Abstract
In this paper, a novel pipette tip micro-solid phase extraction based on molecularly imprinted polymer as a selective sorbent was developed and applied for extraction, pre-concentration and high-performance liquid chromatographic determination of trace amounts of malachite green (MG), rhodamine B (RB), methyl orange (MO) and acid red 18 (AR) dyes in seawater samples. Different parameters affecting the extraction efficiency such as type and volume of eluent solvent, sample volume, number of cycles of extraction and desorption, amount of sorbent and pH of the sample solution were evaluated using one-variable-at-a-time and response surface methodology. In order to optimize dyes extraction, seven factors in three levels were used for Box–Behnken experimental design. Under optimum extraction condition, pH of sample solution was 3.1 for MG, 3.0 for RB, 7.1 for MO and 6.1 for AR; volume of eluent solvent (HCl, 0.5 mol L−1) was 200 µL; volume of the sample solution was 10 mL (for MG) and 4 mL (for RB, MO and AR); the concentration of triton X-114 was 0.085 (MG), 0.10 (RB), 0.08 (MO) and 0.075 (AR) % v/v; the number of extraction cycles was 10 (MG), 6 (RB), 5 (MO) and 7 (AR); the number of elution cycles was 10, 6, 5 and 9, respectively, for MG, RB, MO and AR; NaCl concentration was 0.4 mol L−1; and amount of MIP was 2.0 mg for all dyes. The linear range of calibration curves was 0.5–250.0 µg L−1 for malachite green and methyl orange and 0.5–150.0 µg L−1 for both rhodamine B and acid red 18. The detection limits calculated to be 0.083, 0.10, 0.12 and 0.17 µg L−1 for MG, RB, MO and AR, respectively. The developed protocol was successfully used for determination of dyes in seawater of Chabahar Bay. The mean recoveries were ranged between 76.1 and 97.3% by mean relative standard deviations of 1.2–7.1%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, synthetic dyes are extensively applied in many fields of up-to-date technology, e.g., paper, leather, textile, food, cosmetic and pharmaceutical industries. Large quantities of dyes are continuously entering the environment from these industries [1, 2].
Some of these dyes are quite harmful and poisonous, and even those that are not toxic, like acid red 18, can be hazardous if applied in excess [1, 3]. So, the importance of monitoring of environmental samples for trace determination of these analytes [4,5,6] is obvious.
Malachite green (MG, Fig. 1a) a cationic triphenylmethane dye that is soluble in water has found extensive utilization all over the world as fungicide, parasiticide and antiseptic in the aquaculture. This compound may cause human carcinogenesis and mutagenesis [2]. Rhodamine B (RB, Fig. 1b), soluble in water, methanol and ethanol, is among the oldest and most commonly applied synthetic dyes used as, fluorophore, laser dyes and fluorescent analytical reagents. This compound is also applied widely as a colorant in textile and plastic industries, in foodstuffs as a tracer dye and within water to determine the rate and direction of its flow and transport. RB is dangerous for humans and animals [2, 7]. Due to their low cost and high effectiveness, these dangerous and toxic compounds are widely utilized all over the world. Acid red 18 (AR, Fig. 1c) is a popular azo dye which has uses in food coloring [8]. Methyl orange (MO, Fig. 1d) has been used as textile dyeing stuff and staining agents at laboratories. AR and MO as dyes are of the most abundantly applied dyeing gents all over the world and so can find their way to environmental samples as hazardous pollutants. Various methods such as liquid chromatography–mass spectrometry (LC–MS) [9, 10], liquid chromatography–tandem mass spectrometry (LC–MS/MS) [11] gas chromatography–mass spectrometry (GC–MS) [12], capillary electrophoresis [12], high-performance liquid chromatography (HPLC) [12,13,14,15], high-performance liquid chromatography–mass spectrometry (HPLC–MS) [16] and spectrophotometry [1, 2] have been proposed for determination of trace amounts of dyes in different samples. However, due to their low concentration in the sample or the severe matrix interferes in real samples such as seawater, the direct determination of dyes is challenging. Applying a sample preparation/extraction step before chromatographic analysis can overcome these disadvantages, because it can pre-concentrate the target compounds and also eliminate the interfering elements at the same time [2, 17, 18]. For this purpose, techniques such solvent extraction [19], solid-phase extraction (SPE) [20, 21], molecularly imprinted polymer [12, 15], liquid–liquid extraction (LLE) [22, 23], dispersive liquid–liquid microextraction (DLLME) [24,25,26] and cloud point extraction (CPE) [27, 28] have been developed.
