-25.ix.2021, 1 ♂; same label data as previously, except ix. 2022 T. Neyra, 1 ♀; same label data as holotype, except 10°26′52.68″S, 76°31′37.18″W, 4071 m, ix.2022, T. Neyra, 1 ♀.
Holotype condition. Good. Detached terminalia (Figs. 13f–g and 14a–f) based on paratype.
Taxonomic discussion. This species has a brown coloration and the dense pruinosity on thorax and abdomen give to it a grayish appearance, besides, the microtrichia on wing is nearly absent. In this regard, it resembles Z. maidecita sp. nov. and Z. mariae sp. nov. However, in this species the gonocoxite is not projected apically (it barely extends to the apex of the hypandrium) and the apical scutellar macrosetae are black and usually only one pair is present, while in the other two species the gonocoxite is long projected and the apical scutellar macrosetae are white and usually present in numbers of 5–6. Another useful feature is the presence of a unique anterior macroseta in the mid tibia, although this trait is shared with Z. mariae sp. nov. In other Zabrotica species there are three anterior macrosetae on mid tibiae, except in Z. floresi sp. nov. that has two, but as this latter species is based on a single specimen, this needs to be confirmed with additional material. Lastly, the female of Z. hulli sp. nov. possess the central spermathecae slightly larger than the lateral ones, like in Z. maidecita sp. nov. and some species of Alyssomyia (Artigas 1971; Artigas and Parra 2006).
Zabrotica maidecita sp. nov. (Figs. 21 and 22)
Zabrotica maidecita sp. nov. a–f. Male terminalia, based on paratype, after treatment with KOH. a. Hypandrium; b–c. Gonocoxite and gonostylus, lateral and ventral views; d–e. Phallus, lateral and ventral views; f. Cercus and subepandrial sclerite; g–h. Female. g. Habitus, lateral view; h. Terminalia after treatment with KOH and spermathecae
urn:lsid:zoobank.org:act:09415078-656D-4876-8958-ED0F872D5433
Diagnosis. A brownish species with microtrichia nearly absent on wing; facial gibbosity pruinose, except in the upper fourth (Fig. 21c–d); scape and pedicel reddish brown; usually 5–6 apical scutellar macrosetae, white; R4+5 bifurcation posterior to apex of discal cell; femora black anteriorly and reddish posteriorly, a pattern that extends to the tibiae and the tarsi; pleura silvery pruinose; gonocoxite with apex long projected, blunt and curved backwards, extending well beyond the apex of the hypandrium and almost meeting the opposite one (Figs. 21g–h and 22b).
Description of the male holotype (Fig. 21a–b). Length: body, 13.5 mm; wing, 11 mm.
Head (Fig. 21c–d). Black, except on sides and above facial gibbosity, reddish; scape and pedicel reddish, the former slightly longer than the latter, black setose, except dorsally, mostly with white setae; postpedicel black, about 1.2 times as long as scape and pedicel combined and about five times the length of stylus, the latter plus an apical seta-like sensory article; face about a third the width of head at level of antenna, slightly wider bellow, yellowish gray pruinose except on the upper fourth of the facial gibbosity, sparsely pruinose to apruinose; mystacal macrosetae mostly white, except the lateral and upper ones, black; proboscis black in the basal half and brown distally, white setose at ventral base and pale yellow setose apically; palpus black, white setose, except some black setae apically; frons, vertex and occiput golden pruinose; ocellar tubercle black setose anteriorly and white setose posteriorly, 5 pairs of long setae about 1.3 times as long as scape and pedicel combined; about 30 postocular white macrosetae, a few black above. Thorax. Reddish brown, except some black spots on pleura; silvery pruinose, except the dark paramedial and lateral scutal stripes and golden pruinosity between these on anterior half. Chaetotaxy: Antepronotum covered with pale yellow macrosetae, postpronotum white setose; postpronotal lobe mostly covered with macrosetae, mixed black and yellow, shorter than scutal and scutellar ones; acrostichal and dorsocentral setae mostly black (except the posterior