Abstract
Mucinous borderline ovarian tumors (MBOTs) have a very low recurrence rate and a good prognosis, especially in the early stages, but some MBOTs occasionally recur with the progression to mucinous ovarian carcinomas (MOCs). Here, we present a case of MBOT that recurred as invasive MOC within 3 years. To examine the reason for the progression from MBOT to MOC, whole-exome sequencing of our case identified identical mutations and copy number alterations in KRAS, CDKN2A, and TP53 in both the MBOT and recurrent MOC. The recurrent MOC had a greater copy number alteration burden compared to the primary MBOT. These findings suggest that MBOT may have progressed to MOC via recurrence, wherein the increased burden of copy number alterations could be its key driver. It was also suggested that TP53 mutations already present in MBOT may contribute to the increased copy number alterations leading to MOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucinous ovarian tumors show clear progression from benign, to borderline, and then to carcinoma [1]. Mucinous ovarian carcinoma (MOC) is a rare subtype of epithelial ovarian carcinoma [2]. It is often detected at an early stage and has a good prognosis; however, its prognosis at advanced stage or recurrence with peritoneal dissemination was severe because MOC is refractory to conventional platinum-based chemotherapy regimens [3, 4]. Mucinous borderline ovarian tumors (MBOTs) rarely recur, usually after a prolonged period of more than 5 years, with the prognosis typically being good [3]. Furthermore, even after recurrence, they often remain as MBOTs and do not progress to MOC, though some of MBOTs sometimes recur with the progression to MOC. Here, we report a case of MBOT that recurred as invasive MOC with multiple peritoneal metastases, approximately 2 years after the initial surgery. We also investigated gene mutations and copy number alterations in three components (i.e., cystadenoma and MBOT at initial onset, and MOC at recurrence) to gain insight into its underlying biology and the reasons for its switch from MBOT to MOC.
Case report
Clinical course
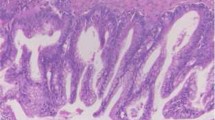
A 73-year-old woman, who was on peritoneal dialysis for chronic renal failure due to immunoglobulin A nephropathy and was under observation for 10 years at our hospital for a right ovarian tumor of 11 cm in size, suddenly developed right lower abdominal pain (Supplementary Fig. 1A). On the same day, her peritoneal dialysis drainage volume increased; a laparoscopic bilateral adnexectomy was performed with suspected rupture of the right ovarian tumor. Intraperitoneally, the right ovarian tumor had ruptured and her left ovary was normal. The final pathological diagnosis was a MBOT of the right ovary, pT1c2NXMX, stage IC2, with a mucinous cystadenoma–borderline tumor sequence (Fig. 1A, B). Immunohistochemical analysis of both components of the ovarian tumor (cystadenoma and borderline tumor components) showed positive staining for PAX8 and negative staining for CDX2, indicating that the ovarian tumor was derived from the ovaries (Supplementary Fig. 2A and B and Table 1). p16 (CDKN2A) was only expressed in the cystadenoma (Fig. 1C). The borderline component showed wild-type pattern, but focally diffuse with the basal layer of the borderline tumor cells, for p53 nuclear staining (Fig. 1D). An insulin-like growth factor mRNA-binding protein 3 (IMP3), a marker for an ovarian mucinous borderline tumor and carcinoma, was expressed only in borderline component (Supplementary Fig. 2C). Since the patient was on peritoneal dialysis, we decided to follow-up the patient without additional surgery or treatment. Two years later, the patient was switched from peritoneal dialysis to hemodialysis.
The pathological findings of the primary tumor. A The initial mucinous borderline tumor (MBOT) with mucinous cystadenoma by hematoxylin–eosin (HE) staining. B Left; cystadenoma, right; borderline tumor by HE staining. C p16 (CDKN2A) cytoplasmic immunostaining. D p53 nuclear immunostaining. Scale bars: A, C, D 100 μm, B 50 μm
Thirty-one months after initial onset, the patient was urgently admitted to the hospital because of increasing ascites effusion. Following ascites puncture, cancer cells were detected. Position emission tomography (PET)–CT suggested carcinomatous peritonitis (supplementary Fig. 1B). Exploratory laparoscopy revealed that the entire abdominal wall and ascending colon was covered with white peritoneal metastasis and a subcutaneous nodule was observed in the left lower abdomen (Supplementary Fig. 1B). The peritoneal and subcutaneous nodules were sampled, wherein MOC was detected in both lesions (Fig. 2A and B). The immunostaining pattern was similar to that in the borderline component at 1st surgery, wherein Ki67 labeling index within the hot spots was increased from 25 to 46% and 50% of tumor cells were positive for p53 nuclear staining, still remained wild-type pattern (Fig. 2C, D and Supplementary Fig. 2). The patient was diagnosed with the recurrence of MBOT with the progression to MOC. The patient died 32 months after the initial onset and 1 month after recurrence.