Molecularly imprinted polymers (MIPs) have been exploited in a number of applications such as their use as separation materials for solid-phase extraction of organic compounds in complex matrices and as recognition elements in biosensors for assay of different analytes due to its stability, ease of preparation and low cost [29,30,31]. High selectivity is obtained by employing them for the extraction and pre-concentration of various analytes from complex matrices [30, 32, 33]. In molecular imprinting, cross-linked polymers are formed by free-radical copolymerization of functional monomers by an excess of cross-linker around a molecule that acts as a target analyte (template). After desorption of template analyte, the polymer can be applied as a selective binding medium for the target compound (template) or structurally related analytes [34,35,36]. The mechanisms with which these polymers specifically bind the print analyte, and related ligands are attributed to the formation of functional groups in a specific arrangement within the polymer that corresponds to the target molecule and to the presence of shape-selective cavities. Due to simplicity, convenience, significant reduction consumption of sorbent, reagent, time, organic solvent and increasing sample throughout, recent trends in SPE methods focus on miniaturization of techniques and devices. As one of the most promising methods, recently pipette tip micro-solid phase extraction (PT-SPE) has been used for pre-concentration of compound from complex samples which is a miniaturized version of conventional SPE. The target compounds are micro-extracted using repeated extraction and desorption of the sample solution. The method has unique advantages including low cost, limited consumption of organic solvent, faster integrate extraction and more simplicity than conventional SPE cartridges. What is needed for doing PT-SPE is simply a pipettor and pipette tips by small inner diameter that requires tiny amount of adsorbent [37, 38].
Application of multivariate experimental design technique is becoming increasingly widespread in analytical chemistry. Multivariate design that permits the simultaneous optimization of several control variables are quicker to use and more cost-effective than traditional unvaried approaches. The Box–Behnken design is a second-order multivariate method based on three-level incomplete factorial design which received widespread application for evaluation of critical experimental conditions, which is, the minimum of maximum of response functions. The number of runs (N) needed for the development of this technique is defined as N = 56 + nc, where (k) is the factor number and nc is the replicate number of the central point [39, 40].
The aim of this paper was to develop a simple, fast, sensitive and highly selective molecularly imprinted polymer pipette tip micro-solid phase extraction (MIP-PT-µSPE) for pre-concentration of malachite green, rhodamine B, methyl orange and acid red 18 dyes from seawater samples followed by their HPLC analysis. The method was optimized using one-variable-at-a-time, response surface methodology and Box–Behnken design. MIP was prepared using a combination of methacrylic acid as functional monomer, ethylene glycol dimethacrylate as cross-linker and 2,2ʹ-azoisobutyronitrile as initiator.
Experimental
Chemicals and materials
Methacrylic acid (MAA), ethylene glycol dimethacrylate (EDMA) and 2,2ʹ-azoisobutyronitrile (AIBN) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Malachite green (MG), rhodamine B (RB), methyl orange (MO), acid red (AR) and all salts, acids and organic solvents were of analytical grade and were obtained from Merck KGaA (Germany) and used as received. The HPLC grade methanol, acetonitrile and water were also purchased from the same company. Milli-Q® water (18.3 MʹΩ/cm) was utilized throughout the experiment after filtering through 0.22 mm nylon membrane. Figure 1 indicates the structurally related dyes used in this work. Triton X-114 (5% v/v) solutions were prepared at 70:30 (V/V) water/methanol. Stock solutions of each dye containing 500 mg L−1 dye were prepared using dissolving of 0.050 g of dyes in Milli-Q®water in 100-mL flasks and diluting to the mark, individually. Working solutions were prepared daily with suitable dilution of this solution.
