Abstract
Purpose of Review
The purpose of this article is to review clinical trials evaluating the efficacy and safety of IL-23 inhibitors in the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis.
Recent Findings
Guselkumab, tildrakizumab, and risankizumab have been approved for the treatment of psoriasis. Mirikizumab is still under investigation in phase 3 studies. IL-23 inhibitors show excellent efficacy and outperform other biologic therapies in multiple head-to-head trials. Network meta-analyses have demonstrated that IL-23 inhibitors have superior long-term efficacy, while IL-17 inhibitors have superior short-term efficacy. Guselkumab is the only IL-23 inhibitor approved for use in patients with psoriatic arthritis. Risankizumab and tildrakizumab are currently under investigation for psoriatic arthritis.
Summary
Phase 3 clinical studies have shown excellent efficacy and safety of IL-23 inhibitors in the treatment of psoriasis. While IL-17 inhibitors have faster onset of action, IL-23 inhibitors are more effective long-term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory skin disease that affects approximately 120 million people worldwide [1]. While psoriasis predominantly affects the skin, it is a systemic inflammatory disease, comorbidities of which include rheumatologic, cardiovascular, psychological, and gastrointestinal diseases [1]. Biologic therapy is used for the treatment of moderate and severe psoriasis, which cover 3–10% and greater than 10% of the body’s surface area, respectively [1].
Researchers have explored multiple immune-regulated pathways to identify the pathogenesis of psoriasis. The interleukin-23 (IL-23)-mediated activation of the Th-17 cells is recognized as the dominant pathway [2]. IL-23 is a proinflammatory multicomplex cytokine that consists of a p19 subunit unique to itself and a p40 subunit that it shares with interleukin-12 (IL-12) [3]. IL-23 maintains and increases Th-17 cell populations, which in turn release additional cytokines, including interleukin-17 (IL-17). Together, these cytokines cause epidermal hyperplasia and keratinocyte proliferation [3].
In 2009, Ustekinumab, a fully human monoclonal antibody, which targets the shared IL-12/23 p40 subunit, was approved for the treatment of psoriasis [4]. Ustekinumab has good efficacy [5] and is superior to earlier biologic agents, including etanercept, an inhibitor of tumor necrosis factor alpha (TNF-ɑ) [6]. However, new studies discovered that IL-12 has a protective role in limiting skin inflammation [7], immune defense [8], and antitumor activity [9]. There was evidence that IL-23 plays a stronger role in the pathogenesis of psoriasis [8], and preclinical studies suggested selective blockade of IL-23 would have more efficacy and fewer risks than combined blockade of IL-12/23 in patients with psoriasis.
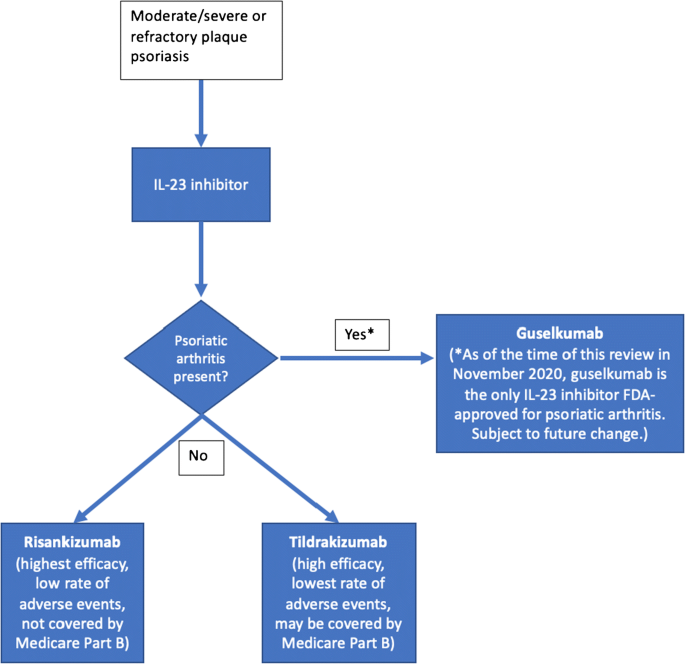
Biologic agents that bind to the p19 subunit specific to IL-23 have since been developed for psoriasis. In this review, we discuss the relevant clinical data for three FDA-approved IL23 inhibitors (guselkumab, tildrakizumab, risankizumab) and one unapproved IL-23 inhibitor (mirikizumab). We highlight the pertinent phase 2 and 3 efficacy and safety data of these IL-23 inhibitors in patients with psoriasis, emphasizing head-to-head comparison trials (Table 1). We also include analyses of IL-23 inhibitor use in patients with psoriatic arthritis (Table 2). Furthermore, we provide an algorithm describing how to select drugs within the IL-23 inhibitor class (Fig. 1).
