Abstract
Purpose of Review
Rates of childhood obesity have been soaring in recent decades. The association between obesity in adulthood and excess morbidity and mortality has been readily established, whereas the association of childhood and adolescent obesity has not. The purpose of this review is to summarize existing data regarding the association of the presence of obesity in childhood/adolescence and early-onset adverse outcomes in adulthood, with specific focus on young adults under the age of 45 years.
Recent Findings
Diabetes, cancer, and cardiometabolic outcomes in midlife are closely linked to childhood and adolescent obesity.
Summary
Childhood and adolescent obesity confer major risks of excess and premature morbidity and mortality, which may be evident before age 30 years in both sexes. The scientific literature is mixed regarding the independent risk of illness, which may be attributed to childhood BMI regardless of adult BMI, and additional data is required to establish causality between the two. Nonetheless, the increasing prevalence of childhood and adolescent obesity may impose an increase of disease burden in midlife, emphasizing the need for effective interventions to be implemented at a young age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of childhood obesity has been soaring in the past three decades, with recent data showing a persistent upward trend [1]. The United States Centers for Disease Control and Prevention (CDC) estimates that more than one in five American teenagers live with obesity in 2020 [2]. In adults, obesity is a known major predisposing factor for cardiovascular morbidity and mortality, diabetes, hypertension, cancer, and other unfavorable cardiometabolic alterations [3]. While there is solid evidence linking obesity with adverse outcomes in adults, data regarding the consequences of adolescent obesity on development of disease in young adulthood are limited. Childhood obesity is associated with numerous complications and comorbidities; with its rising prevalence, diseases traditionally surfacing only in adulthood are becoming increasingly common early in life.
The presentation of some diseases at earlier ages is associated with a higher propensity toward complications and excess mortality, as is exemplified in the case of type 2 diabetes, which presents with a more aggressive clinical course compared to those who develop the disease later in life [4]. The importance of this issue lies in its detrimental implications on young adults of the working age population, affecting individual, economic, social, and public health matters. It is therefore of clinical and public health interest to recognize the pivotal role of childhood obesity as a major driver of excess disease as early as midlife. Therefore, the focus of this review is the association between childhood and adolescent obesity and morbidity such as diabetes, cancer, and cardiovascular disease in early adulthood (< 45 years of age).
Methods
We searched for studies that addressed the association between childhood obesity and morbidity in early adulthood. For the major outcomes, we used PubMed to find relevant articles published through December 2020. Keywords were used to identify appropriate titles regarding obesity (“Obesity,” “Obese,” “BMI,” “Body mass index,” “Fatness,” or “Adiposity”), adolescents (“Adolescent,” “Adolescence,” “Childhood,” or “Pediatric”), and outcomes of interest (“Cancer,” “Diabetes,” “Type 2 diabetes,” “Coronary artery disease,” “Coronary heart disease,” “Congestive heart failure,” “Heart failure,” “Cardiomyopathy,” “Hypertension,” “Blood pressure,” “Cardiovascular,” or “Cardiometabolic”). We limited the search to manuscripts in English and relating to humans. Additional articles were added by manually searching through the references of relevant articles and publication records of established research groups.
We included longitudinal and cross-sectional studies in which data on childhood or adolescent body mass index (BMI) were available. We excluded studies that included individuals with cancer, diabetes, or a relevant chronic illness at baseline, as well as individuals who were prescribed a chronic medical treatment at baseline.
The inclusion of studies in this review was not limited to a specific definition of obesity and there was considerable variation in the classification of obesity. BMI was calculated using CDC, International Obesity Task Force (IOTF) percentile charts, or the World Health Organization (WHO) BMI tables. Childhood overweight and obesity were defined as within the following criterion (IOTF percentiles derived from WHO adult cutoffs of ≥ 25 kg/m2 for overweight and ≥ 30 kg/m2 for obesity; CDC: ≥ 85th percentile for overweight, ≥ 95th percentile for obesity). We also included articles that classified BMI groups according to z-scores.
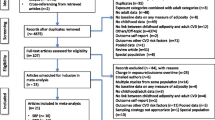
Two authors (AH and AMT) independently screened the titles and abstracts, if available, of all results from the search. Full manuscripts of relevant reports were retrieved. The same reviewers independently assessed the full manuscripts. Disagreements were discussed and resolved by consensus. Fig. 1 shows the selection process according to the PRISMA statement [5].
One author (AH) extracted data from each study using a pre-specified table. The extracted information included, but was not limited to, age at baseline, male-to-female ratio, length of follow-up, outcome, number of cases, effect estimates, and BMI classification.
Results
Diabetes
Three decades ago, type 2 diabetes was thought of as a disease confined solely to adults [6]. Paralleling obesity trends, the global prevalence of diabetes is expected to rise by 51%, from 463 to 700 million by 2045 [7].