Preparation of MIP and NIP
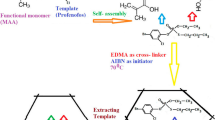
MIP was prepared individually with bulk polymerization technique with mixing 0.5 mmol of each dye as template, 2 mmol of MAA as functional monomer, 20 mmol of EGDMA as cross-linker and 60 mg of AIBN as initiator dissolved in 5 mL methanol as porogen. After that, the mixture deoxygenized using nitrogen stream for 10 min and polymerization was carried out in a thermostated water bath at 65 °C for 6 h. After polymerization, the polymers were ground in a mortar, dried and sieved to get particles by diameters in the range of 55–75 µm, with suspending polymer material in methanol and decantation of methanol for three times, fine particles were removed. For removal of the templates, the product was treated with 0.5 mol L−1 HCl until no dyes was detected by HPLC. Finally, the polymer was washed by methanol for two times and dried under vacuum at room temperature. Figure 2 indicates the preparation scheme of MIP for each dye. The non-imprinted polymer (NIP) was prepared using the same protocol without template molecule.
Assembly of pipette tip
Pipette tip is suitable to be used as a µSPE cartridge because of its special conical shape using various diameters in two ends. In this research, a bigger pipette tip was inserted into a small one to set up a new cartridge. Two dried pipette tips (20 and 100 µL, polypropylene) were used for construction of the pipette tip cartridge. A mass of 2 mg polymer was packed into the 20 µL pipette tip as the sorbent, and then the tip of the larger pipette was cut and connected by the packed tip and a glass syringe was connected to the top of the pipette to suck the sample solution into pipette tip. Before use, MIP-PT-µSPE cartridge was washed successively by methanol (0.5 mL) and Milli-Q® water (0.5 mL) several times.
MIP-PT-µSPE procedure
10 mL (for MG) and 4 mL (for RB, MO and AR) of an aliquot of the sample solution containing appropriate amounts of four dyes (malachite green, rhodamine B, methyl orange and acid red 18) were transferred individually into a 10 mL vial and proper amount of triton X-114 (0.085 (MG), 0.10 (RB), 0.08 (MO) and 0.075 (AR) % v/v) was added to it. Then, pH of this solution was adjusted to the desired value (pH = 3.1 for MG, 3.0 for RB, 7.1 for MO and 6.1 for AR) by drop-wise addition of either 1 mol L−1 HCl or 1 mol L−1 NaOH. Then, 0.10 g of NaCl was added to the solution. MIP-PT-µSPE was performed by loading the sample solution into the cartridge for 10 (MG), 6 (RB), 5 (MO) and 7 (AR), and then washing by 1.0 mL of methanol–water (1:1) to eliminate non-reacted matrix molecules. Then, the target compounds retained on cartridge were eluted using 200 µL of 0.5 mol L−1 HCl for 10, 6, 5 and 9, respectively, for MG, RB, MO and AR. This solution was filtered through a 0.45 µm filter and was injected into HPLC for determination of dyes.
Instrumentation
A Knauer HPLC (Germany) was utilized for all analyses which consisted of a EA4300F smart line pump, fitted with a smart line autosampler 3950, a spectrophotometric detector (applied at wavelengths of 448 nm for MO, 510 nm for AR, 555 nm for RB and 618 nm for MG) and a 250 × 4.6 mm Eurospher 100-5 C18 analytical column equipped with a pre-column with a similar stationary phase. ChromGate V3.1.7 software was used for chromatographic data handling. A 20-µL injection loop was used. pHs were determined using a model 630 Metrohm (Switzerland) pH meter. A Fourier transform infrared (FTIR) spectrometer made by PerkinElmer (Bucks, UK) was used for qualitative spectra interpretations as well as for structure elucidation.
Results and discussion
Chromatographic conditions
Several various mobile phases were studied consisting of methanol, acetonitrile and water in various combinations and pH settings. Finally, a gradient of 85% B at 0–3.5 min and 100% B at 3.5–10 min was selected in which eluent A was water and eluent B was acetonitrile that was adjusted to the pH 5.25 using acetic acid methanol/water (40:60). The injection loop volume was 20 µL, and the mobile phase flow rate was 0.8 mL min−1. The column oven temperature was maintained at room temperature, and the mobile phase was degassed with a stream of helium prior to use.
Infrared spectra
The FTIR spectra of leached, unleached MIP and NIP for each dye were recorded. The absorption peaks of leached MIP and NIP materials were similar, which means that no dyes as template molecules were retained on the MIP.
