Abstract
Kidney transplantation is the encouraged kidney replacement therapy due to providing more prolonged survival with a better quality of life. Unfortunately, kidney transplant recipients are susceptible to infections because of long-term utilization of immunosuppression. Despite dermatophyte infections are generally not life-threatening, the clinical significance has been recently enhanced by an increasing number of immunocompromised patients. We have presented a rare dermatophytosis course, Majocchi's granuloma, that spreads to all extremities during the early post-transplant period. A young kidney transplant recipient was exposed to intensive immunosuppression therapy due to acute rejection in the early period of post-transplantation. After four months, numerous nodular skin lesions were raised on various body parts. An invasive fungal infection was identified in the skin biopsy. Also, Trichophyton rubrum was isolated in the tissue cultures. Consequently, the patient was diagnosed with Majocchi’s granuloma. An effectual treatment was attained with an oral terbinafine tablet. Majocchi’s granuloma is a distinct form of dermatophytosis characterized by the spreading of infection into the dermis. In this unexpected case, we alerted physicians to opportunistic infections in the kidney transplant recipient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Kidney replacement therapy can be required after the glomerular filtration rate (GFR) decreases below 15 ml/min/1.73 m2. Two treatment options are available dialysis or transplantation. Kidney transplantation is the highlighted treatment due to providing more prolonged survival, a better quality of life, and more cost-effectiveness [1].
The immunosuppression agents after kidney transplantation have ensured minimizing both acute rejection and chronic allograft nephropathy [2]. However, these drugs have unfavorable side effects and cause opportunistic infections. Therefore, kidney transplant recipients (KTR) should be closely monitored in the post-transplant period for complications.
KTR are susceptible to infections and wide a variety of pathogens can be responsible. The clinical and radiological manifestations constituted by microbial invasion may be unmarked due to impaired inflammatory response. For this reason, most patients are initially mild symptomatic, and the diagnosis is delayed [3].
Superficial and invasive fungal infections are notable members of opportunistic infections. The fungal infections occur as a wide clinical spectrum in diverse localizations in organ transplant recipients. Moreover, Candida and Aspergillus infections can lead to severe systemic infections and mortality [4]. Dermatophytes are frequently located in the stratum corneum layer of the skin, hair/nails, and the spillover into the deep tissues is rare. Dermatophytes are the most common causes of superficial fungal infection and cause localized infections in the general population.
Although severe progress of dermatophytosis is reported in immunocompromised individuals, it is rare in normal populations. In this case, we have presented a rare and atypical form of dermatophytosis in a young KTR.
Case presentatıon
A 33-year-old male underwent kidney transplantation from a living donor (his mother) seven months ago. The etiologic disease of end-stage kidney disease (ESKD) was focal segmental glomerulosclerosis (FSGS). Before transplantation, he received hemodialysis for one year. Kidney transplantation was ABO compatible. Before transplantation, complement-dependent cytotoxicity (CDC) and flow-cytometric crossmatch were negative. He was exposed to intensive immunosuppression therapy due to the acute rejection after kidney transplantation. The patient had no other systemic diseases, such as diabetes. The induction regimen consisted of anti-thymocyte globulin (ATG), 200 mg for five days, and methylprednisolone 500 mg bolus (afterward tapering). He was discharged with the maintenance regimen that includes tacrolimus 3 mg twice a day, mycophenolate mofetil (MMF) 1000 mg twice a day, and oral 20 mg prednisolone tablet.
In the third postoperative week, 250 mg methylprednisolone was administered for 3 days due to considering acute T-cell mediated rejection (not confirmed by allograft biopsy). Since there was a complete response to pulse steroid, creatinine level decreased to baseline.