Guselkumab
Guselkumab is a fully human IgG1 lambda monoclonal antibody that binds to the p19 subunit of IL-23 and inhibits the intracellular and downstream signaling of IL-23 [10]. It is indicated for moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy and active psoriatic arthritis. Dosage form comes in an injectable solution in a single-dose, prefilled syringe. For both psoriasis and psoriatic arthritis, it is administered 100 mg subcutaneously at week 0, week 4, and every 8 weeks thereafter [11]. Guselkumab may be administered alone or in combination with disease-modifying anti-rheumatic drugs (DMARDs) in adult patients with psoriatic arthritis.
Guselkumab has been evaluated in several phase 3, randomized, multicenter, double-blind, placebo- and comparator-controlled studies. Early studies such as VOYAGE I and II compared the efficacy and safety of guselkumab with adalimumab and placebo in patients with moderate-to-severe psoriasis, including interrupted treatment and switching adalimumab nonresponders to guselkumab [10]. NAVIGATE evaluated the efficacy of guselkumab in patients who had an inadequate response to ustekinumab [12]. The primary endpoints for these three studies were the proportion of patients achieving an investigator’s global assessment (IGA) score of cleared/minimal (0/1) and psoriasis area and severity index (PASI) 90 at week 16 compared with the guselkumab and placebo groups. VOYAGE I and II concluded that guselkumab showed greater efficacy compared with adalimumab in patients with psoriasis through 1 year (IGA 0/1: 95.1% vs. 65.9% [p < .001]) and that the adalimumab nonresponders benefited significantly from switching to guselkumab by week 16 (IGA 0/1: 84.1% vs. 67.7% [p < .001]). NAVIGATE also demonstrated that ustekinumab nonresponders benefited greatly from switching to guselkumab by week 16 (IGA 0/1 42 vs. 19%).
The phase 3 ECLIPSE study compared the efficacies of guselkumab and secukinumab, an IL-17A inhibitor, in patients with moderate-to-severe plaque psoriasis [13]. One thousand forty-eight patients were randomized 1:1 to receive guselkumab or secukinumab at FDA-approved dosing. The primary endpoint of the study was the proportion of patients in the intention-to-treat population who achieved PASI 90 at week 48. Guselkumab showed long-term efficacy with a greater proportion of patients achieving PASI 90 at week 48 compared with that of patients receiving secukinumab (84 vs. 70% [p < .0001]). However, superiority was not established for the major secondary endpoint PASI 75 at weeks 12 and 48 (85 vs 80% [p = 0.0616]).
The first phase 2 study to report the efficacy of the novel therapy using guselkumab for the treatment of psoriatic arthritis was conducted in seven countries, including Canada, Germany, Poland, Romania, Russia, Spain, and the USA [14]. Out of 149 patients who showed inadequate response or intolerance to other biologic treatments, 100 were randomly assigned to receive 100 mg guselkumab and 49 received placebo at week 0, 4, and every 8 weeks for 24 weeks. Twenty-nine patients in the placebo group crossed over and received guselkumab at week 24. At week 24, 58% of patients in the guselkumab group vs 18% patients in the placebo group achieved American College of Rheumatology (ACR) 20 [p < .0001]; 34% of patients in the guselkumab group vs 10% of patients in the placebo group achieved ACR 50 [p = 0.0021]; and 14% of patients in the guselkumab group vs 2% of patients in the placebo group achieved ACR70 [p = 0.0228]. Also, dactylitis resolved in 55% of patients treated with guselkumab compared with 17% on placebo [p = .0014] and enthesitis resolved in 57% of patients treated with guselkumab compared with 29% of patients on placebo [p = .0124].
There were several other studies that demonstrated the benefits of guselkumab in treating patients with psoriatic arthritis. The phase 3 study conducted in Japanese patients with moderate to severe plaque-type psoriasis who were assigned to either guselkumab or placebo also assessed the proportion of patients achieving ACR 20, ACR 50, and ACR 70 (improvement from baseline) in a subset of patients with active psoriatic arthritis (PsA) [15]. Out of the 31 patients diagnosed with PsA, 10 patients received placebo, 10 received 50 mg guselkumab, and 11 received 100 mg guselkumab. All of the 3 patients with active PsA in each guselkumab group achieved ACR 20, compared with 0 of the 4 patients with active PsA in the placebo group. Guselkumab treatment groups showed improvements in joint signs and symptoms from week 16 to week 52.