Mechanism of dyes uptake by MIP
Because of ability of MAA for hydrogen and ionic bond interactions with the analytes during polymerization, it was selected as the functional monomer prior to polymerization. A stable donor–receptor complex between dyes (target template) and MAA (monomer) is formed. Electrostatic interaction is the dominant driving force for the molecular recognition, which occurs between carboxy group of MAA and the target molecule (amine of MG and RB, and sulfonate in MO and AR). Weak interaction including electrostatic and hydrophobic interactions may also contribute to the adsorption of dyes by MIP [12]. After a thermal imprinting process, AIBN was utilized as free-radical initiator. Half-life of AIBN at 65 °C is 6 h and so it is suitable for polymer formation.
Optimization of MIP-PT-µSPE
With the aim of achieving the best efficiency of the proposed technique, various factors affecting extraction efficiency were investigated using both one-variable-at-a-time and response surface methodology; including eluent type and volume, the amount of sorbent, volume and pH of sample solution, the number of aspirating/dispensing cycles, and ionic strength of the sample solution. Each experiment was performed in triplicates. The optimization of method was performed with standard solutions containing 250.0 µg L−1 for malachite green and methyl orange, and 150.0 µg L−1 of rhodamine B and acid red 18 in deionized water.
Effect of type of the eluent solvent
Elution conditions are very important in solid-phase extraction, and selection of appropriate solvent will greatly influence elution performance. So, a suitable solvent should be selected to elute maximum amount of the adsorbed target analytes. In order to select an appropriate eluent, different general SPE-eluting solvents such as methanol, acetonitrile, ethanol, methanol/H2O (1:1), methanol/H2O (2:1), methanol/H2O (1:1), methanol/acetic acid (1:2), methanol/acetic acid (2:1), methanol/acetic acid (1:2), HCl (0.5 mol L−1), acetone and diethylene glycol were used. The experimental results showed that HCl (0.5 mol L−1) is the best eluting solvent among them which can desorb dyes from miniaturized column most properly.
Effect of the amount of sorbent
In this research, the amount of molecularly imprinted polymer which is packed into the pipette tip cartridge is a critical factor which affects extraction efficiency. To get the MIP-PT-µSPE cartridge more effective and at lowest possible consumption of sorbent, various amounts of MIP in the range of 1.0–2.5 mg were packed into it. The best extraction efficiency was obtained when the amount of MIP increased to 2.0 mg and further increase in MIP loading decreased recovery and also prolonged the time required for sample passage (since sample passage and eluent of pipette tip by increasing of mass of MIP is more difficult). Therefore, 2.0 mg of MIP was used as packing polymer in the following experiments.
Effect of type of salt
The salting-out effect is widely utilized in traditional liquid–liquid extraction because it makes the solubility of analytes in the aqueous phase decrease; so, more analytes can enter into the extracting phase [41]. In this work, the effect of type of salt on extraction process was investigated. NaCl, Na2SO4 and KCl were selected as common salts. The results showed that NaCl gives higher extraction efficiency than the other salts. (NaCl showed the best effect on forming the turbid solution as was expected because it can imply more ionic strength on water solution.) Therefore, NaCl was employed as salt in the following studies.
Response surface modeling for optimization of the microextraction of dyes
Response surface methodology (RSM) is an affordable and reliable technique to optimize certain processes. This technique leads to reduction in number of experiments needed to investigate the effect of operation factors. To investigate the parameters affecting the extraction efficiency of dyes, the three levels, three-factorial Box–Behnken experimental design was applied. The input variables were pH of sample solution (A) (2–4 for MG and RB, 6–8 for MO and 5–7 for AR), the concentration of triton X-114 (B) (0.05–0.10% for MG, RB, MO and AR), salt concentration (C) (0.2–0.6 mol L−1 for MG, RB, MO and AR), the number of extraction cycles (D) (7–11 for MG and 3–7 for RB, MO and 5–9 for AR), the number of elution cycles (E) (7–11 for MG and AR and 3–7 for RB and MO), volume of eluent solvent (F) (150–250 µL for MG, RB, MO and AR) and volume of the sample solution (G) (8–12 mL for MG and 2–6 mL for RB, MO and AR). The factor levels were coded as − 1 (low), 0 (central point) and 1 (high) (Table 1). Table SI1 indicates the design of real experiments of Box–Behnken.
The behavior of the system is showed by the following quadratic equation.
where Y is the output or process response (dependent variable), β0 is the constant, X1, X2, …, Xk are the coded independent variables, βi is the linear effect, βii is the quadratic effect, βij is the interaction effect and e is the random error or allows for description or uncertainties between predicted and determined value [42].