A recent longitudinal study [8•] analyzed the association between adolescent BMI and incident type 2 diabetes in a nationwide sample of 1.46 million Israeli adolescents. Of these, 2177 were diagnosed with type 2 diabetes at a mean age of 28 years. The population attributable risk (PAR%) for type 2 diabetes related to adolescent overweight and obesity was 57% for men and 61% for women, emphasizing the importance of abnormally high BMI in adolescence. The point estimates ranged from 6- to 26-fold increased risk for type 2 diabetes among men and from 10- to 44-fold risk among women with overweight or severe obesity, respectively (Fig. 2). Another longitudinal study that followed healthy children of varying BMI values for development of diabetes before age 20 reported a relative risk of 5.7 (95% CI: 1.9–17.2) in boys and 2.3 (95% CI: 0.9–5.8) in girls with a BMI between 100 and 120% of the 95th percentile compared to those of normal weight [9]. With increased severity of obesity, the relative risk increased up to 10- to 18-fold in those with a childhood or adolescent BMI > 140% of the 95th percentile. The relative risks for the same cohort to develop diabetes before age 45 showed a similar trend relating to BMI, though with lower point estimates. A large scale Danish cohort study reached similar conclusions, with hazard ratios (HRs) for early adulthood type 2 diabetes ranging from 1.65 (95% CI: 1.47–1.85) to 2.84 (95% CI: 2.31–3.49) per BMI z-score above average among women and from 1.38 (95% CI: 1.25–1.52) to 2.49 (95% CI: 2.07–3.01) among men [10].
Hazard ratios of young-adulthood morbidity in relation to childhood or adolescent obesity. Studies presented in this figure were longitudinal cohort studies with a baseline BMI measurement in childhood or adolescence and a calculated hazard ratio for relevant outcomes. Reference groups (HR = 1) had BMI within the normal range as defined in each study unless stated otherwise. * HR per increase in z-score of BMI in childhood. † reference group BMI < 18.50 kg/m2. ‡ HR shown for BMI 30–35 kg/m2
Obesity confers an excess risk for diabetes that is independent of coexisting metabolic abnormalities [11]. In a cohort of 33,939 young men, an adjusted model showed a 10.6% (95% CI: 1.08–1.12) increase in diabetes risk with every 1-unit increase in BMI, as well as a lower incidence of diabetes among those with obesity compared with normal weight metabolically healthy individuals [11]. Compared to normal weight individuals, young adults with BMI in the obesity range had an excess risk of early-onset type 2 diabetes in subsequent years despite normoglycemia at baseline, with point estimates ranging from 3.42 to 8.29 [12]. It has been reported that childhood BMI alone was as good a predictor of midlife type 2 diabetes as other cardiometabolic outcomes compared with pediatric metabolic syndrome [13], suggesting an independent risk conferred by elevated BMI.
Cross-sectional studies provided confirmatory evidence of the importance of the degree of obesity severity [14]. There was an inverse linear relationship between the degree of obesity and the age of diabetes onset-adolescent BMI of 39 kg/m2 was associated with type 2 diabetes onset before age 45, compared with a BMI of 33 kg/m2 for disease diagnosed after age 45. Similarly, prevalence of type 2 diabetes among adolescents rises with increasing severity of obesity [15]. A study that characterized the longitudinal BMI profiles of children into adulthood indicated that BMI trajectories in adolescence had a significant positive association with young adult diabetes and hyperglycemia (measured at a mean age of 33–35 years) [16]. These findings suggest that the association between early-life BMI and adult diabetes may not be strictly due to BMI levels but also relate to patterns of weight change during these critical years.
The question whether childhood obesity confers a risk for early-onset diabetes that is independent of adult BMI is a point of debate in the literature. Based on a cohort of men awaiting military draft, Tirosh et al. [17] reported that adolescent BMI was predictive of type 2 diabetes (HR 2.76, 95% CI: 2.11–3.58; mean age of type 2 diabetes onset, 37 years), but when adjusted for BMI in adulthood, this effect was attenuated. These findings were confirmed in several American and European cohorts; in an analysis [18] that included over 6000 children with over 20 years of follow-up from Bogalusa, Muscatine, Childhood Determinants of Adult Health, and Young Finns Study cohorts, the risk of normal weight adults who had obesity as children was comparable to those with no history of obesity. A population-based study from Denmark reported that remission of overweight and obesity before age 13 normalized diabetes risk [19], while those who remained in the overweight category after this age retained an increased risk of type 2 diabetes. On the contrary, a US longitudinal study demonstrated that women who developed obesity before age 16 were more likely to have type 2 diabetes before age 30 compared with those who developed obesity at or after age 18 (OR 2.77, 95% CI: 1.39–5.52) [20]. Another Chinese longitudinal study reported an odds ratio of 2.8 (95% CI: 1.2–6.3) for children with obesity who did not have obesity as adults to develop early-onset diabetes, while the odds ratio for those with persistent obesity was 4.3 (95% CI: 2.2–8.1) [21]. Notably, over 95% of persons with incident early-onset diabetes had overweight or obesity at time of diagnosis [8•].
