Abstract
This study proposed a fast, feasible, and sensitive method for nickel preconcentration and separation in different actual samples through the use of deep eutectic solvent based dispersive liquid–liquid microextraction (DES-DLLME). This method involves dissolving a suitable extraction solvent in a dispersive solvent and its rapid syringing into water sample to obtain a turbid solution. Phase separation could be then carried out by centrifugation and the analyte can be determined. 2,2′-furildioxime was employed as the chelating agent through formation of a hydrophobic complex with nickel. To reach maximum recovery, some variables including type and volume of dispersive solvent, volume of extraction solvent, pH, 2,2′-furildioxime concentration, and salt concentration were optimized. Under optimal conditions, nickel calibration graph was linear in the range of 5.0–100 µg L−1. The detection limit and preconcentration factor were obtained as 1.7 µg L−1 and 40 µg L−1, respectively. Finally, this method was successfully applied for the extraction and determination of nickel in water samples with a relative recovery of 98.8–101.0%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important trace element in nature, nickel can be either essential or toxic for many biological systems depending on its concentration range. Nickel is the metallic constitute of urease enzyme and, hence, should be regarded as an essential substance for plants and some domestic animals. The essential role of nickel for human health has not yet been proven. Nickel enters waters from dissolution of rocks and soils, biological cycles, atmospheric fallout, especially from industrial processes and waste disposal [1]. These facts explain the importance of monitoring nickel concentration in natural waters and food samples from public health and environmental point of view. In comparison with other transition metals, nickel is relatively toxic and has been classified as one of the 13 priority metallic pollutants by US EPA [2]. According to the international regulation on water quality, the approved content of nickel in drinking water is 20.0 µg L−1 [1]. Allergic reactions, respiratory system cancer, skin disorder known as nickel-eczema can be mentioned as some of the nickel-induced effects on human health [3, 4]. Thus, the determination of nickel in various real samples is inevitable.
Owing to their enough detection limits, techniques like electrothermal atomic absorption spectrometry (ETAAS), inductively coupled plasma mass spectrometry (ICP-MS) or inductively coupled plasma-atomic emission spectrometry (ICP-AES) are capable of direct nickel detection in real samples. However, the required instruments are expensive, with high maintenance cost and various types of inherent interferences. Among numerous analytical methods for the determination of metals, flame atomic absorption spectrometry (FAAS) has been extensively employed due to its fast analysis, relative simplicity, and lower cost. However, its sensitivity is usually not enough to detect low concentrations of an analyte in real samples. Initial preconcentration and separation procedures have been carried out for achieving concentration within the range of FAAS detection limits [5, 6].
Some of the common techniques for nickel separation and preconcentration include liquid–liquid extraction [7], coprecipitation [8], ion-exchange [9], solid-phase extraction [10,11,12,13,14,15,16,17], cloud point extraction [18], and liquid phase microextraction [19]. However, some of these methods are typically time consuming and labor-intensive and have multiple procedures that prone to loss of analytes, need high volume of sample and large amounts of high-purity organic solvents which are harmful to health and cause environmental problems. A rapid microextraction procedure known as dispersive liquid–liquid microextraction (DLLME) was first introduced in 2006 [20]. This procedure involves the fast injection of a mixture of high-density organic extraction solvent and a water miscible disperser solvent into an aqueous sample. The resultant cloudy solution provides a huge interface area between the fine droplets of extraction solvent and the bulk sample solution. In this extraction method, any component in the solution, directly or indirectly after previous derivatization reactions, interacts with the fine droplets of extraction solvent, and consequently, gets extracted from the initial solution. Usual extraction solvents in conventional DLLME are common volatile organic solvents with potential toxicity for humans and the environment. To overcome this problem, room temperature ionic liquids (ILs) have been recently introduced as the substituting solvents in DLLME and other sample preparation methods, due to their unique chemical and physical properties such as negligible vapor pressure, non-flammability, good extractability for different organic compounds and metal ions as neutral or charged complexes, in addition to adjustable viscosity and miscibility with water and organic solvents which makes them an attractive choice for separation purposes [21,22,23,24,25,26]. In spite of all these benefits, these solvents have some drawbacks like toxicity, poor biodegradability, and high cost. Therefore, their application in routine analysis is not recommended.