The equations in supplementary data show the relationship of the seven variables for four selected dyes such as MG, RB, MO and AR to response as peak area.
The critical point in the surface response are achieved applying solving these equation systems for condition of (∂Y/∂A) = 0, (∂Y/∂B) = 0, (∂Y/∂C) = 0, (∂Y/∂D) = 0, (∂Y/∂E) = 0, (∂Y/∂F) = 0 and (∂Y/∂G) = 0. The obtained values for the critical point are as follows: pH of sample solution = 3.06 for MG, 3.0 for RB, 7.1 for MO and 6.1 for AR, volume of eluent solvent = 196 µL (MG), 200 µL (MO), 199 µL (for RB and AR), volume of the sample solution = 10 mL (for MG) and 4 mL (for RB, MO and AR), the concentration of triton X-114 = (0.08% for MG, 0.10 for RB, 0.078 for MO and 0.075 for AR), the number of extraction cycles = 9.7 (MG), 6 (RB), 5 (MO) and 7 (AR), the number of elution cycles = 9.5, 6, 5 and 9, respectively, for MG, RB, MO and AR and salt concentration = (0.45, 0.48, 0.43 and 0.44 mol L−1, respectively, for MG, RB, MO and AR).
Table 2 indicates the results of the response surface model fitting in the form of analysis of variance (ANOVA). The ANOVA results show which the model was significant, as evident from the Fisher’s F test (Fmodel = 51.80, 11.30, 17.86 and 10.43 for MG, RB, MO and AR) by a very low probability value (pmodel = < 0.0001). Values of “Prob > F” less than 0.0500 indicate model terms are significant. Values greater than 0.1000 indicate the model terms are not significant.
The lack-of-fit characterize the failure of the model to represent value in the experimental domain at point which is not included in the regression [39]. The nonsignificant data of lack-of-fit (> 0.05) revealed that the quadratic model is statistically significant for the response. The “Lack-of-Fit F-value” of 1.71, 2.03, 3.64 and 2.36 implies the lack-of-fit is not significant relative to the pure error. By the correlation coefficient (R2), the goodness of the model was checked. The values of determination coefficient [R2 = 0.9859 (MG), 0.9383 (RB), 0.9601 (MO) and 0.9335 (AR)] show good relation between the experimental and predicted values of the response. The adjusted R2 value of 0.967 (MG), 0.855 (RB), 0.906 (MO) and 0.844 (AR) showed that only 3.3, 14.5, 9.4 and 15.6% for, respectively, MG, RB, MO and AR of the total variation were not described with the model. A high degree of precision and a good deal of the reliability of the conducted run were described by a low value of the coefficient of variation (CV = 4.62, 1.48, 14.06 and 27.13 for, respectively, MG, RB, MO and AR) [39]. Two-dimensional response surface as the functions of other variable for selected analytes is indicated in Fig. 3.
Analytical performance
Linear range, limit of detection and enrichment factor
Under optimum condition, the MIP-PT-µSPE methodology was investigated in respect to its linearity, limit of detection (LOD) and enrichment factor. Results are summarized in Table 3. The calibration curve was linear over a concentration range of 0.5–250.0 µg L−1 for malachite green and methyl orange, 0.5–150.0 µg L−1 for rhodamine B and acid red 18. LOD was calculated based on the three Sd m−1 criteria, in which Sd is the standard deviation of seven consecutive measurements of the blank and m is the slope of the calibration curve. LOD of the proposed technique for the dyes was calculated 0.083, 0.10, 0.12 and 0.17 µg L−1, for MG, RB, MO and AR, respectively. The enrichment factors (defined as the ratio of calibration curve slopes after and before microextraction by MIP-PT-µSPE) achieved 107-fold for MG, 87 for AR, 43 for MO and 32 for RB. Furthermore, the comparison of the proposed technique by those using various pre-concentration methods for dyes determination is indicated in Table 4.