The pathological findings of the recurrent mucinous carcinoma (MOC). A, C and D the peritoneal metastasis and (B) the subcutaneous tumor. A and B The recurrent mucinous carcinoma (MOC) by HE staining. C p16 (CDKN2A) cytoplasmic immunostaining. (D) p53 nuclear immunostaining. Scale bars: A, C and D 100 μm B 50 μm
Whole-exome sequencing
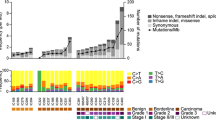
DNA was extracted from paraffin-embedded sections using a laser-capture microdissection system. Whole-exome sequencing was performed for three specimens: cystadenoma and borderline components at 1st surgery as well as carcinoma at recurrence. Ovarian stroma was used as a germline control. All the tumors shared many genetic mutations, indicating that they originated from the same clone (Fig. 3A, B). Cystadenoma displayed only the p.G12C somatic mutation in the KRAS gene as a driver mutation, whereas both MBOT at initial onset and MOC at recurrence shared driver events, including the p.G12D mutation in KRAS, p.E154K mutation in TP53, and p.A21Gfs*24 mutation in CDKN2A. The patient had no pathological germline mutations. The proportion of the genome displaying copy number alterations increased stepwise from mucinous cystadenoma to MBOT to MOC (Fig. 3C). MBOT and MOC shared a gain of 12p including KRAS and losses of heterozygosity of CDKN2A (9p) and TP53 (17p), despite wild-type pattern of p53 nuclear staining. We found whole-genome doubling and more copy number alterations including amplification of the KRAS mutant allele only in MOC, which suggests one of the reasons for the progression from MBOT to MOC.
The genetic analyses of three components in our case. A Somatic mutations and their variant allele frequencies in three components; mucinous cystadenoma, borderline tumor (MBOT) in the initial onset tumor and recurrent mucinous carcinoma (MOC). Each column represents an equivalent mutation. Driver mutations are illustrated. B Clonal ordering based on somatic mutations and copy number aberrations. Driver mutations in each clone were noted. C Copy number aberration analysis based on whole-exome sequencing data. Blue dots represent total copy numbers. Green and red dots represent the B-allelic frequencies. Black lines on blue dots represent copy number changes
Discussion
MBOTs may recur but are rarely fatal. Most MBOTs are stage I, with a survival rate of almost 100% with or without intraepithelial carcinoma [5]. For MOC progress from benign epithelium to a pre-invasive lesion to carcinoma with shared genetic events, MBOTs often have components of benign cystadenoma, MBOT, and MOC. Considering the possibility of inadequate sampling in our case, MBOT at the 1st surgery were re-sectioned and retrospectively examined, and a continuum of MBOT was found from mucinous cystadenoma, but no malignant component was found.
To investigate why it recurred with the progression to MOC, we examined genetic mutations and copy number alterations in the cystadenoma, borderline tumor, and carcinoma components. All three shared somatic mutations (Fig. 2), suggesting that they were derived from the same clone. Mucinous cystadenoma showed only a p.G12C mutation in KRAS as a driver mutation, whereas borderline tumors and recurrent MOCs shared driver mutations in KRAS, TP53, and CDKN2A. Some gene mutation data were consistent with the immunostaining patterns of the components (Fig. 1). However, p53 nuclear staining showed wild-type pattern, albeit focally diffuse, in both MBOT and recurrent MOC.
KRAS and CDKN2A mutations were found in 95% of MBOTs and 80–90% of MOCs, which were not thought to be the key drivers of progression to MOC [6,7,8,9]. In contrast, as key drivers of progression from borderline precursors to invasive MOC, TP53 mutations and copy number alterations have been previously reported by genetic analyses of MOC, including cystadenoma and borderline tumors [9]. TP53 mutation has been reported as the key driver in a previously reported case of a ruptured MBOT with rapid recurrence as an invasive MOC of the contralateral ovary [10]. In addition, a high copy number alteration burden was associated with a worse prognosis in MOC [9]. In our case, the TP53 mutation already existed in MBOT; the only difference was that the recurrent MOC had more copy number alterations and whole-genome doubling. Copy number alterations contribute to carcinogenesis and TP53 mutations have been suggested to be associated with copy number alterations [11]. Given that TP53 mutations increase copy number alterations, borderline tumors with TP53 mutations may be considered cancer-like. Kang et al. reported that TP53 mutation in MBOT was associated with a higher risk of recurrence and overall survival (HR = 4.6, 95% CI 1.5–14.3, p = 0.0087), whereas TP53 mutation in MOC was not associated with overall survival [12]. Our case suggest that increased copy number alterations could contribute to carcinogenesis from MBOT to MOC, and that TP53 mutation may support this.