In recent years, green and new types of ionic liquid solvents, termed deep eutectic solvents (DESs), have drawn considerable attention in analytical and engineering fields [27, 28]. The use of DESs was first reported by Abbott et al. in 2003 [29], which involved mixing a hydrogen bonding acceptor (HBA) with a hydrogen bonding donor (HBD) following continuous heating and stirring. This eutectic mixture benefits from a far lower melting point in comparison with the original HBA and HBD. Owing to their characteristics similar to those of ionic liquids, such as eco-friendliness, small volatility, and tunable viscosity, DESs are always labeled as ‘ionic liquid analogues’ [30, 31]. In comparison with ionic liquids, which are the most popular green solvents [32,33,34], DESs are benefited by low cost, simple synthesis, biodegradability [35], strong solubility [36, 37] and even lower toxicity [38, 39]. Thus, DESs have become favorite solvents for scientific research and substituted conventional volatile organic solvents and ionic liquids.
This research applied deep eutectic solvent based dispersive liquid–liquid microextraction method for a sensitive and precise determination and preconcentration of nickel in water samples. In this method, a water-immiscible DES was used as the extraction solvent in the DLLME of nickel from water samples for the first time. Effective parameters were studied and optimized to achieve high-extraction efficiency.
Experimental
Reagents
In this study, analytical reagent grade chemicals were applied. 1000 mg L−1 nickel in 0.5 mol L−1 HNO3 stock standard solution was provided using nickel nitrate (Merck, Darmstadt, Germany). Lower concentrations of standard solutions were also prepared by diluting the stock standard solution.
The other materials including nitric acid, sodium hydroxide, ethanol, methanol, acetonitrile, acetone, sodium nitrate, 2,2′-furildioxime, (2-hydroxyethyl)-trimethylammonium chloride (choline chloride) (ChCl), and 4-boromo phenol (BPh) were supplied from Merck (Darmstadt, Germany). Certified reference materials NIST SRM 1643e (National Institute for Standards and Technology, Gaithersburg, MD, USA) were also utilized. The glassware was washed by deionized water and then kept in 10% (v/v) nitric acid (for 24 h), followed by several times washing with deionized water.
Instruments
Nickel determination was carried out by use of an atomic absorption spectrometer, SensAA (GBC, Australia), at a wavelength of 232.0 nm. Background was corrected by deuterium lamp. Phase separation was achieved by a centrifuge (Hettich, EBA 20, Germany). PH was measured by a Metrohm digital pH-meter model 827 (Metrohm, Herisau, Switzerland) combined with a glass electrode.
DES preparation
ChCl (1.39 g) and BPh (3.46 g) were added to a 10-mL screw cap tube. It was then placed into a water bath at 75 °C for 10 min after closing its cap. Afterwards, it was sonicated for 5 min. This heating/sonicating cycle was repeated once again to obtain a homogeneous liquid. The prepared DES had the viscosity and density of 1.32 Pa S and 1.21 g cm−3, respectively, at 20 °C.
Extraction procedure
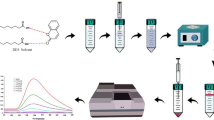
40.0 mL of analyte-containing solution, 0.8 × 10−3 mol L−1 2,2′-furildioxime, and 1 mol L−1 ammonia buffer (pH 9) were added to the glass test tube. Then, the mixture of 0.1 mL methanol (dispersive solvent) and 0.1 mL of the DES (extraction solvent) were rapidly injected to the aqueous solution utilizing a glass syringe. Injection resulted in the turbidity of the solution due to dispersion of small DES droplets in the solution. After centrifugation at 4000 rpm for 5 min, the dispersed DES droplets formed sediments at the bottom of the tube. DES sedimentations were made up to 1 mL by adding 1.0 mol L−1 ethanolic HNO3. The resultant solution was then manually injected into FAAS. Schematic diagram of the synthesis of DES and nickel preconcentration by DES-DLLME is depicted in Fig. 1.
Results and discussion
The effect of pH
In the process of ions extraction, the complex and extraction efficiencies are highly related to pH value. The pH range of 1.0–10.0 was investigated to find the optimal pH value. The results indicated nearly constant recovery in the pH range of 7–9 (Fig. 2). Lower recovery at pH values lower than 7 could be attributed to the competition between H+ and Ni2+ in forming the complex [40]. Finally, pH 9 was chosen as the optimal value and pH adjustment was accomplished by use of 1 mol L−1 of ammonia buffer.
The effect of chelating agent concentration
To optimize 2,2′-furildioxime concentration, the range of 2 × 10−5–1.0 × 10−3 mol L−1 was examined and extraction efficiencies were obtained (Fig. 3). Results revealed that the complete extraction of nickel occurred at concentrations over 6.0 × 10−5 mol L−1. In this regard, 0.8 × 10−3 mol L−1 2,2′-furildioxime was selected to overcome other extractable species.