ones, white), dorsocentral macrosetae present on most part of scutum, becoming longer and stouter on posterior two thirds; 4 black notopleural macrosetae (alongside with several long notopleural setae), 3 supra-alar macrosetae, also black, and 5 postalar, white; discal scutellar setae absent, 6 apical scutellar macrosetae, white; pleura with some white setae on anepimeron (posteriorly and on its superoposterior angle), metanepisternum and mixed with the katatergal macrosetae, also white. Wing. Veins brown; wing membrane with microtrichia nearly absent; crossvein r-m slightly posterior to middle of discal cell; R4+5 bifurcation posterior to apex of discal cell; cell cua narrowly open; halter yellow. Legs. Coxae reddish, silvery pruinose, except some apruinose areas; femora, tibiae and tarsi black anteriorly and reddish brown posteriorly; claws black, empodia and pulvilli yellowish. Chaetotaxy: Coxae white setose; femora white setose, with white macrosetae; fore femur with 1 posterodorsal, preapical, 1–2 dorsally; mid femur with 3 anterior and 1 posterodorsal, preapical; hind femur with 4 anterior, 4 anteroventral, and 1 posteroventral; tibiae with white setae and macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 2 long ventral macrosetae, mid tibia with 4 anterodorsal, 4 posterodorsal, 4 posteroventral, 2 ventral, and 1 anteroventral macrosetae; hind tibia with 4 anteroventral, 4 anterodorsal, 1 dorsal, 3 posterodorsal and 3 posteroventral macrosetae; tarsi with black and white macrosetae. Abdomen. Dark brown dorsally, reddish ventrally, more or less laterally compressed; covered with yellowish gray pruinosity, more abundant on posterior margins of tergites 2–5, forming yellowish fringes which do not stand out from the background; setae white, lateromarginal ones longer on tergites 1–3; tergite 1 with 6–7 lateromarginal white macrosetae. Terminalia (Figs. 21e–h and 22a–f). Reddish brown, white setose; epandrium curved downwards (Fig. 21g–h); hypandrium triangular with acute apex, longer than wide (Figs. 21f and 22a); gonocoxite subtriangular with apex blunt, long projected and curved backwards, extending well beyond the apex of hypandrium and almost touching the opposite gonocoxite (Figs. 21g–h and 22b); gonostylus with a lobe-shaped process (Fig. 22c); phallus single pronged, curved downwards, ejaculatory apodeme narrow in lateral view, slightly wide and rounded posteriorly (Fig. 22d–e); cercus with curved margins posteriorly (Fig. 22f).
Female (Fig. 22g–h). Similar to male, except terminalia; three sclerotized oval spermathecae, the central one slightly bigger.
Variation. In some paratypes, a few lateromarginal macrosetae on tergite 1 are weakened; most specimens have 4–6 scutellar macrosetae, although one specimen presents only one pair.
Etymology. The specific epithet is a feminine noun in apposition. This species is named after my beloved grandmother Haydé Roldán, known as “Maidecita” by all her grandchildren.
Distribution (Fig. 1b). Peru, department of Cajamarca, Cajamarca and Hualgayoc provinces. The type locality (Fig. 23), dominated by shrublands and grasslands, is located on the western Andean slopes of northern Peru. The known specimens were collected in July, August and September (Table 1).
Type material. 8 ♂, 5♀. Holotype. PERU: CA [Cajamarca department]. Mina Yanacocha [Cajamarca province], 7°0′22.77″S, 78°33′1.85″W, 3540 m, 19.ix.2020, roquedal, G. Sarabia leg./ HOLOTYPE ♂ Zabrotica maidecita Sánchez (MUSM). Paratypes: PERU: CA. Cajamarca, Cajamarca 6°58′48.95″S, 78°34′7.89″W, 3470 m, 30.viii.2022 Manual T. Neyra leg (4♂, 3 ♀); PERU: CA. Baños del Inca, 6°59′49.49″S, 78°30′22.08″W, 3886 m, 22.ix.2021, F. Molina leg. (♀); PERU: Cajamarca, China Linda [La Encañada], 31.viii.2018 6°54′24.96″S, 78°29′34.37″W 3801 m. Pitfall trap C. Rossi (2♂); PERU: CA. Hualgayoc, Hualgayoc 3790 m 6°45′49.8″S, 78°39′6.5″O 19–21.vii.2019 Pajonal andino Pitfall P. Sánchez (♂); PERU: CA. Hualgayoc, Chugur 06°43′13.98″S, 78°41′02.63″W 3721 m ix. 2022 I. Medina (♀).
Holotype condition. Good. Detached terminalia (Figs. 21h and 22a–f) based on paratype.