Mendelian randomization (MR) studies have become an important tool to establish causal relations between environmental risk factors and diseases. However, MR studies focused specifically on early-onset type 2 diabetes are limited. MR analysis supported causality between childhood BMI and incident lifetime type 2 diabetes. It was reported that one standard deviation increase in childhood BMI was associated with an 83% increase in risk of type 2 diabetes (odds ratio (OR) 1.83 (95% CI: 1.46–2.30)) [22•]. However, another MR study reported no evidence for causal association between childhood adiposity and fasting glucose or insulin levels [23]. Another large MR study did not demonstrate excess risk of type 2 diabetes among those with obesity as children when an adjustment was made for adult BMI [24]. Causality between childhood BMI and early-onset diabetes was demonstrated through a mediation analysis model that was implemented on the Bogalusa Heart Study cohort [25]. It provided evidence of a one-directional temporal relationship between higher childhood BMI and higher insulin levels, which was more significant among those who developed metabolic syndrome and type 2 diabetes in adulthood.
There is mounting evidence that the pathophysiology of early-onset type 2 diabetes is more aggressive compared to the adult-onset type. A rapid course that is difficult to control [4, 26] as well as reduced adherence to medical treatment [27] contribute to greater glucose dysregulation. Among adolescents with diabetes and obesity who were followed for 20 months, beta cell failure was rapid with a 20% decline annually [28]. In comparison, a 7% yearly reduction in beta cell function was reported in adults with diabetes and obesity [29]. Severe impairment in insulin secretion was also reported in young adolescents after a relatively short duration of diabetes [30]. In concordance, glycemic control was worse among those with an earlier diagnosis of type 2 diabetes (before age 45) compared to those of later diagnosis [26]; the former also had a greater rate of renal complications. In patients with type 1 and early-onset type 2 diabetes (between ages 22 and 25), there was significant excess of mortality among the type 2 diabetes group that occurred after a shorter disease duration and at a younger age [31]. Adolescent obesity was a major predictor of type 2 diabetes-related mortality in midlife, with a 20-fold increased hazard ratio compared to adolescents with normal BMI (HR 20.38, 95% CI: 14.20–29.23) [32] (Fig. 3).
Hazard ratios of young-adulthood mortality in relation to childhood or adolescent obesity. Studies presented in this figure were longitudinal cohort studies with a baseline BMI measurement in childhood or adolescence and a calculated hazard ratio for relevant outcomes in early adulthood. Reference groups (HR = 1) had BMI in the normal range as defined in each study. * HR per increase in z-score of BMI in childhood
The complete list of studies used for the review on diabetes is available in Online Resources 1, 2, 3.
Cancer
The tumor microenvironment is important in the development and growth of cancer, and the obesogenic environment has been suggested to facilitate pro-carcinogenic processes [33]. Adult obesity has been established as an independent causal factor for 12 types of cancer — pharynx, esophageal adenocarcinoma, stomach, pancreas, gallbladder, liver, colorectal, postmenopausal breast, ovarian, endometrial, advanced prostate, and renal cancer [34] and is predicted to replace smoking as the leading modifiable cause of cancer [35].
While there is a strong body of evidence supporting the link between adult obesity and cancer, only a limited portion of studies examined the exposure to an elevated adolescent or childhood BMI. A nationwide Israeli study [36•] analyzed the incidence of approximately 55,000 cases of cancer in a cohort of 2.3 million adolescents with a maximal follow-up length of 45 years. This study provided a comprehensive assessment of the association between adolescent BMI and incident cancer, although lacked smoking data. Baseline BMI was measured at mean age of 17 years and participants were followed for cancer incidence, with a mean age at cancer diagnosis of 43.2 years for men and 40 years for women. Adolescent overweight and obesity conferred HRs of 1.11 (95% CI: 1.02–1.21) and 1.26 (95% CI: 1.18–1.35) for any cancer among men, respectively, and HRs of 1.21 (95% CI: 1.14–1.28) and 1.27 (95% CI: 1.13–1.18) for any cancer among women (apart from cervix and premenopausal breast cancer, with obesity having been postulated as a protective factor for the latter [37, 38]), respectively (Fig. 2). Importantly, these point estimates were almost unaffected by socioeconomic status and were well apparent among adolescents with overweight of both sexes by age 27 years (men, HR = 1.14 (95% CI: 1.01–1.29); women, HR = 1.22 (95% CI: 1.08–1.39)). The projected population attributable risk for cancer for adolescent overweight and obesity was 5.1% (4.2–6.1) for men and 5.7% (4.2–7.3) for women. Cancer-related mortality was also higher in those who had obesity as adolescents (Fig. 3). Strikingly, data in this report demonstrated a significant period effect in the association between adolescent obesity and cancer incidence, thereby raising the possibility that the aforementioned point estimates may be an underestimation of the true risk in today’s population.
Other studies based on the same cohort that analyzed the association between adolescent obesity and specific cancer types reported higher adjusted point estimates for the following cancers: gastroesophageal and esophageal carcinoma, HR 7.60 (95% CI: 2.68–21.5) [39]; male breast cancer, HR 5.08 (95% CI: 2.62–9.86) [40]; renal cell carcinoma, HR 2.43 (95% CI: 1.54–3.83) [41]; pancreatic cancer, HR 3.75 (95% CI: 2.66–5.28) [42]; non-cardia gastric cancer, HR 1.78 (95% CI: 1.12–2.83) [43]; rectal cancer, HR 1.78 (95% CI: 1.21–2.62) [44]; colon cancer, HR 1.53 (95% CI: 1.18–1.98) [44], and non-Hodgkin lymphoma, HR 1.28 (95% CI: 1.04–1.56) [45]. A population-based study in Sweden reported a HR of 1.15 (95% CI: 1.00–1.34) for every one-unit increase in standard deviation scores for adolescent BMI for the development of thyroid malignancy in early adulthood [46]. The combination of these findings suggests a link between early-life obesity and carcinogenesis, an effect that may be more profound in certain cancer types.