Evaluation of the recognition power of MIP
In order to further study the recognition ability of MIP in separation of each dye from other analytes, the same dye solutions containing all target compounds were extracted with both MIP and non-imprinted polymer (NIP). Under the same optimum condition for pre-concentration of each selected dyes, each synthesis polymer showed good selectivity and the best recovery for target molecule. The MIP contains carboxylic groups at the surface of the polymer skeleton ability for target analytes than its analogues due to specific interaction, hydrophobic interactions, hydrogen bonds and ionic exchange. Results are described in Fig. 4. Enhancement of sensitivity of MIP-PT-µSPE is obvious.
Determination of dyes in seawater
To assess the performance of the MIP-PT-µSPE technique, extraction and determination of the target analytes in seawater were investigated. No salt was added for seawater sample, because it is salt saturated with itself. Tested samples were taken from five stations beside Chabahar Bay (southern east of Iran). To study the influence of sample matrices on recovery, these real samples were spiked at the concentration of 20 µg L−1 by the target compounds. Figure 5 shows sample chromatograms for a sample taken from station 1. Allowing the pre-concentration of each dye by high selectivity (Fig. 5b), extraction by NIP showed no such selectivity (Fig. 5c). As can be seen in Fig. 5, MIP-PT-µSPE enables to accurately analyze dyes, because of its high enrichment factor. Therewith, it can also eliminate the matrix interferences successfully. The results are again tabulated in Table 5, which indicate good recoveries for samples taken from five stations in the range of 76.1–97.3%. Reproducibility of the technique as RSD % was calculated to be in the range of 1.2–7.1% for MG, 2.2–6.1% for RB, 1.2–5.4% for MO and 2.6–5.9% for AR. These results show that the investigated technique can be successfully used for the determination of dyes in very complicated matrices including seawater samples.
Conclusions
In this paper, a very simple miniaturized molecularly imprinted polymer-based pipette tip micro-solid phase extraction method was successfully used for the extraction and pre-concentration of malachite green, rhodamine B, methyl orange and acid red 18 in seawater samples dyes in seawater samples. The method indicates that investigated MIP-PT-µSPE for the extraction of dyes has the potential to be a new type of carrier. The linear ranges cover a wide concentration and MIP could selectively separate dyes for chromatographic analysis even at very low concentrations. The method has the advantages of easy to prepare and use good precision, repeatability and rapid separation, low cost, low sample volume and good selectivity in complex matrix such as seawater.
References
E. Ghasemi, M. Kaykhaii, J. Braz. Chem. Soc. 27, 1521 (2016)
E. Ghasemi, M. Kaykhaii, Spectrochim. Acta A Mol. Biomol. Spectrosc. 164, 93 (2016)
P. Rao, R.V. Bhat, R.V. Sudershan, T.P. Krishna, N. Naidu, Food Addit. Contam. 21, 415 (2004)
S. Combeau, M. Chatelut, O. Vittori, Talanta 56, 115 (2002)
N. Yoshioka, K. Ichihashi, Talanta 74, 1408 (2008)
Z.A. Alothman, Y.E. Unsal, M. Habila, A. Shabaka, M. Tuzen, M. Soylak, Food Chem. Toxicol. 50, 2709 (2012)
P. Qi, Z. Lin, J. Li, C.L. Wang, W.W. Meng, H. Hong, X. Zhang, Food Chem. 164, 98 (2014)
L.E. Arnold, N. Lofthouse, E. Hurt, Neurotherapeutics 9, 599 (2012)