Two months later serum creatinine levels increased again, however, there was no response to pulse steroid (500 mg for three days) treatment. An allograft biopsy was immediately performed and antibody-mediated allograft rejection was determined. Peritubular capillarities, linear immunofluorescence staining for C4d in the peritubular capillaries, and minimal interstitial inflammation were observed in kidney biopsy. Also, the patient had donor-specific antibody (DSA) positivity after transplantation. Plasmapheresis was performed in five sessions and intravenous immunoglobulin (IVIG) was administered (cumulative dose 1000 mg/kg). Lastly, rituximab was administered as a single dose of 500 mg.
After undergoing rejection therapy, the patient experienced partial recovery in allograft functions. The last laboratory results were as follows: glucose: 82 mg/dL, blood urea nitrogen (BUN): 24 mg/dL, creatinine: 1.8 mg/dL, eGFR: 51 ml/min/1.73 m [2], protein: 7.4 g/dL, albumin: 3.9 g/dL, CRP: 44 mg/L, leukocyte count: 8.2 103/μL, hemoglobin: 10.8 g/dL, platelet count: 190 × 103/μL, and tacrolimus through level: 7.3 ng/mL.
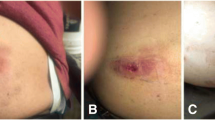
Four months after transplantation, skin rashes appeared, increasing in number. When the rashes did not resolve spontaneously, he was admitted to the hospital 2 weeks later. These nodular lesions were erythematous with fluctuation. The lesions caused pain and itching and were located on the dorsum of the hands, left forearm, left leg, medial thighs, bilateral buttocks, and back. The most prominent lesions, with sharp borders, were observed on the dorsum of the left hand, measuring 3 cm at the widest point. These lesions are illustrated in Fig. 1. Despite the presence of these lesions, the patient was able to maintain his daily activities independently. Additionally, there were no significant findings on physical examination.
A skin biopsy was conducted by the dermatology department. The pathological examination of the tissue sample revealed dermal infection findings and reactive squamous hyperplasia. Furthermore, Trichophyton rubrum, identified as fungal elements in the form of hyphae, was isolated in the microbiological tests. The corresponding images are displayed in Figs. 2 and 3.
The image of skin biopsy in light microscopy. Acanthosis is shown in the epidermis. Squamous epithelial islands without atypia are shown in the dermis. Dense neutrophil-rich mixed inflammatory cells are infiltrating the dermis surrounding these islands. Increased vasculature is in the dermis (400x, H&E stain)
Antifungal therapy was promptly initiated after diagnosis. The patient started taking an oral terbinafine 250 mg tablet once a day. The treatment lasted for eight weeks, during which the tacrolimus level was closely monitored due to the potential for drug interaction. Tacrolimus trough level decreased in the second week of antifungal treatment, so drug dose increased to 4 mg twice a day. Afterwards, tacrolimus trough level measurements remained within the target range (5–7 ng/mL). During this treatment period, the doses of MMF and steroid therapy were not changed. Because the patient had a high immunologic risk for rejection, immunosuppressive regimen was not alleviated. The lesions showed regression and healing during the follow-up period. Figure 4 displays the last status of the lesions on the hand dorsum, taken three months after the beginning of treatment. Due to the absence of a life-threatening infection and the previous rejections, we did not attenuate an immunosuppressive regimen. The patient's medical history is summarized in Fig. 5.
Dıscussıon
We presented a rare manifestation of dermatophytosis in the immunocompromised host. In this case, pathological and microbiological findings established the diagnosis. A comprehensive clinical approach led to recognition of an unusual clinical entity. MG is a rare form of fungal infection and primarily caused by dermatophytes in the dermis [5]. Normally, dermatophytic infections are confined to the stratum corneum of the epidermis. Additionally, nodular formation is uncommon for dermatophytosis. This report has showcased the aggressive attitude of a commonplace infection due to immunosuppression.
KTR requires close monitoring after transplantation due to the susceptibility to serious infection and malignancy by immunosuppressive therapy. Both physical examinations and laboratory tests are essential during the follow-up period to promptly recognize complications. Infections are a major cause of death following organ transplantation. The most intense immunosuppression exposure is in the first six-month period after transplantation, and life-threatening infections often occur during this period. The fungal infections remain a major cause of mortality. In particular, Candida spp. is the most common cause of fungal infections in solid organ transplant recipients [6]. Antiviral, antibacterial, and antifungal prophylactic treatments are widely used during this crucial period.