Taxonomic discussion. This species resembles Z. hulli sp. nov. and Z. mariae sp. nov. due to its grayish appearance and the absence of microtrichia on wing. However, it can be readily distinguished from the first one due to its long projected gonocoxite that extends well beyond the apex of the hypandrium, a feature shared with Z. clarkei and Z. mariae sp. nov. Although, Z. clarkei is quite different in body coloration and the presence of microtrichia on wing, while from Z. mariae sp. nov. it can be differentiated due to its silver pruinosity and differences in the gonocoxite and the hypandrium; in Z. maidecita sp. nov. the gonocoxite is blunt apically and the hypandrium is longer than wide, while in Z. mariae sp. nov. the gonocoxite is pointed apically and the hypandrium is as long as wide. Furthermore, this species is only known from northern Peru, while Z. mariae sp. nov. inhabits localities of southern Peru.
Zabrotica mariae sp. nov. (Figs. 24 and 25)
Zabrotica mariae sp. nov. a–f. Male terminalia, based on paratype, after treatment with KOH. a. Hypandrium; b–c. Gonocoxite and gonostylus, lateral and ventral views; d–e. Phallus, lateral and ventral views; f. Cercus and subepandrial sclerite; g–h. Female. g. Habitus, lateral view; h. Terminalia after treatment with KOH and spermatheca
urn:lsid:zoobank.org:act:09415078-656D-4876-8958-ED0F872D5433
Diagnosis. A brownish species with microtrichia nearly absent on wing; facial gibbosity almost entirely pruinose (Fig. 24c–d); 5–6 notopleural macrosetae, mostly white; usually 5–6 apical scutellar macrosetae, predominantly white; R4+5 bifurcation slightly anterior to apex of discal cell; femora black anteriorly and reddish posteriorly (although sometimes the reddish coloration is only present posteroventrally); mid femur with only one anterior macroseta, preapical; gonocoxite with apex long projected, pointed and curved backwards, extending well beyond the apex of the hypandrium and almost meeting the opposite one (Figs. 24e, h and 25b).
Description of the male holotype (Fig. 24a–b). Length: body, 11 mm; wing, 9.5 mm.
Head (Fig. 24c–d). Black, except on sides and above facial gibbosity, reddish; antenna black, black setose ventrally and white setose dorsally, scape about 1.2 times as long as pedicel; postpedicel slightly longer than scape and pedicel combined and about five times the length of stylus, the latter plus an apical seta-like sensory article; face about a third the width of head at level of antenna, slightly wider below, yellowish gray pruinose; mystacal macrosetae mostly white, except the lateral and uppermost ones, black; proboscis black, white setose at ventral base and pale yellow setose apically; palpus black, white setose, except some black setae apically; frons, vertex and occiput yellowish gray pruinose, the ocellar tubercle sparsely pruinose; the latter mixed black and yellow setose, 4 pairs of long setae about 1.3 times as long as scape and pedicel combined; about 30 postocular white macrosetae. Thorax. Mixed reddish brown and black; gray pruinose, except the dark paramedial and lateral scutal stripes and golden pruinosity between these on the anterior half. Chaetotaxy: Antepronotum covered with pale yellow macrosetae, postpronotum pale yellow setose; postpronotal lobe mostly covered with yellow macrosetae, subequal to scutal and scutellar ones; acrostichal and dorsocentral setae mainly black, present and well developed on most part of scutum; 5–6 yellowish notopleural macrosetae (one mixed black), 2–3 supra-alar, and 4 postalar, also yellowish; discal scutellar setae absent, 4 apical scutellar macrosetae, white; pleura with some white setae on anepimeron, posteriorly and on its superoposterior angle, metanepisternum and mixed with the katatergal macrosetae, also white. Wing (Fig. 24b). Veins brown; wing membrane with microtrichia