The effect of extraction solvent type
The component of DES has significant influence on its physicochemical properties, such as polarity, viscosity, and dissolving capacity, which directly affects the extraction efficiency of target compounds. Therefore, three different DESs including ChCl:4-bromophenol (DES-1) (1.39 g:3.46 g), ChCl:4-chlorophenol (DES-2) (1.39 g:2.56 g), and ChCl:p-cresol (DES-3) (1.39 g:2.16 g) were examined as extraction solvents [27]. In all cases, DESs were synthesized according to “DES preparation”. Comparison of the extraction recoveries obtained with different DESs [DES-1 (99.9%), DES-2 (95.3%), DES-3 (85.6%)] showed that DES-1 (ChCl: 4-bromophenol) was the best extraction solvent and it was used as the optimum extraction solvent in further experiments.
Structural characterization of extraction solvent
The structural characterizations of prepared DES were conducted through use of Fourier transform infrared spectroscopy (FT-IR). Figure 4 displays the infrared spectrum of DES in which a broad band at 3206 cm−1 is associated with the stretching vibration of O–H groups of BPh in the DES. Due to intermolecular hydrogen bonding between BPh and ChCl, the wave number of the OH group in DES was shifted in comparison with BPh.
The effect of extraction solvent amounts
To evaluate the extraction solvent volume influence, several volumes of DES in the range of 0.1–0.9 mL were assessed. The impact of this parameter on the recovery of nickel is represented in Fig. 5, in which by increasing the DES volume from 0.1 to 0.3 mL, the recovery remained constant and then declined. In higher DES volumes, the organic phase volume rose. The larger volume of organic phase resulted in the enhancement of the viscosity of the samples as diluted to 1.0 mL with ethanolic HNO3. The enhanced viscosity seriously reduced the sampling efficiency of FAAS’s pneumatic nebulization [41, 42]. Hence, 0.1 mL of DES was selected as extraction solvent.
The effect of disperser solvent type and volume
Miscibility, toxicity, and price are among the important factors influencing the choice of the disperser solvent. The miscibility in the extraction solvent and the aqueous sample has a direct relationship with the formation of the turbid solution. In this content, acetone, acetonitrile, methanol, and ethanol were addressed. Based on the results [acetone (92.0 ± 2.5%), methanol (100.0 ± 2.2%), acetonitrile (87.0 ± 2.3%), and ethanol (93.0 ± 2.4%)], methanol was chosen due to its higher recoveries as demonstrated in Fig. 6.
After the determination of the disperser solvent type, its volume had to be studied. In this regard, various experiments were conducted with different volumes of methanol (0.1–1.1 mL) in different amounts of DES. Figure 7 demonstrates the nickel recovery versus the methanol volume. Clearly, when the volume of methanol was increased from 0.1 to 0.3, the recovery remained constant and then reduced. The lower recoveries at larger methanol volumes could be attributed to the enhanced solubility of the complex in water. Accordingly, 0.1 mL of methanol was considered as the optimal value.
The effect of the type of diluting solvent
For selecting the best type of diluting solvent, acetone, acetonitrile, methanol, and ethanol were investigated. Figure 8 demonstrates the absorbance versus type of diluting solvent. According to obtained results, we chose methanol because of the higher sensitivity.
The effect of ionic strength
Salt addition to the aqueous solution will generally lead to a decline in the organic compounds’ solubility in water; hence improving the analyte extraction. Various experiments in the presence of 0–35% w/v NaNO3 were carried out to examine the effect of ionic strength on DES-DLLME performance. The results revealed no significant effect of ionic strength on the extraction efficiency and sensitivity up to 35.0%.
The effect of matrix
The impacts of different cations and anions on Ni2+ recovery were also addressed in this study. Changes in recovery higher than ± 5% were regarded as interference for nickel preconcentration and determination. Table 1 verifies that this method can lead to acceptable results in nickel determination for real samples.
Figures of merit
Table 2 lists the figures of merit obtained for nickel DES-DLLME: Dynamic linear range for Ni determination varies from 5.0 to 100.0 µg L−1 (A = 0.0053 C + 0.0197, R2 = 0.9998); the relative standard deviation (RSD) and limit of detection (LOD) were also 2.0% (n = 10, C = 20 µgL− 1) and 1.7 µg L−1 (3Sb/m), respectively. The preconcentration factor that was calculated by dividing the aqueous phase volume into the final volume of the diluted phase was 40 for 40 mL sample solution.
Validation of the proposed methodology
This method was validated by analyzing CRM NIST SRM 1643e. The results are tabulated in Table 3. No significant difference was observed between the result of this study and the certified values, showing the capability of this preconcentration technique in nickel determination from water samples.