Immunohistochemical nuclear staining for p53 is usually performed to examine TP53 mutation status since TP53 target sequencing is not routinely clinically accessible. In our case, MBOT was diagnosed with wild-type pattern for p53 nuclear staining because p53 positive cells were focally diffuse with the basal layer of the MBOT, not positive in the superficial cells. Kang et al. reported that the criteria for endometrial carcinoma and high grade serous ovarian carcinoma were not concordant in MBOTs and MOCs to account for terminal differentiation and intratumoral heterogeneity [12, 13]. They suggest that the cases with a minimum of overexpression in 5% of tumor cell nuclei within the basal layer of MBOTs be regarded as abnormal p53 immunohistochemistry pattern, because the cases with a TP53 missense mutation showed overexpression in 5 to 100% of tumor cell nuclei. According to their criteria, MBOT in our case should have been diagnosed with p53 mutation pattern since more than 5% of borderline tumor cells within the basal layer of borderline tumor cells were positive for p53 staining, compared to mucinous cystadenoma component (Fig. 1D).
In conclusion, MBOTs sometimes recur as invasive MOCs. High copy number alterations can contribute to the progression of MBOTs to MOCs, especially if MBOTs have TP53 mutations. For MBOTs with TP53 mutations, careful follow-up may be advisable, given the possibility of recurrence as invasive MOC refractory to chemotherapy.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Morice P, Gouy S, Leary A (2019) Mucinoud ovarian carcinoma. N Engl J Med 380:2356–2266
Kurnit KC, Frumovitx M (2022) Primary mucinous ovarian cancer: options for surgery and chemotherapy. Int J Gynecol Cancer 32:1455–1462
Sherman ME, Mink PJ, Curtis R et al (2004) Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis. Cancer 100:1045–1052
Gorringe KL, Cheasley D, Wakefield MJ et al (2020) Therapeutic options for mucinous ovarian carcinoma. Gynecol Oncol 156:552–560
Hauptmann S, Friedrich K, Redline R et al (2017) Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch 470:125–142
Zeissig MN, Ashwood LM, Kondrashova O et al (2023) Next batter up! Targeting cancers with KRAS-G12D mutations. Trends Cancer 9:955–967
Gemignani ML, Schlaerth AC, Bogomolniy F et al (2003) Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol 90:378–381
Therachiyil L, Anand A, Azmi A et al (2022) Role of RAS signaling in ovarian cancer. F1000Res 11:1253
Cheasley D, Wakefield MJ, Ryland G et al (2019) The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun 10:3935
Yang Y, Guan Y, Xu M et al (2022) Rapid recurrence of a ruptured mucinous borderline ovarian tumor harboring. Heliyon 8:e10877
Steele CD, Abbasi A, Ismam SMA et al (2022) Signatures of copy number alterations in human cancer. Nature 606:984–991
Kang EY, Cheasley D, LePage C et al (2021) Refined cut-off for TP53 immunohistochemistry improves prediction of TP53 mutation status in ovarian mucinous tumors: implications for outcome analyses. Mod Pathol 34:194–206
Köbel M, Ronnett BM, Singh N et al (2019) Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol 38(Suppl 1):S123–S131
Funding
This research was funded by the Japan Science and Technology Agency (JST): Fusion Oriented REsearch for disruptive Science and Technology (FOREST) Program (JPMJFR215V to N.K.), the Moonshot Research and Development Program (JPMJMS2022 to N.K.), the Japan Agency for Medical Research and Development (AMED): The Core Research for Evolutional Science and Technology (CREST) (grant no. JP22gm1110011 to S.O.) and the Moonshot Research and Development Program (grant no. JP22zf0127009 to S.O.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest related to this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from the participant included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13691_2024_722_MOESM1_ESM.tif
Supplementary file1 Supplementary fig. 1. Images of the initial tumor and the recurrent tumor. (A) The initial tumor, top: T2-weighted magnetic resonance image (MRI), bottom: macroscopic images, (B) The recurrent mucinous carcinoma, top: PET-CT images, bottom left: T2-weighted MRI and bottom right: intraperitoneal image. (TIF 4293 KB)
13691_2024_722_MOESM2_ESM.tif
Supplementary file2 Supplementary fig. 2. The immunostaining findings of the initial tumor and the recurrent tumor. (A-D) The initial mucinous borderline tumor (MBOT) with mucinous cystadenoma. (E-H) The recurrent mucinous carcinoma (MOC). (A, E) PAX8 nuclear staining, (B, F) CDX2 nuclear staining, (C, G) IMP3 cytoplasmic immunostaining, and (D, H) Ki67 nuclear immunostaining. Scale bars: 100μm. (TIF 7049 KB)
About this article
Cite this article
Wakazono, E., Taki, M., Watanabe, K. et al. A case report of mucinous borderline ovarian tumor with recurrence as invasive carcinoma with high copy number alterations. Int Canc Conf J (2024). https://doi.org/10.1007/s13691-024-00722-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13691-024-00722-1