Y.-J. Xu, X.-H. Tian, X.-Z. Zhang, X.-H. Gong, H.-H. Liu, H.-J. Zhang, H. Huang, L.-M. Zhang, J. Chromatogr. Sci. 50, 591 (2012)
E. Guerra, M. Celeiro, J.P. Lamas, M. Llompart, C. Garcia-Jares, J. Chromatogr. A 1415, 27 (2015)
N. Hidayah, F. Abu Bakar, N.A. Mahyudin, S. Faridah, M.S. Nur-Azura, M.Z. Zaman, Inter. Food Res. J. 20, 1511 (2013)
Z. Lian, J. Wang, Marine Poll. Bull. 64, 2656 (2012)
N. Vachirapatama, J. Mahajaroensiri, W. Visessanguan, J. Food Drug Anal. 16, 77 (2008)
J. Xie, T. Peng, D.D. Chen, Q.J. Zhang, G.M. Wang, X. Wang, Q. Guo, F. Jiang, D. Chen, J. Deng, J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 913–914, 123 (2013)
C. Long, Z. Mai, Y. Yang, B. Zhu, X. Xu, L. Lu, X. Zou, J. Chromatogr. A 1216, 2275 (2009)
K. Mitrowska, A. Posyniak, J. Zmudzki, J. Chromatogr. A 1207, 94 (2008)
M. Nassiri, M. Kaykhaii, S.H. Hashemi, M. Sepahi, Iran. J. Chem. Chem. Eng. 37, 89 (2018)
S.H. Hashemi, Z. Monfaredzadeh, Iran. J. Chem. Chem. Eng. Articles in Press
A. Author, Analyst 88, 864 (1963)
S.M. Plakas, K.R. El Said, G.R. Stehly, W.H. Gingerich, J.L. Allen, Can. J. Fish. Aquat. Sci. 53, 1427 (1996)
M.J.M. Bueno, S. Herrera, A. Uclés, A. Agüera, M.D. Hernando, O. Shimelis, M. Rudolfsson, A.R. Fernández-Alba, Anal. Chim. Acta 665, 47 (2010)
T. Zou, P. He, A. Yasen, Z. Li, Food Chem. 138, 1742 (2013)
C. Long, Z. Mai, X. Yang, B. Zhu, X. Xu, X. Huang, X. Zou, Food Chem. 126, 1324 (2011)
B. Xu, D. Song, Y. Wang, Y. Gao, B. Cao, H. Zhang, Y. Sun, J. Sep. Sci. 37, 1967 (2014)
Z. Gao, T. Liu, X. Yan, C. Sun, H. He, S. Yang, J. Sep. Sci. 36, 1112 (2013)
P. Biparva, E. Ranjbari, M.R. Hadjmohammadi, Anal. Chim. Acta 674, 206 (2010)
B. Haddou, N. Guitri, A. Debbab, C. Gourdon, Z. Derriche, Sep. Sci. Technol. 46, 734 (2011)
C.C. Wang, A.N. Masi, L. Fernández, Talanta 75, 135 (2008)
M. Khajeh, S. Afzali Moghaddam, M. Bohlooli, M. Ghaffari-Moghaddam, E-Polymers 16, 243 (2016)
H. Hashemi, M. Khajeh, M. Kaykhaii, Anal. Methods 5, 2778 (2013)
M. Khajeh, M. Kaykhaii, H. Hashemi, M. Mirmoghaddam, Polym. Sci. Ser. B 51, 344 (2009)
S.H. Hashemi, M. Kaykhaii, F. Tabehzar, J. Chil. Chem. Soc. 63, 4032 (2018)
S.H. Hashemi, M. Kaykhaii, M. Khajeh, Anal. Lett. 48, 1815 (2015)
M. Khajeh, M. Bohlooli, H. Hashemi, J. Macromol. Sci. 46, 1 (2009)
M. Kaykhaii, M. Khajeh, S.H. Hashemi, J. Anal. Chem. 70, 1318 (2015)
M. Khajeh, M. Kaykhaii, M. Mirmoghaddam, H. Hashemi, J. Environ. Anal. Chem. 89, 981 (2009)
M. Kaykhaii, H. Yahyavi, M. Hashemi, M.R. Khoshroo, Anal. Bioanal. Chem. 408, 4907 (2016)
M.R. Rezaei Kahkha, M. Kaykhaii, M.S. Afarani, Z. Sepehri, Anal. Methods 8, 5978 (2016)
K. Yetilmwzsoy, S. Demirel, R.J. Vanderbei, J. Hazard. Mater. 171, 551 (2009)
D.C. Montgomery, Design and Analysis of Experiments, 4th edn. (Wiley, New York, 1997)
S.H. Hashemi, M. Kaykhaii, F. Tabehzar, J. Iran. Chem. Soc. 13, 733 (2016)
V. Hosseinpour, M. Kazemeini, A. Mohammadrezaee, Appl. Catal. A 394, 166 (2011)
Acknowledgements
We gratefully acknowledge the financial support received from the Research Council of Chabahar Maritime University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashemi, S.H., Kaykhaii, M., Jamali Keikha, A. et al. Application of response surface methodology to optimize pipette tip micro-solid phase extraction of dyes from seawater by molecularly imprinted polymer and their determination by HPLC. J IRAN CHEM SOC 16, 2613–2627 (2019). https://doi.org/10.1007/s13738-019-01724-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01724-0