Comparison
The performance of this DES-DLLME method was also compared with other preconcentration techniques (Table 4). As can be seen, the obtained detection limit by the proposed procedure is the best. Relative standard deviation (RSD) and preconcentration factor (PF) are comparable to most of those reported in the other researches in Table 4. Linear dynamic range (LDR) of the proposed method has a better lower limit than almost all because of good sensitivity. Due to these good analytical characteristics, obviously, the performance of this method is comparable with those of other reported methods. Rapidity, cost-effectiveness, feasibility, and being timesaving are among the advantages of this method, introducing it as a suitable candidate for nickel analysis.
Nickel determination in real samples
The proposed DES-DLLME method was employed in nickel determination and preconcentration in several water samples (i.e., tap, river, mineral, and sea water) (Table 5).
To analyze the water samples, a 0.45 µm millipore membrane was utilized to filter collected water samples (river and sea water) before extraction. Table 5 clearly indicates that 5.0 µg L−1 nickel exists in sea water samples while tap, river, and mineral water showed no nickel contamination. To accredit the accuracy of the proposed method, 25 and 50 µg L−1 of nickel were spiked to the samples before extraction. The relative recoveries and RSDs varied in the range of 98.8–101.0% and 2.0–2.5%, respectively. Therefore, this technique seems to be a promising technique for ultra-trace level determination of nickel in water samples.
Conclusion
Deep eutectic solvent based dispersive liquid–liquid microextraction (DES-DLLME) coupled with FAAS was developed for sensitive nickel determination in real samples. DESs have attracted considerable attention as green solvents for the extraction and separation of target compounds on account of their excellent properties. They have attracted increasing attention because they possess similar physical and chemical properties, but are much cheaper, safer, and easier to obtain than ILs. The proposed method proved its cost-effectiveness, sensitivity, rapidity, feasibility, and being environment-friendly. It was also proven to possess a good recovery and a high preconcentration factor. The developed method is a promising approach in the determination of ultra-trace levels of nickel in water samples with low LOD, high accuracy (recovery > 98%), and precision (RSD < 2.5%). Its sensitivity could be even increased by the application of GF-AAS as the detection step.
References
Guide lines for Drinking Water Quality, Health Criteria and Other Supporting Information (vol, 2, 2nd edn. (World Health Organization, Geneva, 1998)
P. Patnaik, Handbook of Environmental Analysis: Chemical Pollutants in Air, Water, Soil, and Solid Wastes (CRC Press, Boca Raton, 1997)
D. Templeton, Biological Monitoring of Chemical Exposure in the Workplace (World Health Organization, Geneva, 1990)
H.A. Mckenzie, L.E. Smythe, Quantitative Trace Analysis of Biological Materials (Elsevier, Amsterdam, 1988)
S. Baytak, A.R. Turker, J. Hazard. Mater. B 129, 130 (2006)
M. Soylak, N.D. Erdogan, J. Hazard. Mater. B 137, 1035 (2006)
S.A. Popova, S.P. Bratinova, C.R. Ivanova, Analyst 116, 525 (1991)
H.W. Chen, J.C. Jin, Y.F. Wang, Anal. Chim. Acta 353, 181 (1997)
J.H. Wang, E.H. Hansen, Anal. Chim. Acta 424, 223 (2000)
A. Mirabi, Z. Dalirandeh, A. Shokuhi-Rad, J. Magn. Magn. Mater. 381, 138 (2015)
A. Mirabi, A. Shokouhi Rad, S. Nourani, TrAC Trends Anal. Chem. 74, 146 (2015)