The phase 3 DISCOVER-1 study looked at the effect of guselkumab in patients with active PsA despite standard therapies, such as non-biologic DMARDs, apremilast, and NSAIDs [16]. Three hundred eighty-one patients were divided into 3 study arms: 100 mg guselkumab administered every 4 weeks (n = 128); 100 mg guselkumab administered at week 0, 4, then every 8 weeks (n = 127); and matching placebo (n = 126). The primary endpoint of the study was ACR 20 at week 24. Greater percentage of patients achieved ACR 20 at week 24 in the guselkumab q4w (59.4%) and guselkumab q8w (52.0%) compared with the placebo group (22.2%) [p < .0001]. These results showed significantly improved psoriasis, physical function, and quality of life, and they were consistent amongst patients who did not show adequate response to other tumor necrosis factor inhibitors. The larger DISCOVER-2 study did not suggest that there is a significant difference between guselkumab q4w and q8w dosing regimens in treating the signs and symptoms of PsA [17].
The phase 3 ORION study evaluated the safety and efficacy of the self-administration of guselkumab with the UltraSafe Plus syringe (One-Press) for patients with moderate-to-severe plaque psoriasis [18]. Sixty-two patients received 100 mg guselkumab at weeks 0, 4, 12, 20, and 28, and 16 patients received placebo at weeks 0, 4, and 12 with crossover to 100 mg guselkumab at weeks 16, 20, and 28. The Self-Injection Assessment Questionnaire (SIAQ) was given to assess the One-Press usability and acceptability. The primary endpoint of the study was the proportions of patients achieving IGA 0/1 and PASI 90 responses at week 16. Higher proportions of patients treated with guselkumab achieved IGA 0/1 (80.6 vs. 0% [p < .001]) and PASI 90 (75.8 vs. 0% [p < .001]) compared with those treated with placebo. The study also found that the use of UltraSafe Plus was highly acceptable to patients.
The most recent IXORA-R phase 4 study directly compared the early and complete skin clearance by guselkumab and ixekizumab, an IL-17A inhibitor, in moderate-to-severe plaque psoriasis [19]. Five hundred seven patients were randomly assigned to receive subcutaneous injections of 100 mg guselkumab at weeks 0, 4, and 12. Five hundred twenty patients received 160 mg ixekizumab at week 0, followed by 80 mg every 2 weeks from weeks 2 to 12. To maintain blinding, patients on guselkumab also received one placebo injection at weeks 0, 2, 6, 8, and 10. The primary endpoint of the study was the percentage of patients who achieved PASI 100 at week 12. Major secondary endpoints included the proportion of patients who achieved PASI 50 at week 1, PASI 75 at week 2, and PASI 90 at weeks 4 and 8. Overall, more patients treated with ixekizumab achieved all primary and major secondary endpoints compared with those treated with guselkumab up to week 12. PASI 100 was achieved in 41% of patients in the ixekizumab group vs. 25% of patients in the guselkumab group at week 12 [p < .001].
The most commonly reported adverse events in patients taking guselkumab in the phase 3 clinical trials include nasopharyngitis, upper respiratory infections (URI), injection site erythema, headache, arthralgia, pruritus, and back pain [10, 12, 13, 18]. The types and patterns of adverse events in patients treated with guselkumab were similar to those reported by patients treated with placebo and other comparator drugs throughout the course of the studies. The frequencies of serious infections, malignancies, and major adverse cardiovascular events were low and similar across the treatment groups.
There are several ongoing phase 3 clinical trials of guselkumab further evaluating the efficacy and safety in treatment of moderate-to-severe plaque-type psoriasis (NCT03818035, NCT04080648, NCT03451851, NCT04340076). One study evaluates the use of guselkumab in facial or genital psoriasis (NCT04439526) and in palmoplantar-non-pustular psoriasis (NCT03998683). The effects of guselkumab during pregnancies are also being monitored in the OTIS Autoimmune Diseases in Pregnancy Study for pregnant women who have been treated with either guselkumab or ustekinumab (NCT02103361). Guselkumab is also being considered as a treatment of pityriasis rubra pilaris (NCT03975153). It is also studied in many different gastrointestinal conditions including Crohn’s disease (NCT04397263, NCT03466411), ulcerative colitis (NCT03662542, NCT04033445), and familial adenomatous polyposis (NCT03649971).
Tildrakizumab
Tildrakizumab is a fully humanized monoclonal anti-IL-23 antibody which selectively binds to the p19 subunit, inhibiting IL-23 from interacting with its receptor [20]. It is currently approved by the FDA for treatment of adults with moderate-to-severe plaque psoriasis. The recommended dosing regimen of Tildrakizumab is 100 mg subcutaneous injection at weeks 0 and 4 followed by 100 mg every 12 weeks thereafter. The FDA also states that it should only be administered by a healthcare provider [21].