nearly absent; crossvein r-m slightly posterior to middle of discal cell; R4+5 bifurcation slightly anterior to apex of discal cell; cell cua narrowly open; halter yellow. Legs. Coxae mostly reddish brown, some black in fore and mid coxa, gray pruinose; femora black anteriorly and reddish brown posteriorly, although the black extends partially posterodorsally; tibiae and tarsi brown, the former lighter basally; claws black, empodia and pulvilli yellowish. Chaetotaxy: Coxae white setose; femora white setose, with white macrosetae; fore femur with 1 posterodorsal, preapical, 1–2 dorsally; mid femur with 1 anterior and 1 posterodorsal, both preapical; hind femur with 4 anterior, 1 anteroventral, and 2 posteroventral; tibia with white setae and macrosetae, fore tibia with vertical rows of 4–5 anterodorsal, posterodorsal and posteroventral macrosetae, besides 2 long ventral macrosetae, mid tibia with 4 anterodorsal, 4 posterodorsal, 4 posteroventral, 2 ventral, and 1 anteroventral macrosetae; hind tibia with 3 anteroventral, 4 anterodorsal, 5 posterodorsal and 2 posteroventral macrosetae; tarsi mostly with white macrosetae. Abdomen. Dark brown, more or less laterally compressed; covered with yellowish gray pruinosity, more abundant on posterior margins of tergites 2–5, forming yellowish fringes that do not stand out from the background; setae white, lateromarginal ones longer on sides of tergites 1–3; tergite 1 with 4 pale yellow lateromarginal macrosetae. Terminalia (Figs. 24e–h and 25a–f). Brown, white setose; epandrium gently curved downwards (Fig. 17e, h); hypandrium triangular, as long as wide (Figs. 24f and 25a); gonocoxite subtriangular with apex pointed, long projected and curved backwards, extending well beyond level of apex of hypandrium and close to the opposite one (Figs. 24e, h and 25b); gonostylus with a lobe-shaped process (Fig. 25c); phallus with single prong, curved downwards, ejaculatory apodeme slightly wide in lateral view and rounded distally (Fig. 25d–e); cercus with curved margins posteriorly (Fig. 25f).
Female (Fig. 25g–h). Similar to male, except terminalia; three sclerotized oval spermathecae of similar size.
Variation. The number of apical scutellar macrosetae varies from 4 to 6 and in some specimens the red coloration of femora is only present posteroventrally.
Etymology. The specific epithet is a feminine noun in the genitive case. This species is named after my beloved grandmother María Pérez.
Distribution (Fig. 1b). Peru, departments of Apurímac (Cotabambas province), Cuzco (Chumbivilcas and Espinar provinces) and Puno (Azángaro province). The type locality (Fig. 26), mainly dominated by grasslands, is located on the eastern Andean slopes of southern Peru. The known specimens were collected in July, August, September and October (Table 1).
Type material. 4 ♂, 8♀. Holotype. PERU: AP [Apurímac]. Cotabambas, [Tambobamba] Chalhuahuacho, CC [Comunidad Campesina]. Chicñahui, 14°7′9.03″S, 72°20′41.11″W, 4194 m, 20.viii..2021, A. Eime / HOLOTYPE ♂ Zabrotica mariae Sánchez (MUSM). Paratypes: PERU: AP. [Cotabambas] Tambobamba, Chalhuahuacho, 14°4′40.2″S, 72°15′43.6″O 4204 m 27–29.vii.2019 L. Pérez (♂); PERU: AP. Cotabambas, Tambobamba, CC. Choquecca, 14°4′4.35″S, 72°16′9.34″W 4083 m 9.ix.2021. M. Rodríguez (♀); PERU: CU [Cuzco]. Espinar 4006 m 14°54′35.6″S, 71°22′53.4″O 24.ix.2017 M. Cárdenas (2 ♀); PERU: CU. Chumbivilcas 4184 m 14°31′48.1″S, 71°47′0.98″O 03.ix.2017 M. Cárdenas(4 ♀); PERU: CU. Espinar, Espinar, 14°59′25.2″S, 71°16′26.03″O, 4139 m. 5.x.2019 I. Medina (♂); PERU: CU. Espinar, Qbra [Quebrada]. Chaisamayo 14°59′46.15″S, 71°16′25.93″W, 4167 m. x.2013 Pastizal. I. Medina (♂); PERU: PU [PUNO]. Azángaro, San Antón 14°34′31.40″S, 70°19′1.27″W, 4062 m 20.iix.2021 L. Villena (♀).
Holotype condition. Good. Detached terminalia (Figs. 17h and 18a–f) based on paratype.