The complete list of studies used for the review on cancer is available in Online Resource 4.
Cardiovascular Risk Factors
There is solid evidence that childhood and adolescent obesity are causally linked with early onset of hypertension, coronary artery disease, stroke, and other types of cardiovascular morbidity and mortality in early adulthood [47]. MR studies also support a causal relationship between childhood obesity and dyslipidemia [23]. Wurtz et al. [48] demonstrated a strong association of BMI with multiple cardiometabolic risk factors in young adulthood even below the threshold for obesity, emphasizing the causality of BMI in the progression of disease.
In several cohorts, adolescent BMI was suggested as a principal risk factor for dysmetabolism in young adulthood [49, 50]. Childhood obesity between 3 and 9 years of age was associated with an OR of 3.0 (95% CI: 2.0–4.7) to develop metabolic syndrome in early adulthood, while adolescent obesity between 12 and 18 years of age yielded an OR of 2.1 (95% CI: 1.4–3.1) [49]. In other cohorts addressing childhood obesity, elevated BMI was associated with an increased adult BMI, waist-to-hip circumference, ankle-brachial index, total cholesterol, LDL cholesterol, insulin and insulin resistance, as well as blood pressure measurements in adulthood compared with children of normal weight [51, 52].
Hypertension
Hypertension is the most common comorbidity associated with adolescent overweight and obesity [53]. Juonala et al. [18] demonstrated, in a study that included four cohorts, that children with overweight and obesity had a relative risk of 1.8 (95% CI: 1.5–1.2) to develop hypertension before age 46. The correlation between BMI and blood pressure is strongest among those in the overweight and obesity BMI ranges [54]. In a cross-sectional study, Skinner et al. [15] demonstrated a dose-dependent relationship between the degree of obesity and prevalence of hypertension, reaching a prevalence of 11.1% among children with class III obesity aged 3 to 19 years. The latter was confirmed for the adolescent population elsewhere [55], with the prevalence of blood pressure in the hypertensive ranges increasing from 22.1 among overweight males to 42.4% in males with class III obesity, and from 19.2 to 43.9% among females. The correlation coefficient between childhood BMI and systolic blood pressure is estimated to be 0.34 [56].
Increasing BMI deciles were significantly associated with increasing values of both systolic and diastolic blood pressure in both men and women in longitudinal and cross-sectional studies [57, 58], with an OR of 1.108 (95% CI: 1.107–1.110) to develop systolic hypertension (systolic blood pressure > 130 mmHg) in males and 1.114 (95% CI: 1.139–1.146) in females for every 1-unit increase in BMI [58].
Of note, the risk for hypertension attributed to adolescent obesity may depend on adult BMI. A pooled analysis of three British cohorts [59] used childhood and adult BMI data and showed that the odds ratio for hypertension in early adulthood (age 34–53 years) was comparable among those who were persistently in the overweight category (OR 2.56; 95% CI: 1.40–4.68) and those with overweight only in adulthood (OR 2.28; 95% CI: 1.76–2.95). Overweight confined to childhood or adolescence was not associated with increased odds of hypertension in adulthood after considering adult BMI. A Taiwanese study that followed children with baseline BMI measurements up to early adulthood found an OR of 6.51 (95% CI: 3.36–12.36) for children and young adults with persistent obesity for early onset of hypertension, while the OR for hypertension among children with obesity who had normal adulthood BMI was reduced to 2.23 (95% CI: 0.89–5.58) [60]. Similarly, Israeli men and women who had obesity as children had a HR of 3.65 (95% CI: 2.87–4.63) and 7.23 (95% CI: 2.68–19.51), respectively, which were reduced to 2.59 (95% CI: 2.02–3.33) and 4.81 (1.13–20.53) respectively after adjustment for adulthood BMI was made [61] (Fig. 2). MR studies are scarce but report no causality between elevated childhood BMI and early-onset adult hypertension [23].
In a cohort of 2.3 million adolescents with a mean age of 17.3 years and a mean follow-up of 18 years, hazard ratios for hypertensive heart disease mortality were 1.9, 4.1, and 8.0 for high normal, overweight, and obesity BMI categories, respectively [62].
Coronary Artery Disease
The excess risk for coronary artery disease that is imposed by obesity is independent of metabolic abnormalities; Twig et al. [63] demonstrated that among metabolically healthy young men with obesity, there was a threefold risk of incident coronary artery disease compared to normal weight metabolically healthy individuals in their late 20s to early 30s.