Tidrakizumab was evaluated for efficacy and safety in 2 three-part, double-blinded, randomized, placebo-controlled phase III trials, reSURFACE 1 and reSURFACE 2. The primary endpoints in both studies were the proportion of patients achieving PASI 75, 90, and 100 at week 12, and the secondary endpoint was a physician’s global assessment (PGA) score of 0/1 at week 12. In reSURFACE 1 tildrakizumab was compared with placebo in a total of 772 patients, and in reSURFACE 2 it was compared with etanercept and placebo in 1090 patients.
reSURFACE 1 established that when compared with placebo, both doses (100 mg and 200 mg) of tildrakizumab led to significantly higher proportions of PASI 75 (64% on 100 mg, 62% on 200 mg, 6% on placebo [p < 0.0001]), PASI 90 (35% on 100 mg, 35% on 200 mg, 3% on placebo [p < 0.0001]), and PASI 100 (14% on 100 mg, 14% on 200 mg and 1% on placebo [p < 0.0001]), as well as achievement of PGA 0/1 (58% on 100 mg, 59% on 200 mg, 7% on placebo [p < 0.0001]) [22].
Additionally, reSURFACE 2 supported these findings with patients on tildrakizumab (100 mg and 200 mg) achieving statistically superior results at week 12 when compared to etanercept and placebo for PASI 75 (61% on 100 mg, 66% on 200 mg, 48% on etanercept, 6% on placebo), PASI 90 (39% on 100 mg, 37% on 200 mg, 21% on etanercept, 4% on placebo) and PASI 100 (12% on 100 mg, 12% on 200 mg, 5% on etanercept, 0% on placebo). When compared with etanercept, treatment with tildrakizumab 200 mg led to a significantly higher proportion of patients achieving PGA 0/1 at week 12 (59% on 200 mg, 48% on etanercept [p < 0.0031]), but tildrakizumab 100 mg was not significantly different in the outcome (55% on 100 mg [p < 0.0663]) [22].
Long-term safety and efficacy of tildrakizumab was evaluated in the extension studies of the original reSURFACE 1 and reSURFACE 2 trials. The primary endpoint was maintained response of PASI 75, 90, and 100 at week 148 in responders (≥ PASI 75 at week 28) and partial responders (PASI 50–75) to tildrakizumab. Additionally, outcomes of partial or non-responders (PASI <50 at week 28) to etanercept who were switched to tildrakizumab were evaluated at week 148. PASI 75, 90, and 100 responses were well maintained through week 148 in both tildrakizumab 100 mg-responders and 200 mg-responders (72.6, 53.8, and 28.9% on 100 mg, 80.2, 59.9, and 32.6% on 200 mg, respectively). Partial responders or nonresponders to etanercept who were switched to tildrakizumab achieved PASI 75, 90, and 100 responses in 66.9, 43.8, and 14.9 of patients at week 148 [23•].
The most common adverse events noted in the 148-week long extension study were nasopharyngitis, upper respiratory tract infections, influenza, bronchitis, and sinusitis. Nasopharyngitis was the most common adverse in all treatment groups occurring at 10.2 events per 100 patient years (PYs) in tildrakizumab 100 mg, 9.8 events per 100 PYs in tidrakizumab 200 mg, 22.4 events 100 PYs in placebo, and 41.1 events per 100 PYs in etanercept. Over the 148-week extension study, there was no increased risk over placebo of severe infections, malignancies, major adverse cardiovascular events, hypersensitivity reactions, nonmelanoma skin cancer, or melanoma skin cancer [23•]. A systematic review and meta-analysis comparing the safety of IL-12/23, IL-23, and IL-17 inhibitors in the treatment of plaque psoriasis found that when compared with placebo, tildrakizumab 100 mg had one of the lowest risks of adverse events related to achieving ≥ PASI 75 and PGA 0/1. The only other biologic that had a slightly lower risk of adverse events related to achieving PGA 0/1 was ustekinumab 45 mg [24].
Further clinical trials investigating tildrakizumab are ongoing. Long-term results from the 5-year extension studies of reSURFACE 1 and reSURFACE 2 have not been reported. A Phase 2b clinical trial (NCT02980692) evaluating safety and efficacy of tildrakizumab for active psoriatic arthritis is complete, but full data has yet to be published. Additionally, 2 phase III randomized, double blind, placebo-controlled studies to evaluate the safety and efficacy of tildrakizumab in psoriatic arthritis are still under research (INSPIRE 1: NCT04314544 and INSPIRE 2: NCT04314531). Tildrakizumab is also currently being investigated for the treatment of nail psoriasis (NCT03897075), scalp psoriasis (NCT03897088), pediatric patients with chronic plaque psoriasis (NCT03997786), bullous pemphigoid (NCT04465292), psoriatic arthritis with concomitant ankylosing spondylitis or non-radiographic axial spondyloarthritis (NCT03552).
Of note, one study evaluated cost-effectiveness between tildrakizumab and other commonly used first line treatments for moderate-to-severe plaque psoriasis, including biologics and apremilast. A model was developed to compare the calculated direct medical costs over 10 years for those undergoing treatment who achieved a PASI 75 response. It showed that tildrakizumab was one of the most cost-effective biologic agents, with only brodalumab and infliximab described as more affordable options [25].