Taxonomic discussion. As previously explained, this species is similar in appearance to Z. hulli sp. nov. and Z. maidecita sp. nov. but the first species presents a short gonocoxite, not extending beyond the apex of the hypandrium, while Z. mariae sp. nov. has the gonocoxite long projected apically, like in Z. clarkei and Z. maidecita sp. nov. However, while Z. clarkei can be readily differenced because of its black coloration and the presence of microtrichia on wing, Z. maidecita sp. nov. presents a gonocoxite with the apex blunt and hypandrium longer than wide, while in Z. mariae sp. nov. the gonocoxite has pointed apex and the hypandrium is as long as wide. Additionally, this species stands out from others because of the number of notopleural macrosetae that are usually 5–6 (in other species there are normally three and exceptionally four alongside with short or long setae) and the macrosetae on postpronotal lobe, subequal in length to the scutal and scutellar ones. Z. hulli sp. nov. and Z. maidecita sp. nov. present postpronotal macrosetae too, but these are comparatively shorter than scutal and scutellar ones.
Key to the species of Zabrotica (including known females)
-
1. Abdomen sparsely pruinose to apruinose … 2
-
- Abdomen almost entirely covered with pruinosity … 4
-
2. Facial gibbosity apruinose, shining black (Figs. 3c and 5g); male terminalia mostly black setose (Fig. 3g); epandrium markedly curved downwards; gonocoxite long projected apically and markedly curved backwards, extending beyond apex of hypandrium and usually touching or surpassing the apex of the opposite gonocoxite (Figs. 3g and 4a–c) (females frequently with reddish or partially reddish femora) … clarkei Hull (Peru and Chile).
-
- Facial gibbosity pruinose at least on lower half; male terminalia mostly yellow setose (Figs. 9f and 15e); epandrium only gently curved downwards; gonocoxite straight and at most shortly projected apically, only extending to apex of hypandrium (Figs. 9g and 15f) … 3
-
3. Facial gibbosity almost entirely pruinose (Fig. 9d–e); apical scutellar macrosetae yellowish; all femora black, tibiae uniform in color; proximal half of abdomen sparsely pruinose to apruinose dorsally (Fig. 9a); hypandrium with truncate apex (Fig. 10a–b) … artigasi sp. nov. (Central Peru).
-
- Facial gibbosity apruinose in the upper half (Fig. 15c–d); apical scutellar macrosetae black; fore femur almost entirely black, the mid and hind femora black anteriorly and reddish posteriorly, a pattern that extends to the tibiae and tarsi, including fore legs (Fig. 15a); abdomen almost entirely apruinose (Fig. 15a–b); hypandrium with acute apex (Fig. 16a) … hockingi sp. nov. (Central Peru)
-
4. Facial gibbosity apruinose, shining black, mystacal macrosetae almost entirely black, very few white (Fig. 12c–d); postpronotal lobe without macrosetae; wing membrane densely covered with microtrichia on anterior half (Fig. 12b); male terminalia black setose (Fig. 12e) … floresi sp. nov. (Central Peru to Bolivia [?]).
-
- Facial gibbosity pruinose, at most apruinose on the upper fourth, mystacal macrosetae mixed black and white (Figs. 18c, 21c, and 24c); postpronotal lobe with macrosetae; microtrichia on wing membrane nearly absent (Figs. 18b, 21b, and 24b); male terminalia white setose (Figs. 18e, 21e, and 24e) … 5
-
5. Thorax dark brown; usually two black apical scutellar macrosetae; gonocoxite not projected apically, straight and barely extending to the apex of hypandrium (Fig. 18e–f) … hulli sp. nov. (Central Peru).
-
- Thorax reddish or partially reddish, usually 4–6 white apical scutellar macrosetae; gonocoxite long projected apically, curved backwards and extending well beyond the apex of the hypandrium (Figs. 21h and 24h) … 6
-
6. Scape and pedicel reddish (Fig. 21c); thorax mostly silvery pruinose, especially on pleura; 3–4 notopleural macrosetae; abdomen black dorsally and reddish ventrally; epandrium strongly curved downwards (Fig. 21g–h); gonocoxite blunt apically (Fig. 22b); hypandrium longer than wide (Fig. 22a) … maidecita sp. nov. (Northern Peru).
-
- Antenna black (Fig. 24c); thorax yellowish gray pruinose; usually 5–6 notopleural macosetae; abdomen dorsally and ventrally concolorous, black; epandrium only gently curved downwards (Fig. 24e, h); gonocoxite apically pointed (Fig. 25b); hypandrium as long as wide (Fig. 25a) … mariae sp. nov. (Southern Peru).