Dermatophyte infections are common worldwide, affecting approximately 20–25% of the world’s population with skin mycoses. While dermatophyte infections are generally not complicated, their clinical significance has recently been heightened due to an increasing number of immunocompromised patients, the aging population, and the use of broad-spectrum antibiotics.
Pathogens responsible for skin mycoses are primarily anthropophilic and zoophilic dermatophytes from the genera Trichophyton, Microsporum, and Epidermophyton. The diagnosis of cutaneous dermatophyte infections is frequently based on physical examination. The potassium hydroxide (KOH) method can be used to confirm dermatophyte infections. Additionally, fungal culture serves as an alternative method for diagnosis [7]. Trichophyton rubrum is the most commonly identified dermatophyte in superficial fungal infections of the skin, primarily causing tinea pedis, onychomycosis, tinea corporis, and tinea capitis. Serious infections caused by Trichophyton are rare; however, a case of disseminated infection in kidney transplant recipients has been reported [8].
T cells play a pivotal role in both direct and indirect allorecognition in transplant immunology. Inactivation or depletion of T lymphocytes through drugs or antibodies, such as ATG, leads to reduced graft rejection. Glucocorticoids disrupt the phagocytic ability of monocytes and neutrophils and impair T cell functions. Calcineurin inhibitors (CNI) selectively inhibit T-cell activation, with minimal effects on phagocytic cells. Mycophenolic acid induces apoptosis in activated T lymphocytes [9]. The role of CD4 + T cells against fungal infections has been elucidated in immunocompetent individuals. Th1 and Th17 helper cells are the most prominent subgroups of T lymphocytes in antifungal immunity. Therefore, T-cell depletion due to immunosuppression increases the susceptibility to fungal infections in organ transplant recipients [10]. Also, environmental factors are considerable determinants in the epidemiology of fungal infections. The incidence of invasive fungal infection was reported as 1–10% among KTR [11].
A higher rate of skin lesions in organ transplant recipients according to the general population has been reported in previous studies. The malignancy possibility should be considered in the differential diagnosis of all new-onset skin lesions. In the analysis of 116 KTR, fungal infections have been reported as the second most common skin lesion in five five-year follow-up periods afterward transplantation. [12]
MG is a distinct form of dermatophytosis and characterized by the spread of epidermal infection into the dermis. MG is an inflammatory and granulomatous condition. MG can rarely progress to generalized invasive infection and internal organ involvement. The most frequently identified organism in MG etiology is Trichophyton rubrum [5]. The first form of MG is primarily observed in healthy individuals and is defined as a perifollicular, papular form induced by penetrating trauma, commonly found on the lower extremities. The second form is associated with immunosuppression, presenting as a nodular form and frequently occurring on the upper extremities [13]. Our case report aligns with the characteristics of the second form.
Treatment of MG is similar to other dermatophytoses and can be achieved through topical or systemic antifungal drugs. The majority of cutaneous dermatophytosis were limited to the epidermis, and can be cured with topical therapy. Oral antifungal agents are employed for severe or refractory infections and cases where the infection has spread into follicles or the dermis [14]. Terbinafine, itraconazole, fluconazole, and griseofulvin are among oral antifungal drugs. We successfully treated the patient with terbinafine. Dose adjustment of terbinafine is not necessary in patients with creatinine clearance ≥ 50 ml/min [15]. Antifungal agents have the potential to increase the levels of CNI through cytochrome P450 microsomal enzyme inhibition, as seen with drugs like ketoconazole [14]. Therefore, drug concentrations should be closely monitored. In this case, we monitored tacrolimus levels twice a month, and no significant alterations were detected during antifungal therapy.