There is evidence supporting the notion that childhood and adolescent BMI may be predictive of coronary and cardiovascular morbidity regardless of adult BMI. A study from New Zealand with periodic weight and height measurements from age 3 to 38 years assessed endothelial dysfunction at end of follow-up using peripheral arterial tonometry [64]. Individuals with overweight had a higher degree of endothelial dysfunction than those with normal weight, and individuals with obesity had even worse values. BMI at age 3 years was already predictive of endothelial dysfunction at age 38, with children with one standard deviation elevation in BMI having a greater extent of disease. This effect was only partly attenuated after adjustment for BMI and cardiovascular factors at age 38 years. Another 23-year longitudinal study showed significant association between childhood BMI and BMI trajectory with intima-media thickness, indicating that subclinical cardiovascular disease might be rooted in childhood [65].
Notably, data from four major longitudinal cohorts was somewhat contradictive; the predictability of early adulthood intima-media thickness was significant only after the age of 9 [66]. In another Chinese longitudinal study, the adjustment to adulthood BMI attenuated the positive association between childhood BMI and intima-media thickness in young adulthood, rendering it insignificant [67].
A study on Danish and Finnish cohorts found an independent, strongly positive association between BMI at age 7 and coronary artery disease in adulthood [68]. In a cohort study of 37,000 career army personnel, Tirosh et al. showed that adolescent obesity was associated with incident angiography-proven coronary heart disease later in life independently of adult BMI [17] (Fig. 2). They reported a hazard ratio of 7.0 for incident coronary disease among adolescents who were in the upper BMI decile versus those at the lower decile and suggested that early exposure to obesity early in life may gradually promote coronary atherosclerosis. Juonala et al. [18] reported a relative risk of 1.7 (95% CI: 1.4–2.2) for individuals with consistent obesity throughout childhood and adulthood, but this risk was diminished in the assessment of individuals who had obesity at childhood, but a normal BMI at adulthood. Among middle-aged Swedish men, those with BMI ≥ 30kg/m2 in adolescence had an adjusted HR of 3.1 (95% CI: 2.2–4.4) for coronary artery disease [69].
An MR study reported a 28% increase in risk of coronary artery disease with every increase in one standard deviation in childhood BMI (kg/m2), essentially proving causality between the two variables [22•]. Another MR analysis found a 7% increase in OR for CAD with every 1-unit increase in the log-odds of having childhood obesity [70].
Coronary heart disease-related mortality has also been described in relation to childhood BMI (Fig. 3). Must et al. [71] reported a relative risk of 2.3 (95% CI: 1.4–4.1) in male adolescents with overweight for coronary-related mortality by late adulthood (usually after 7th decade of life). These findings were confirmed by a Danish study [72] that showed BMI at age 7 predicted coronary-related mortality, with increasing HRs until age 13 years. The hazard ratio among girls, for example, showed a graded increase from 1.10 to 1.23 for every unit increment in BMI z-score, with similar estimates among boys. Applying the same analytical approach, a nationwide Israeli study reported an adjusted HR of 1.54 (95% CI: 1.46–1.62), consistent with a stronger association with BMI in later adolescence than in earlier childhood [73•]. The latter study showed that adolescent obesity conferred a HR of 4.9 for coronary artery disease (95% CI: 3.9–6.1; low normal BMI was the reference group). Adolescents with obesity had a HR of 3.5 (95% CI: 2.9–4.1) for cardiovascular mortality (a composite outcome including coronary, stroke, and sudden death) and there was a twofold risk for cardiovascular mortality before age 30 years. The population attributable risk for coronary-related and for total cardiovascular mortality among those who had overweight and obesity as adolescents was 27.4% and 20.4%, respectively. Longitudinal studies performed on a similar cohort described a hazard ratio for cardiovascular mortality ranging from 5.4 (95% CI: 4.60–6.33) in men to 3.9 (95% CI: 2.47–6.14) in women with adolescent BMI in the obese range [62, 74].
Heart Failure
The rising global rates of obesity are paralleled by a concomitant increase in incidence of heart failure among young adults under 45 years of age [1, 75, 76].
In a study that followed 2.3 million adolescents, there were 94 deaths due to heart failure after a mean follow-up of 18.4 years, with a hazard ratio of 5.43 for death due to heart failure among individuals with obesity at baseline [62]. In another study [77], individuals with a BMI of 30–35 kg/m2 at age 18 had a HR of 6.47 (5.39–7.77) for heart failure development at an average age of 44.5 years (Fig. 2). Those with a BMI over 35 kg/m2 had a HR of 9.21 (6.57–12.92) and developed the disease at a slightly younger age of 42.8 years. The PAR% for obesity in heart failure was 24%.
Cardiomyopathy
The prevalence of cardiomyopathy among young adults doubled over the end of the previous century and the beginning of this one [75], affecting 20% of heart failure patients under the age of 45 years. Individuals with a baseline BMI in the obesity range had a 2.26 (95% CI: 1.22–4.17) and 6.86 (95% CI: 2.93–16.02) HR for BMI group 30–35 and > 35 kg/m2, respectively, to develop heart failure with concomitant cardiomyopathy in early adulthood [77]. Another study performed on the same cohort affirmed the strong association between increasing BMI and cardiomyopathy, especially the dilated form, with an 8-fold increased risk among those with BMI > 35 kg/m2 versus teenagers with BMI in the normal range [78] (Fig. 2). Changes in myocardial function and geometry are already evident among children with obesity, indicating that the unfavorable effects on cardiac remodeling are early and may be independent of ischemic heart disease [79].