Discussion
This work increases to seven the number of known species of Zabrotica, previously monotypic, and demonstrate the need for further research on the taxonomy of this family in the Neotropical region for a better understanding of its diversity in order to avoid misidentifications, something frequent in faunistic studies, as recognized by Vieira et al. (2019). Additionally, it is worth mentioning that most of the specimens of the new species described were deposited in the MUSM as part of heritage studies performed as a requirement for economic development projects. These projects are usually located in Andean localities poorly or never sampled for insects, and normally include two yearly excursions during brief periods, in the wet and dry seasons, using methods like Moericke and pitfall traps, and opportunistic sampling with entomological net, but not specifically addressed for catching Asilidae or other dipteran taxa. Consequently, it also gives an insight into how understudied the Neotropical Asilidae fauna is, and how diverse this group can be in the Andean region, known to be a center of endemism and speciation (Larsen et al. 2011).
In the most recent classification of Asilidae, based on a phylogenetic analysis using morphological evidence (Dikow 2009a), Stenopogoninae was recovered as non-monophyletic and Tillobromatini (tribe within Stenopogoninae) was elevated to subfamily status. However, Tillobromatinae, and many other subfamilies in this classification, cannot be readily identified using external morphology and, as a result, genera not evaluated by Dikow are currently unplaced at the subfamily level. Subsequent phylogenetic analyses using molecular evidence (Dikow 2009b; Cohen et al. 2021) recovered Stenopogoninae and Tillobromatinae as non-monophyletic. Although, genera assigned to Tillobromatinae (e.g., Tillobroma Hull, 1962), Stenopogoninae (e.g., Microstylum) and Brachyrhopalinae (Agrostomyia Londt, 1994) have being recovered by Cohen et al. (2021) together in a clade, suggesting that Tillobromatinae could be expanded to encompass the entire group, but only after a reevaluation of Stenopogoninae and Brachyrhopalinae. Therefore, Zabrotica, might be corroborated as belonging to this expanded concept of Tillobromatinae, because Artigas and Papavero (1991a, b, c) included it in Tillobromatini as well as in Enigmomorphini (as Aymarasilus) together to other genera morphologically similar, such as Alyssomyia, Araujoa, Creolestes and Prolepsis, this latter recovered as sister of Tillobroma by Cohen et al. (2021).
About this, Artigas and Papavero (1991a, c) allocated in Tillobromatini genera with cell m3 open (or closed at wing margin) and stylus absent or composed of a single article fused to the postpedicel. So, although Zabrotica presents cell m3 closed and petiolate, it was considered as belonging to Tillobromatini due to its “second flagellomere (stylus) broad and thick, fused or absent and vertex unescavated”. This is ambiguous and confusing, as in Zabrotica the stylus is present and not fused to postpedicel, hence, the use of the key provided by these authors results in its identification as Aymarasilus. Therefore, in this work this is revised thanks to the access to insect collections through visitation or the digitization of their specimens, a promising resource that will certainly help to reveal more possible cases of synonymy for a better understanding of the taxonomy of robber flies.
Data Availability
Open access to data of specimens deposited in CNC and NMNH are available at https://www.cnc.agr.gc.ca/taxonomy/TaxonSearch.php and https://collections.nmnh.si.edu/search/ento/respectively.