Only a few case reports of the condition have been documented in transplant recipients in THE literature. Burg et al. reported a 39-year-old man who had undergone kidney transplantation 14 years ago who had a fungal infection that was manifested as a bilateral inguinal granuloma. The lesions were surgically removed and Trichophyton rubrum were detected microbiologically. Immunosuppressive treatment was not changed and local antimycotic treatment was succesful in this case [16]. In another case, a 53-year-old male who underwent liver transplantation was reported. Acute cellular rejection was diagnosed five weeks after transplantation and 5 days of ATG (1.5 mg/kg/day) was administered. Afterward, multiple distinct violaceous fluctuant nodules were present in the inguinal and genital areas. The fungal culture of the specimen confirmed the diagnosis by isolating Trichophyton rubrum. These lesions were improved with oral terbinafine 250 mg daily for six weeks [17].
In conclusion, we have emphasized the diverse range of posttransplant skin lesions and their wide etiology. Transplantation physicians should conduct thorough examinations of the skin during each time. Being proactive in this regard contributes to early diagnosis in immunocompromised populations.
References
Gordon EJ. Patients’ decisions for treatment of end-stage renal disease and their implications for access to transplantation. Soc Sci Med. 2001;53(8):971–87.
Zand MS. 2005 Immunosuppression and immune monitoring after renal transplantation. Paper presented at: Seminars in dialysis2005.
Fishman JA. Infection in renal transplant recipients. Paper presented at: Seminars in nephrology 2007.
Saral R. Candida and Aspergillus infections in immunocompromised patients: an overview. Rev Infect Dis. 1991;13(3):487–92.
Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives. Infect drug Resist. 2018;11:751–60.
Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45(4):305–20.
Levitt JO, Levitt BH, Akhavan A, Yanofsky H. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis. Dermatol Res Pract. 2010;2010:764843.
Trottier CA, Jhaveri VV, Zimarowski MJ, Blair BM, Alonso CD. Beyond the superficial: disseminated Trichophyton rubrum infection in a kidney transplant recipient. Paper presented at: Open Forum Infectious Diseases 2020.
Schmidt S, Hogardt M, Demir A, Röger F, Lehrnbecher T. Immunosuppressive Compounds affect the fungal growth and viability of defined Aspergillus species. Pathogens (Basel, Switzerland). 2019;8(4):273.
Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transplant. 2011;17(S3):S34–7.
Seok H, Huh K, Cho SY, et al. Invasive fungal diseases in kidney transplant recipients: risk factors for mortality. J Clin Med. 2020;9(6):1824.
Engin B, Alagoz S, Fenjanchi A, et al. Evaluation of cutaneous manifestations according to the time in renal transplant recipients. Turkderm-Turk Arch Dermatol Venerol. 2013;47(2):88.
Radentz WH, Yanase DJ. Papular lesions in an immunocompromised patient. Arch Dermatol. 1993;129(9):1191–2.
Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166(5):353–67.
Irimie M, Tătaru A, Oantă A, Moga M. In vitro susceptibility of dermatophytes isolated from patients with end-stage renal disease: a case–control study. Mycoses. 2014;57(3):129–34.
Burg M, Jaekel D, Kiss E, Kliem V. Majocchi’s granuloma after kidney transplantation. Exp Clin Transplant Off J Middle East Soc Organ Transplant. 2006;4(2):518–20.
Gega A, Ketsela G, Glavin F, Soldevilla-Pico C, Schain D. Majocchi’s granuloma after antithymocyte globulin therapy in a liver transplant patient. Transpl Infect Dis. 2010;12(2):143–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of İnterest
All the authors have declared no competing interest.
Ethical approval
Not applicable to this case report.
Consent for publication
Fully informed written consent for the publication of his case was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Uysal, C., Oguz, H., Cifci, H. et al. Widespread form of Majocchi’s granuloma in a kidney transplant recipient. CEN Case Rep (2024). https://doi.org/10.1007/s13730-024-00883-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13730-024-00883-1