Risankizumab
Risankizumab is a humanized IgG1 monoclonal antibody targeting the p19 subunit of IL-23 approved by the FDA for the treatment of moderate-to-severe plaque psoriasis [26]. The drug is administered at a dose of 150 mg (two 75 mg injections) subcutaneously at week 0, 4, and every 12 weeks after.
The phase 3 clinical trials, ULTIMMA-1 and ULTIMMA-2, demonstrated the excellent efficacy of Risankizumab, where it achieved primary endpoints of PASI 90 at week 16 (75.3 and 74.8% [p < .0001]), static physician’s global assessment (sPGA) 0/1 at week 16 (87.8 and 83.7%), and PASI 90 at week 52 (81.9 and 80.6% [p < .0001]) [27].
Multiple trials have demonstrated risankizumab’s superiority over other biologic agents. In a phase 2 trial conducted in 2017 [28], patients were randomized to receive risankizumab (at a single 18 mg dose at week 0; 90 or 180 mg doses at week 0, 4, and 16) or ustekinumab (45 or 90 mg at weeks 0, 4, and 16). Primary endpoint was PASI 90 at week 12. Investigators analyzed the pooled data of patients on risankizumab (90 mg or 180 mg) or ustekinumab (45 or 90 mg) at week 12. Results showed that 77% of patients on risankizumab achieved PASI 90 at week 12 in comparison to 40% of patients on ustekinumab [p < .001]. Furthermore, risankizumab achieved superior PASI 100 (45 vs. 18% [p < .001]) and sPGA 0/1 (89 vs. 62% [p < .001]) at week 12 in comparison to ustekinumab.
In the IMMvent trial, a randomized, double-blind, active-comparator-controlled phase 3 trial, risankizumab was compared with adalimumab for patients with moderate-to-severe plaque psoriasis [29]. In part A of the trial, patients were either assigned to receive 150 mg risankizumab subcutaneously at week 0 and 4 or 80 mg adalimumab at weeks 1, 3, and 5 and then weekly until week 16. During part B of the study, adalimumab intermediate responders (those who achieved PASI ≥ 50 but < 90) were re-randomized to continue 40 mg adalimumab or switch to 150 mg risankizumab until week 44. Primary endpoints for part A included PASI 90 and sPGA 0/1 at week 16 and for part B, PASI 100 at week 44. During part A, risankizumab demonstrated superior PASI 90 (72% vs. 47% [p<.0001]) and sPGA 0/1 (84% vs. 60% [p < .0001]) at week 16 in comparison to adalimumab. In part B, PASI 100 was achieved in 40% of adalimumab intermediate responders who were re-randomized to risankizumab. Only 7% of patients who continued treatment on adalimumab achieved PASI 100 at week 44 [p < .0001].
In the IMMerge trial, a phase 3 randomized, open-label, efficacy assessor-blinded clinical trial, risankizumab was compared with secukinumab in patients with moderate-to-severe plaque psoriasis [30•]. Here, patients were administered 150 mg of risankizumab at weeks 0, 4, and every 12 weeks after until week 40 or 300 mg of secukinumab at weeks 0, 1, 2, 3, 4, and every 4 weeks after until week 48. Primary endpoints were PASI 90 at week 16 demonstrating noninferiority of risankizumab and PASI 90 at week 52 demonstrating superiority of risankizumab. Both endpoints were achieved as PASI 90 of risankizumab demonstrated noninferiority at week 16 (73.8 vs. 65.6%) and superiority at week 52 (86.6 vs. 57.1% [p < .001]) in comparison to secukinumab.
A new trial has evaluated the efficacy and safety of continuous risankizumab therapy versus treatment withdrawal in patients with plaque psoriasis [31]. This phase 3 randomized trial was divided into two parts. In part A, patients were assigned to receive 150 mg risankizumab or placebo at weeks 0 and 4. All patients received risankizumab at week 16. During part B, at week 28, patients who achieved sPGA 0/1 on risankizumab were re-randomized to continue Risankizumab treatment or receive placebo every 12 weeks until week 88. Primary endpoints for part A included PASI 90 and sPGA 0/1 at week 16. For part B, primary endpoint included sPGA 0/1 at week 52. Of note, secondary endpoints included time to loss of PASI 90 and time to relapse (sPGA ≥ 3). For part A, PASI 90 at week 16 was achieved in 88.7% of patients on risankizumab compared with 7% of patients on placebo while sPGA 0/1 at week 16 was achieved 83.5% of patients on risankizumab compared with 7% of patients on placebo [p < .001]. For part B, sPGA 0/1 at week 52 was achieved in 87.4% of patients on risankizumab compared to 61.3% of patients on placebo [p < .001]. Time to relapse (sPGA ≥ 3) was 295 days and the median time to loss of PASI 90 was 210 days in patients re-randomized to placebo.