References
Amorim DS (2009) Neotropical diptera diversity: richness, patterns, and perspectives. In: Bickel D, Pape T, Meier R (eds) Diptera diversity: status, challenges and tools. Koninklijke Brill NV, Leiden, pp 71–97. https://doi.org/10.1163/ej.9789004148970.I-459.17
Artigas JN (1970) Los Asílidos de Chile:(Diptera: Asilidae). Gayana Zool 17:1–472
Artigas JN (1971) Las estructuras quitinizadas de la spermatheca y funda del pene de los Asílidos y su valor sistemático a través del estudio por taxonomía numérica. Gayana Zool 18:1–106
Artigas JN (1974) Aymarasilus inti n. sp., nuevo género y especie de asílido de Chile (Diptera – Asilidae). Bol Soc Biol Concepción 47:227–232
Artigas JN, Papavero N (1988) The American genera of Asilidae (Diptera): Keys for identification with an atlas of female spermathecae and other morphological details. I. Key to the subfamilies and subfamily Leptogastrinae. Gayana Zool 52(1–2):95–114
Artigas JN, Papavero N (1991) The American Genera of Asilidae (Diptera): Keys for identification with an atlas of female spermathecae and other morphological details. VII.1. Subfamily Stenopogoninae Hull. A preliminary classification into tribes. Gayana Zool 55(2):139–44
Artigas JN, Papavero N (1991b) The American Genera of Asilidae (Diptera): Keys for identification with an atlas of female spermathecae and other morphological details. VII.4. Subfamily Stenopogoninae Hull – Tribe Enigmomorphini, with descriptions of three new genera and species and a catalogue of the Neotropical species. Bol Soc Biol Concepción 62:27–53
Artigas JN, Papavero N (1991c) The American Genera of Asilidae (Diptera): Keys for identification with an atlas of female spermathecae and other morphological details. VII.5. Subfamily Stenopogoninae Hull – Tribe Tillobromini, with descriptions of three new genera and two new species and a catalogue of the Neotropical species. Rev Chilena Ent 19:17–27
Artigas JN, Parra LE (2006) Phylogeny and review of the genus Alyssomyia Hull, with descriptions of two new species (Diptera, Asilidae, Stenopogoninae, Enigmomorphini). Stud Neotrop Fauna Environ 41(2):97–116. https://doi.org/10.1080/01650520500250301
Artigas JN, Vieira R (2014) New genus and species of Neotropical robber flies (Diptera, Asilidae, Asilinae). Zootaxa 3774:282–288. https://doi.org/10.11646/zootaxa.3774.3.5
Carayon J (1969) Emploi du noir chlorazol en anatomie microscopique des insectes. Ann Soc entomol Fr 5(1):179–193. https://doi.org/10.1080/21686351.1969.12278919
Carrera M, Papavero N (1965) Sôbre os gêneros Pritchardia Stuardo, Alyssomyia Hull e Hypenetes Loew (Diptera, Asilidae). Pap Avulsos Zool 18(6):47–55
Castañeda E, Gonzales P (2022) One hundred and two conspicuous birds across an elevation gradient in the western Andes of Central Peru. Arnaldoa 29(2):291–318. https://doi.org/10.22497/arnaldoa.292.29107
Cohen CM, Noble K, Cole TJ, Brewer MS (2021) The phylogeny of robber flies (Asilidae) inferred from ultraconserved elements. Syst Entomol 46(4):812–826. https://doi.org/10.1111/syen.12490
Cumming JM, Wood DM (2017) Adult morphology and terminology. In: Kirk-Spriggs AH, Sinclair BJ (eds) Manual of afrotropical diptera, vol 1. Suricata 4. South African national biodiversity institute, Pretoria, pp 89–133
Dikow T (2009a) Phylogeny of Asilidae inferred from morphological characters of imagines (Insecta: Diptera: Brachycera: Asiloidea). Bull Am Mus Nat Hist 319:1–175. https://doi.org/10.1206/603.1
Dikow T (2009b) A phylogenetic hypothesis for Asilidae based on a total evidence analysis of morphological and DNA sequence data (Insecta: Diptera: Brachycera: Asiloidea). Org Divers Evol 9(3):165–188. https://doi.org/10.1016/j.ode.2009.02.004
Dikow T (2021) Asiloid Flies. Deciphering their diversity and evolutionary history. https://asiloidflies.si.edu/. Accessed 30 Nov 2021
Dufour PC, Willmott KR, Padrón PS, Xing S, Bonebrake TC, Scheffers BR (2018) Divergent melanism strategies in Andean butterfly communities structure diversity patterns and climate responses. J Biogeogr 45(11):2471–2482. https://doi.org/10.1111/jbi.13433
Fisher EM (2009) Asilidae (Robber flies, Assassin flies, Moscas cazadoras, Moscas ladronas). In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA (eds) Manual of central American Diptera, vol 1. National Research Council Press, Ottawa, pp 585–632
Gonzales FN, Craven D, Armesto JJ (2023) Islands in the mist: A systematic review of the coastal lomas of South America. J Arid Environ 211:1–16. https://doi.org/10.1016/j.jaridenv.2023.104942