The complete list of studies used for the review on cardiometabolic disease is available in Online Resources 5, 6, 7.
Other Outcomes
Apparently healthy adolescents with obesity are more prone to early-adulthood mortality related to kidney disease [80], with an estimated hazard ratio of 8.4 (95% CI: 5.1–13.8) compared to adolescents with normal weight. Adolescent BMI has also been linked to excess mortality in relation to infectious diseases, especially among men [81].
The complete list of studies used for the review on other outcomes is available in Online Resource 8.
Conclusion
Obesity during childhood and adolescence is linked to major cardiometabolic morbidity and mortality emerging in early young adulthood. Excess morbidity related to diabetes, certain types of cancers, and a spectrum of cardiovascular diseases before the age of 45 in both men and women has been associated with childhood or adolescent obesity in several Western cohorts. Notably, these studies usually treated adolescent obesity as a single group without considering the severity of obesity. Given the dramatic increase in the prevalence of severe obesity in the recent decade, it is likely that the reported point estimates in this review may be an underestimation of the risk conferred by adolescents with an advanced degree of obesity. The substantial risk of cancer, diabetes, and cardiovascular mortality attributed to excessive BMI in the first two decades of life underscores the importance of obesity as a primary target for intervention aimed to reduce the burden of chronic morbidity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Cockrell Skinner A, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC, Skinner C. Prevalence of obesity and severe obesity in US children. Pediatrics. 1999;141:1–18. https://doi.org/10.1542/peds.2017-4078.
National Center for Health Statistics, National Health and Nutrition Examination Survey. [cited 2020 Dec 2] https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm#table2
Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–65. Mayo Foundation for Medical Education and Research. Available from:. https://doi.org/10.1016/j.mayocp.2016.09.017.
Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. Available from:. https://doi.org/10.1016/S2213-8587(17)30186-9.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–6. Available from:. https://doi.org/10.1136/bmj.b2535.
American KR, Diabetes Association. Type 2 diabetes in children and adolescents: consensus conference report. Diabetes Care. 2000;105:671–80.
International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: https://www.diabetesatlas.org, n.d.
• Twig G, Zucker I, Afek A, Cukierman-Yaffe T, Bendor CD, Derazne E, et al. Adolescent obesity and early-onset type 2 diabetes. Diabetes Care. 2020;43:1487–95 A nationwide study that assessed type 2 diabetes risk for different degrees of adolescent obesity during the third and fourth decades of life. It shows a substantial risk of incident diabetes for both sexes regardless socioeconomic status and other coexisting morbidities.
Tanamas SK, Reddy SP, Chambers MA, Clark EJ, Dunnigan DL, Hanson RL, et al. Effect of severe obesity in childhood and adolescence on risk of type 2 diabetes in youth and early adulthood in an American Indian population. Pediatr Diabetes. 2018;19:622–9.
Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sørensen TIA, Baker JL. Childhood body mass index and development of type 2 diabetes throughout adult life—a large-scale danish cohort study. Obesity. 2017;25:965–71.
Twig G, Afek A, Derazne E, Tzur D, Cukierman-Yaffe T, Gerstein HC, et al. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care. 2014;37:2989–95.
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–62.
Magnussen CG, Koskinen J, Chen W, Thomson R, Schmidt MD, Srinivasan SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:1604–11.
Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes. Diabetes Care. 2001;24:1522–7.
Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373:1307–17.
Zhang T, Xu J, Li S, Bazzano LA, He J, Whelton PK, et al. Trajectories of childhood BMI and adult diabetes : the Bogalusa Heart Study. Diabetologia. 2019;62:70–7.
Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;55:296–7.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85.
Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. 2018;378:1302–12.
The NS, Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care. 2013;36:865–72.
Liang Y, Hou D, Zhao X, Wang L, Hu Y, Liu J, et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine. 2015;50:87–92. Available from:. https://doi.org/10.1007/s12020-015-0560-7.
• Geng T, Smith CE, Li C, Huang T. Childhood BMI and adult type 2 diabetes, coronary artery diseases, chronic kidney disease, and cardiometabolic traits: a Mendelian randomization analysis. Diabetes Care. 2018;41:dc172141 The first Mendelian randomization study that proves causality between obesity and deleterious cardiovascular metabolic comorbidities.
Viitasalo A, Schnurr TM, Pitkänen N, Hollensted M, Nielsen TRH, Pahkala K, et al. Abdominal adiposity and cardiometabolic risk factors in children and adolescents: a Mendelian randomization analysis. Am J Clin Nutr. 2019;110:1079–87.
Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia. 2020;63:2359–71.
Zhang T, Zhang H, Li Y, Li S, Fernandez C, Bazzano L, et al. Long-term impact of temporal sequence from childhood obesity to hyperinsulinemia on adult metabolic syndrome and diabetes: the Bogalusa Heart Study. Sci Rep. 2017;7:1–7.
Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005.
Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–31.
Bacha F. Progressive deterioration of β-cell function in obese youth with type 2 diabetes. Pediatr Diabetes. 2013;23:1–7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf.
Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet Med. 1998;15:297–303.
Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care. 2005;28:638–44.
Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–9.
Twig G, Tirosh A, Leiba A, Levine H, Shor DBA, Derazne E, et al. BMI at age 17 years and diabetes mortality in midlife: a nationwide cohort of 2.3 million adolescents. Diabetes Care. 2016;39:1996–2003.
Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309.
WRCF. Body fatness and weight gain and the risk of cancer. Contin Updat Proj Expert Rep. 2018;2018:3–141 Available from: Available at dietcancerreport.org.
Cancer Research UK. When could overweight and obesity overtake smoking as the biggest cause of cancer in the UK? September, 2018. https://www.cancerresearchuk.org/sites/default/files/obesity_ tobacco_cross_over_report_final.pdf (accessed Dec 2, 2020).
• Furer A, Afek A, Sommer A, Keinan-Boker L, Derazne E, Levi Z, et al. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2·3 million adolescents in Israel. Lancet Diabetes Endocrinol. 2020;8:216–25. https://doi.org/10.1016/S2213-8587(20)30019-XA nationwide study that assessed cancer-specific risk for various groups of adolescent BMI. It shows a substantial risk of incident cancer among adolescents with overweight and obesity that was apparent before age 30 years. Cancer risk for the latter persisted when the study sample was limited to overweight and obese adolescents who had unimpaired health at baseline.
Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7:R314–25.
Keinan-Boker L, Levine H, Derazne E, Molina-Hazan V, Kark JD. Measured adolescent body mass index and adult breast cancer in a cohort of 951,480 women. Breast Cancer Res Treat. 2016;158:157–67.
Levi Z, Kark JD, Shamiss A, Derazne E, Tzur D, Keinan-Boker L, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer. 2013;119:4086–93.
Keinan-Boker L, Levine H, Leiba A, Derazne E, Kark JD. Adolescent obesity and adult male breast cancer in a cohort of 1,382,093 men. Int J Cancer. 2018;142:910–8.
Leiba A, Kark JD, Afek A, Derazne E, Barchana M, Tzur D, et al. Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol. 2013;189:25–9. Available from:. https://doi.org/10.1016/j.juro.2012.08.184.
Zohar L, Rottenberg Y, Twig G, Katz L, Leiba A, Derazne E, et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: a nationwide study of 1.79 million Israeli adolescents. Cancer. 2019;125:118–26.
Levi Z, Kark JD, Twig G, Katz L, Leiba A, Derazne E, et al. Body mass index at adolescence and risk of noncardia gastric cancer in a cohort of 1.79 million men and women. Cancer. 2018;124:356–63.
Levi Z, Kark JD, Katz LH, Twig G, Derazne E, Tzur D, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: a population-based study. Cancer. 2017;123:4022–30.
Leiba M, Leiba A, Keinan-Boker L, Avigdor A, Derazne E, Levine H, et al. Adolescent weight and height are predictors of specific non-Hodgkin lymphoma subtypes among a cohort of 2,352,988 individuals aged 16 to 19 years. Cancer. 2016;122:1068–77.
Kitahara CM, Gamborg M, De González AB, Sørensen TIA, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014;74:235–42.
Sommer A, Twig G. The impact of childhood and adolescent obesity on cardiovascular risk in adulthood: a systematic review. Curr Diab Rep. 2018;18:91.
Würtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765.
Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The Cardiovascular Risk in Young Finns Study. Ann Med. 2008;40:542–52.
Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton lipid research clinics follow-up study. Pediatrics. 2007;120:340–5.
Sundaram MES, Berg RL, Economos C, Coleman LA. The relationship between childhood BMI and adult serum cholesterol, LDL, And ankle brachial index. Clin Med Res. 2014;12:33–9.
Rademacher ER, Jacobs DR, Moran A, Steinberger J, Prineas RJ, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. J Hypertens. 2009;27:1766–74.
Kelly RK, Magnussen CG, Sabin MA, Cheung M, Juonala M. Development of hypertension in overweight adolescents: a review. Adolesc Health Med Ther. 2015;6:171–87.
Movahed MR, Bates S, Strootman D, Sattur S. Obesity in adolescence is associated with left ventricular hypertrophy and hypertension. Echocardiography. 2011;28:150–3.
Twig G, Reichman B, Afek A, Derazne E, Hamiel U, Furer A, et al. Severe obesity and cardio-metabolic comorbidities: a nationwide study of 2.8 million adolescents. Int J Obes. 2019;43:1391–9. Available from:. https://doi.org/10.1038/s41366-018-0213-z.
Pereira PF, Serrano HMS, Carvalho GQ, Lamounier JA, Peluzio MDCG, Franceschini SDCC, et al. Body fat location and cardiovascular disease risk factors in overweight female adolescents and eutrophic female adolescents with a high percentage of body fat. Cardiol Young. 2012;22:162–9.
Sabo RT, Lu Z, Daniels S, SSS. Serial childhood body mass index and associations with adult hypertension and obesity: the Fels Longitudinal Study. Physiol Behav. 2017;176:139–48.
Chorin E, Hassidim A, Hartal M, Havakuk O, Flint N, Ziv-Baran T, et al. Trends in adolescents obesity and the association between BMI and blood pressure: a cross-sectional study in 714,922 healthy teenagers. Am J Hypertens. 2015;28:1157–63.