The most common adverse reactions experienced with risankizumab use are upper respiratory infections, fatigue, injection site reactions, and tinea infections [27, 29, 30•]. Number and types of adverse events were comparable between risankizumab, placebo, and comparator drugs. The frequency of serious adverse events was also similar across treatment groups.
The use of risankizumab has been explored in other comorbid conditions of psoriasis, including psoriatic arthritis. In the SustaIMM phase 2 and 3 trial conducted in Japan, ACR 20 was measured in 11 patients who developed comorbid psoriatic arthritis during the study [32]. For patients on 75 mg risankizumab, 2/5 patients (40%) achieved ACR 20 at week 16, while 1/3 (33%) of the patients achieved ACR 20 on 150 mg of risankizumab. None of the 3 patients on placebo diagnosed with psoriatic arthritis achieved ACR 20 at week 16.
An additional 4 trials are also exploring the efficacy of risankizumab in patients with psoriatic arthritis (NCT03675308, NCT03671148, NCT02719171). One of these studies (NCT02719171) was a multiarm trial comparing the use of risankizumab versus placebo in patients with active psoriatic arthritis. Primary endpoint for this study was ACR 20 at week 16. In patients who received 150 mg of risankizumab every 4 weeks, 150 mg at weeks 0, 4, and 16, 150 mg at weeks 0 and 12, and 75 mg at week 0, ACR 20 was achieved in 57.1%, 61.9%, 59%, and 65% of patients, respectively. In the placebo arm, ACR 20 was achieved in 35.7% of patients.
Trials are also exploring the use of risankizumab in patients with hidradenitis suppurativa (NCT03926169), palmoplantar pustulosis (NCT04451720), Crohn disease (NCT02513459, NCT02031276, NCT03105128, NCT04524611, NCT03105102m NCT03104413), ulcerative colitis (NCT04254783, NCT03914261, NCT03398148, NCT03398135), asthma (NCT02443298), atopic dermatitis (NCT03706040), and ankylosing spondylitis (NCT02047110).
Mirikizumab
Mirikizumab is a humanized monoclonal IgG4 antibody that targets the p19 subunit of IL-23. Its use is currently being studied in patients with plaque psoriasis. Optimal dosing has not yet been provided by the FDA, but a recent phase 2 clinical study demonstrated that 300 mg of mirikizumab achieved the greatest effect [33].
In this study, patients were randomized in a 1:1:1:1 ratio to receive mirikizumab subcutaneously at 30 mg, 100 mg, 300 mg, or placebo every 8 weeks. Primary endpoint was PASI 90 at week 16. Here, patients on 30 mg, 100 mg, 300 mg, and placebo achieved PASI 90 scores of 29, 59, 67, and 0%, respectively. Secondary endpoints at week 16 included PASI 75, PASI 100, and sPGA 0/1. PASI 75 was achieved in 53, 78, 75, and 4% of patients, respectively. PASI 100 was achieved in 16, 31, 31, and 0%, respectively. Finally, sPGA 0/1 was achieved in 37, 71, 69, and 2% of patients, respectively. Patients experiencing at least one adverse event (51, 47, 47, and 48%, respectively) and rates of serious adverse events (2, 2, 4, and 2%, respectively) were similar across all arms, demonstrating a good safety profile for mirikizumab. The most common adverse event reported in this study were upper respiratory tract infections.
A phase 3 clinical trial comparing the efficacy and safety of mirikizumab to secukinumab and placebo in patients with moderate-to-severe plaque psoriasis is ongoing (OASIS-2: NCT03535194). Primary endpoints of this study are PASI 90 and sPGA 0/1 at week 16. Another study assessing the long-term safety and maintenance of treatment effect in moderate-to-severe psoriasis is also ongoing (OASIS-3: NCT03556202). No preliminary data is available on either of these studies.
Mirikizumab is use is also being investigated in ulcerative colitis (NCT004611, NCT03519945, NCT03518086, NCT03524092, NCT04469062, NCT02589665) and Crohn’s disease (NCT04232553, NCT03926130, NCT02891226).
Comparison of Efficacy and Safety Data of IL-23 Inhibitors to IL-17, IL-12/23, and TNF-ɑ Inhibitors: Network Meta-Analyses
Network meta-analyses are emerging as a new tool allowing for the comparison of multiple treatments simultaneously. Here, treatments are compared using both direct comparisons of interventions within randomized controlled trials and indirect comparisons across trials based on a common comparator [34]. Six such analyses have been performed which compare both the short- and long-term efficacy and safety of the IL-23 inhibitors, IL-17 inhibitors, IL-12/23 inhibitors, and TNF-ɑ inhibitors.