A. Mirabi, A. Shokuhi-Rad, M.R. Jamali, N. Danesh, Aust. J. Chem. 69, 314 (2016)
A. Mirabi, A. Shokuhi-Rad, H. Khodadad, J. Magn. Magn. Mater. 389, 130 (2015)
A. Mirabi, A.S. Rad, F. Divsalar, H. Karimi-Maleh, Arab. J. Sci. Eng. https://doi.org/10.1007/s13369-017-3025-x (2018)
A. Mirabi, A.S. Rad, M. Abdollahi, Chem. Select. 2, 4439 (2017)
A. Mirabi, A.S. Rad, Z. Khanjari, M. Moradian, Sens. Actuators B Chem. 253, 533 (2017)
A. Mirabi, A.S. Rad, S.A. Siadati, S.A.A. Tabari, Pakistan J. Sci. Ind. Res. Ser. A 59, 23 (2016)
P. Zh. Suna, Q. Liang, J. Ding, Cao, J. Hazard. Mater. B 137, 943 (2006)
M. Reclo, E. Yilmaz, M. Soylak, V. Andruch, Y. Bazel, J. Mol. Liq. 237, 236 (2017)
M. Rezaee, Y. Assadi, M.R. Milani Hosseini, E. Aghaee, F. Ahmadi, S. Berijani, J. Chromatogr. A 1116, 1 (2006)
A. Heintz, J. Chem. Thermodyn. 37, 525 (2005)
K.N. Marsh, J.A. Boxall, R. Lichtenthaler, Fluid Phase Equilib. 219, 93 (2004)
S. Pandey, Anal. Chim. Acta 556, 38 (2006)
R. Liu, J. Liu, Y. Yin, X.-l. Hu, G.-B. Jiang, Anal. Bioanal. Chem. 393, 871 (2009)
T.L. Greaves, C.J. Drummond, Chem. Soc. Rev. 8, 1709 (2008)
N.V. Plechkova, K.R. Seddon, Chem. Soc. Rev. 1, 123 (2008)
M.A. Farajzadeh, M.R. Afshar Mogaddam, M. Aghanassab, Anal. Methods https://doi.org/10.1039/c5ay03189c (2016)
M.A. Farajzadeh, M.R. Afshar Mogaddam, B. Feriduni, RSC Adv. (2016) https://doi.org/10.1039/C6RA04103E
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Chem. Commun. 1, 70 (2003)
M.A. Kareem, F.S. Mjalli, M.A. Hashim, I.M. AlNashef, J. Chem. Eng. Data 55, 4632 (2010)
A.P. Abbott, D. Boothby, G. Capper, D.L. Davies, R.K.J. Rasheed, Am. Chem. Soc. 126, 9142 (2004)
J. Flieger, Z.A. Czajkowska, Food Chem. 166, 150 (2015)
D. Ge, H.K. Lee, J. Chromatogr. A 1251, 27 (2012)
C.H. Ma, T.T. Liu, L. Yang, Y.G. Zu, X. Chen, L. Zhang, Y. Zhang, C. Zhao, J. Chromatogr. A 1218, 8573 (2011)
K. Radoševi´c, M.C. Bubalo, V.G. Srˇcek, D. Grgasb, T.L. Dragiˇcevi´cb, I.R. Redovnikovi´ca, Ecotox. Environ. Safe 112, 46 (2015)
Y. Dai, G.J. Witkamp, R. Verpoorte, Y.H. Choi, Anal. Chem. 85, 6272 (2013)
G. Li, D. Deng, Y. Chen, H. Shan, N.J. Ai, Chem. Thermodyn. 75, 58 (2014)
M. Hayyan, M.A. Hashim, M.A. Al-Saadi, A. Hayyan, I.M. AlNashef, M.E. Mirghani, Chemosphere 93, 455 (2013)
M. Hayyan, M.A. Hashim, A. Hayyan, M.A. Al-Saadi, I.M. AlNashef, M.E. Mirghani, O.K. Saheed, Chemosphere 90, 2193 (2013)
R. Rahnama, M. Najafi, Environ. Monit. Assess. 188, 150 (2016)
X.D. Wen, L.Q. Ye, Q.W. Deng, L. Peng, Spectrochim. Acta A Mol. Biomol. Spectrosc. 83, 259 (2011)
X.D. Wen, Q.W. Deng, S.L. Ji, S.C. Yang, L. Peng, Microchem. J. 100, 31 (2012)
C.A. Sahin, M. Efecinar, N. Satiroglu, J. Hazard. Mater. 176, 672 (2010)
E.L. Silva, P.D. Santos Roldan, M.F. Giné, J. Hazard. Mater. 171, 1133 (2009)
A. Safavi, H. Abdollahi, M.R. Hormozi Nezhad, R. Kamali, Spectrochim. Acta Part A 60, 2897 (2004)
F. Tokay, S. Bağdat, Appl. Spectrosc. 70, 543 (2016)
A.S. Amin, A.S. AL-Attas, J. Saudi Chem. Soc. 16, 451 (2012)
K. Kocot, B. Zawisza, R. Sitko, Spectrochimica Acta Part B 73, 79 (2012)
A. Mirabi, M.R. Jamali, P. Mehraeen, K. Berijani, Asian J. Chem. 24, 3425 (2012)
Acknowledgements
The authors appreciate the research council of Payame Noor University for its financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rad, A.S., Rahnama, R., Zakeri, M. et al. Dispersive liquid–liquid microextraction based on green type solvents—"deep eutectic solvents"—for highly selective separation and efficient preconcentration of nickel in water samples. J IRAN CHEM SOC 16, 1715–1722 (2019). https://doi.org/10.1007/s13738-019-01643-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01643-0