Park MH, Sovio U, Viner RM, Hardy RJ, Kinra S. Overweight in childhood, adolescence and adulthood and cardiovascular risk in later life: pooled analysis of three British birth cohorts. PLoS One. 2013;8:3–8.
Su TC, Liao CC, Chien KL, Hsu SHJ, Sung FC. An overweight or obese status in childhood predicts subclinical atherosclerosis and prehypertension/hypertension in young adults. J Atheroscler Thromb. 2014;21:1170–82.
Tirosh A, Afek A, Rudich A, Percik R, Gordon B, Ayalon N, et al. Progression of normotensive adolescents to hypertensive adults a study of 26 980 teenagers. Hypertension. 2010;56:203–9.
Twig G, Ben-Ami Shor D, Furer A, Levine H, Derazne E, Goldberger N, et al. Adolescent body mass index and cardiovascular disease-specific mortality by midlife. J Clin Endocrinol Metab. 2017;102:3011–20.
Twig G, Gerstein HC, Shor DBA, Derazne E, Tzur D, Afek A, et al. Coronary artery disease risk among obese metabolically healthy young men. Eur J Endocrinol. 2015;173:305–12.
Williams MJA, Milne BJ, Ambler A, Theodore R, Ramrakha S, Caspi A, et al. Childhood body mass index and endothelial dysfunction evaluated by peripheral arterial tonometry in early midlife. Int J Obes. 2017;41:1355–60.
Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Body mass index trajectories in childhood is predictive of cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart study. Int J Obes. 2018;42:923–5.
Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood. Circulation. 2010;122:2514–20.
Yan Y, Hou D, Liu J, Zhao X, Cheng H, Xi B, et al. Childhood body mass index and blood pressure in prediction of subclinical vascular damage in adulthood: Beijing blood pressure cohort. J Hypertens. 2017;35:47–54.
Andersen LG, Ängquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5:20–2.
Falkstedt D, Hemmingsson T, Rasmussen F, Lundberg I. Body mass index in late adolescence and its association with coronary heart disease and stroke in middle age among Swedish men. Int J Obes. 2007;31:777–83.
Fang X, Zuo J, Zhou J, Cai J, Chen C, Xiang E, et al. Childhood obesity leads to adult type 2 diabetes and coronary artery diseases: a 2-sample Mendelian randomization study. Med (United States). 2019;98:e16825.
Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. N Engl J Med. 1992;326:653–7.
Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007:687–96.
• Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374:2430–40 A nationwide study that assessed the association between adolescent BMI and midlife mortality. It showed that the risk of mortality due to cardiovascular disease was nearly 2- and 4-fold among adolescents with overweight and obesity respectively and was evident before age 30 years. The mortality risk for the latter persisted with extensive sensitivity analyses including limiting the study sample to overweight and obese adolescents who had unimpaired health at baseline.
Furer A, Afek A, Orr O, Gershovitz L, Landau Rabbi M, Derazne E, et al. Sex-specific associations between adolescent categories of BMI with cardiovascular and non-cardiovascular mortality in midlife. Cardiovasc Diabetol. 2018;17:1–10. Available from:. https://doi.org/10.1186/s12933-018-0727-7.
Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35:25–32.
Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133:639–49.
Rosengren A, Åberg M, Robertson J, Waern M, Schaufelberger M, Kuhn G, et al. Body weight in adolescence and long-term risk of early heart failure in adulthood among men in Sweden. Eur Heart J. 2017;38:1926–33.
Robertson J, Schaufelberger M, Lindgren M, Adiels M, Schiöler L, Torén K, et al. Higher body mass index in adolescence predicts cardiomyopathy risk in midlife: long-term follow-up among Swedish men. Circulation. 2019;140:117–25.
Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, et al. Childhood obesity: impact on cardiac geometry and function. JACC Cardiovasc Imaging. 2014;7:1198–205.
Twig G, Vivante A, Bader T, Derazne E, Tsur AM, Levi M, et al. Body mass index and kidney disease-related mortality in midlife: a nationwide cohort of 2.3 million adolescents. Obesity. 2018;26:776–81.
Twig G, Geva N, Levine H, Derazne E, Goldberger N, Haklai Z, et al. Body mass index and infectious disease mortality in midlife in a cohort of 2.3 million adolescents. Int J Obes. 2018;42:801–7. Available from:. https://doi.org/10.1038/ijo.2017.263.
Acknowledgements
This review is dedicated to prof. Arnon Afek for his continuous support of medical research in the Israel Defense Forces by facilitating seminal medical data linkage between various governmental agencies in Israel, making key longitudinal studies on adolescent obesity possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Adi Horesh, Avishai M. Tsur, Aya Bardugo, and Gilad Twig declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Childhood Obesity
Supplementary Information
ESM 1
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Horesh, A., Tsur, A.M., Bardugo, A. et al. Adolescent and Childhood Obesity and Excess Morbidity and Mortality in Young Adulthood—a Systematic Review. Curr Obes Rep 10, 301–310 (2021). https://doi.org/10.1007/s13679-021-00439-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-021-00439-9