Four studies analyzed short-term (week 12 and week 16) efficacy data across biologics. Bai et al.’s study revealed that ixekizumab 80 mg every 2 weeks ranked the highest in short-term achievement of PASI 75, brodalumab 210 mg ranked highest in short-term achievement of PASI 100, and secukinumab 300 mg ranked highest in achievement of sPGA 0/1, IGA 0/1, and PGA 0/1 [35]. Warren et al. identified that IL-17 biologics demonstrated greater cumulative benefits on PASI 75, 90, and 100 for week 12 compared with the IL-23 biologic agents, tildrakizumab, and guselkumab [36]. A similar analysis conducted by Warren et al. demonstrated that brodalumab, ixekizumab, and secukniumab had higher DLQI 0/1 gains at week 12 compared with other biologic agents [37]. Of note, Warren et al. did not review risankizumab data in either study. Sawyer et al. analyzed 77 trials comparing PASI 50, 75, 90, and 100 at week 12 and 16 and concluded that IL-17 inhibitors, guselkumab, and risankizumab were more efficacious than tildrakizumab, ustekinumab, and all TNF-ɑ inhibitors. In this same study, they found that brodalumab, ixekizumab, and risankizumab were significantly more efficacious than secukinumab [38].
Only one network meta-analysis reviewed long-term efficacy data, including PASI 75, 90, and 100 at week 52 [39•]. This study showed that risankizumab, brodalumab, and guselkumab provide the highest PASI 75, 90, and 100. Risankizumab had the highest probability of achieving complete clearance and was determined to be significantly superior to all therapies except brodalumab and guselkumab, where there was no significant association. Brodalumab and guselkumab were determined to be more efficacious than secukniumab, ustekinumab, adalimumab, and etanercept. Of note, tildrakizumab was not included in this analysis.
In terms of short-term (week 12 and week 16) safety data, risk of experiencing an adverse event was higher in brodalumab, secukinumab, ixekizumab, and ustekinumab compared with placebo. Ixekizumab 80 mg every 4 weeks ranked highest in risk of discontinuation due to adverse event (AE) and guselkumab ranked highest for risk of serious adverse event (SAE) during short-term treatment [35]. A network meta-analysis performed by Afach et al. showed that, when “psoriasis worsening leading to hospitalization” is not included as an SAE, anti-TNF-ɑ agents were associated with higher occurrence of SAEs than placebo [40].
Conclusions
IL-23 inhibitors are the newest biologic therapies available for the treatment of psoriasis. Guselkumab, tildrakizumab, and rizankizumab have been approved for the treatment of moderate-to-severe plaque psoriasis. Mirikizumab is still undergoing testing in phase 3 clinical trials. Phase 3 studies have demonstrated excellent efficacy in IL-23 inhibitors. In multiple comparator trials, IL-23 inhibitors have been demonstrated to be superior to combined IL-12/23 inhibitors and TNF-ɑ inhibiting agents. Some trials, in addition to network meta-analyses, have shown that IL-17 inhibitors have higher short-term efficacy than IL-23 inhibitors, while IL-23 inhibitors achieve superior long-term efficacy. Though additional long-term safety data is needed, current data published on early phase 3 and comparator trials show that IL-23 have good safety profiles. Several studies are now investigating IL-23 inhibitors’ dual role in the treatment of common psoriasis comorbidities, including psoriatic arthritis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. https://doi.org/10.1001/jama.2020.4006.
Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33(5 Suppl 93):2–6 https://pubmed.ncbi.nlm.nih.gov/26472336/. Accessed August 24, 2020.
Di Cesare A, Di Meglio P, Nestle FO. The IL-23Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–50. https://doi.org/10.1038/jid.2009.59.
Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human Interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–92. https://doi.org/10.1056/NEJMoa062382.
Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–84. https://doi.org/10.1016/S0140-6736(08)60726-6.
Griffiths CEM, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of Ustekinumab and Etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–28. https://doi.org/10.1056/NEJMoa0810652.
Kulig P, Musiol S, Freiberger SN, Schreiner B, Gyülveszi G, Russo G, et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016;7(1):1–14. https://doi.org/10.1038/ncomms13466.
Levin AA, Gottlieb AB. Specific targeting of interleukin-23p19 as effective treatment for psoriasis. J Am Acad Dermatol. 2014;70(3):555–61. https://doi.org/10.1016/j.jaad.2013.10.043.
Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22(2):237–46. https://doi.org/10.1038/cdd.2014.134.
Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. https://doi.org/10.1016/j.jaad.2016.11.041.
(No Title). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761061s007lbl.pdf. Accessed September 30, 2020.
Langley RG, Tsai TF, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–23. https://doi.org/10.1111/bjd.15750.
Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–9. https://doi.org/10.1016/S0140-6736(19)31773-8.
Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391(10136):2213–24. https://doi.org/10.1016/S0140-6736(18)30952-8.
Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–62. https://doi.org/10.1111/1346-8138.14504.
Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–25. https://doi.org/10.1016/S0140-6736(20)30265-8.
Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–36. https://doi.org/10.1016/S0140-6736(20)30263-4.
Ferris LK, Ott E, Jiang J, Hong HCH, Li S, Han C, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (one-press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. J Dermatolog Treat. 2020;31(2):152–9. https://doi.org/10.1080/09546634.2019.1587145.
Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–58. https://doi.org/10.1111/bjd.18851.
Sinclair R, Thirthar PV. Tildrakizumab for the treatment of psoriasis. Expert Rev Clin Immunol. 2019;15(1):5–12. https://doi.org/10.1080/1744666X.2019.1544493.
(No Title). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf. Accessed September 30, 2020.
Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–88. https://doi.org/10.1016/S0140-6736(17)31279-5.
• Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182(3):605–17. https://doi.org/10.1111/bjd.18232Importance: This 148-week long extension study presented long-term safety and efficacy data for tildrakizumab. There was no increased risk over placebo of no increased risk over placebo of severe infections, malignancies, major adverse cardiovascular events, or hypersensitivity reactions.
Bilal J, Berlinberg A, Bhattacharjee S, Trost J, Riaz I. Bin, Kurtzman DJB. A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J Dermatolog Treat. 2018;29(6):569–78. https://doi.org/10.1080/09546634.2017.1422591.
Wu JJ, Jia X, Zhao Y, Carrico J, Brodtkorb TH, Mendelsohn A, et al. Comparative cost-effectiveness of tildrakizumab and other commonly used treatments for moderate-to-severe psoriasis. J Dermatolog Treat. 2019:1–8. https://doi.org/10.1080/09546634.2019.1698700.
(No Title). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed September 30, 2020.
Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–61. https://doi.org/10.1016/S0140-6736(18)31713-6.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60. https://doi.org/10.1056/NEJMoa1607017.
Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–86. https://doi.org/10.1016/S0140-6736(19)30952-3.
• Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy–assessor-blinded clinical trial. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.19341Importance: A recent head-to-head trial comparing the efficacy of risankizumab to secukinumab demonstrated that risankizumab, an IL-23 inhibitor, had superior long-term efficacy to secukinumab, an IL-17A inhibitor.
Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous Risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–58. https://doi.org/10.1001/jamadermatol.2020.0723.
Ohtsuki M, Fujita H, Watanabe M, Suzaki K, Flack M, Huang X, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–94. https://doi.org/10.1111/1346-8138.14941.
Reich K, Rich P, Maari C, Bissonnette R, Leonardi C, Menter A, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181(1):88–95. https://doi.org/10.1111/bjd.17628.
Li T, Puhan MA, Vedula SS, Singh S, Dickersin K. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011;9. https://doi.org/10.1186/1741-7015-9-79.
Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:1–25. https://doi.org/10.1155/2019/2546161.
Warren RB, Gooderham M, Burge R, Zhu B, Amato D, Liu KH, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2020;82(5):1138–49. https://doi.org/10.1016/j.jaad.2019.12.038.
Warren RB, See K, Burge R, Zhang Y, Brnabic A, Gallo G, et al. Rapid response of biologic treatments of moderate-to-severe plaque psoriasis: a comprehensive investigation using Bayesian and Frequentist network meta-analyses. Dermatol Ther (Heidelb). 2020;10(1):73–86. https://doi.org/10.1007/s13555-019-00337-y.
Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. PLoS One. 2019;14(8). https://doi.org/10.1371/journal.pone.0220868.
• Yasmeen N, Sawyer LM, Malottki K, Levin LÅ, Didriksen Apol E, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2020. https://doi.org/10.1080/09546634.2020.1743811Importance: This network meta-analysis compared brodalumab, ustekinumab, ixekizumab, risankizumab, secukinumab, etanercept, guselkumab, adalimumab, apremilast, certolizumab, and inflixumab. In particular, the researchers evaluated long-term week 52 efficacy data. Risankizumab demonstrated the most superior long-term efficacy data and had the highest probability of achieving complete clearance.
Afach S, Chaimani A, Evrenoglou T, Penso L, Brouste E, Sbidian E, et al. Meta-analysis results do not reflect the real safety of biologics in psoriasis. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.19244.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Psoriasis
Rights and permissions
About this article
Cite this article
Hadeler, E., Mosca, M., Hong, J. et al. Advancements in Biologic Therapy for Psoriasis: the IL-23 Inhibitors. Curr Derm Rep 10, 6–15 (2021). https://doi.org/10.1007/s13671-020-00325-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-020-00325-y