Hadley A (2012) Combine ZP, image stacking software. https://combinezp.software.informer.com/download/. Accessed 1 Aug 2022
Hull FM (1958) Some species and genera of the family Asilidae. Proc Entomol Soc Wash 60(6):251–257
Hull FM (1962) Robber flies of the world: The genera of the Family Asilidae. Bull US Natl Mus 224(1–2):907. https://doi.org/10.5479/si.03629236.224
Lamas G (1972) A catalogue of Peruvian Asilidae (Diptera), with keys to identification and descriptions of two new species. Rev Peru Entomol 15:304–316
Larsen TH, Escobar F, Armbrecht I (2011) Insects of the tropical Andes: diversity patterns, processes and global change. In: Herzog SK, Martínez R, Jørgensen PM, Tiessen H (eds) Climate change and biodiversity in the tropical Andes. Inter–American Institute of Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), pp. 228–244. Available from: https://www.researchgate.net/profile/Gunnar-Brehm/publication/230818242_Phenology_and_interspecific_Ecological_Interactions_of_Andean_Biota_in_the_Face_of_Climate_Change/links/0912f504ef60c7cc53000000/Phenology-and-interspecific-Ecological-Interactions-of-Andean-Biota-in-the-Face-of-Climate-Change.pd♀page=241. Accessed 15 June 2023
Loew H (1858) Bidrag till kännedomen om Afrikas Diptera [part]. Öfversigt af Kongliga Svenska Vetenskaps-akademiens Forhandlingar Stockholm. 14: 337–383
Londt JG (1994) Afrotropical Asilidae (Diptera) 25. A key to the genera of the subfamily Stenopogoninae with new synonymy and descriptions of six new genera. Ann Natal Mus 35(1):71–96
Lynch Arribálzaga E (1881) Asilides argentinos [cont.]. An Soc Cient Argent, 11:17–32, 112–128 [cont.]
Macquart J (1838) Diptères exotiques nouveaux ou peu connus. Mem Soc Sci Agric Lille 2(1):9‒225, 25 pls. https://doi.org/10.5962/bhl.title.15792
Papavero N (2009) Catalogue of neotropical diptera. Asilidae. Neotropical Diptera 17:1–178
Philippi RA (1865) Aufzählung der chilenisschen Dipteren. Verh Zool-Bot Ges Wien 15:595–782
Shorthouse DP (2010) SimpleMappr, an online tool to produce publication-quality point maps. http://www.simplemappr.net. Accessed 10 March 2023
Stuardo Ortiz C (1946) Catálogo de los dípteros de Chile. Ministerio de Agricultura, Impresiones Universitaria, Santiago, 250 p
Vieira R (2012) Ctenodontina Enderlein, 1914 (Diptera, Asilidae, Asilinae): First record for Brazil and description of a new species. ISRN Zool 2012:1–6. https://doi.org/10.5402/2012/517410
Vieira R, Ayala-Landa JM (2014) Aristofolia Ayala-Landa, a valid genus of Asilinae (Diptera, Asilidae). Rev Bras Entomol 58(1):29–31. https://doi.org/10.1590/S0085-56262014000100006
Vieira R, Rafael JA (2014) Longivena, a new robber-fly genus from Brazil (Diptera, Asilidae, Asilinae). ZooKeys 443:119–138. https://doi.org/10.3897/zookeys.443.8324
Vieira R, Camargo A, Pollet M, Dikow T (2019) Updated checklist of French Guianan Asilidae (Diptera) with a focus on the Mitaraka expedition. Zoosystema: 41(23):443–464. https://doi.org/10.5252/zoosystema2019v41a23. http://zoosystema.com/41/23. Accessed 24 Oct 2021
Walker F (1851) Insecta Saundersiana: or characters of undescribed insects in the collection of William Wilson Saunders. Esq. Diptera. Part II. John Van Voorst, London, pp 77–156
Acknowledgements
Thanks to the curators and technical managers of collections for providing access to the material examined and supplying images of specimens of Zabrotica deposited in their institutions: Jeff Skevington, Bradley Sinclair and Michelle Locke in CNC; and Marina Fuentes and Myriam Ramírez in MZUC-UCC. Also, thanks to colleagues that allowed the use of their images of Zabrotica or localities where specimens were collected, and finally, to the reviewers whose suggestions enhanced the quality of this manuscript.
Funding
This work was partially supported by the S. W. Williston Diptera Research of the NMNH.
Author information
Authors and Affiliations
Contributions
The author states sole responsibility for the study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Additional information
Edited by Patrícia J Thyssen
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sánchez, P. Taxonomic Review of Zabrotica Hull 1958 (Diptera: Asilidae: Stenopogoninae), a Senior Synonym of Aymarasilus Artigas 1974, with Descriptions of Six New Species. Neotrop Entomol 53, 110–139 (2024). https://doi.org/10.1007/s13744-023-01096-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-023-01096-4