Abstract
Purpose of Review
Malignant pleural effusions (MPEs) are frequent and are associated with significant morbidity and mortality. This article reviews the most recent literature on the management of MPE.
Recent Findings
With an increasing focus on personalised medicine, the primary treatment aims have changed from the primary aim of recurrence prevention to symptom control and quality of life improvement. Dependent on patient preference, options available to achieve such targets include repeated aspiration, talc pleurodesis (either through a small-bore chest drain or at thoracoscopy), and indwelling pleural catheters (IPCs). There is a consensus regarding the use of IPC in cases of trapped lung and failed talc pleurodesis, but two recent randomised controlled trials have proposed the use of IPCs as first-line therapy.
Conclusion
MPE management should be personalised, considering factors such as predicted survival, performance status, social support, local expertise and, most importantly, patient preference. IPCs are now more widely available as a treatment option and should be explored as a first-line treatment in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
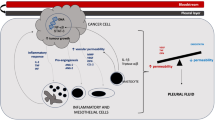
Malignant pleural effusion (MPE) is a common condition associated with significant morbidity and mortality. It is defined as the presence of malignant cells in the pleural space associated with the accumulation of pleural fluid. In the case of known active malignancy associated with pleural effusion but the absence of neoplastic cells in the fluid, it is considered as a paramalignant effusion. Paramalignant pleural effusions can be associated with a variety of conditions (Table 1), but in up to 41% of cases a cause cannot be identified [1] and close follow-up is required to exclude an undiagnosed MPE. Lung cancer is the commonest cause of MPE accounting for 38% of all cases followed by breast cancer (17%) and lymphoma (12%) [2,3,4]. Previous studies estimate that MPE from an unknown primary accounts for 11% of cases [4], but it is likely that this number will fall with the wider use of invasive tests to obtain pleural biopsies and the more recent advances in pathological techniques used for tumour subtyping.
The prevalence of malignancy in the elderly is increasing as patients with chronic diseases live longer and therapies for cancers become more efficient at controlling disease with fewer side effects. The incidence of cancer in patients above 65 years of age has increased 11-fold compared to those younger than 65 [5]. Since the presence of MPE indicates advanced disease, a thorough assessment of elderly patients is required to consider whether, in addition to MPE management strategies, systemic anticancer therapy would be tolerated and warranted. Review by a physician with geriatric oncology interest in elderly patients with cancer has led to a non-oncological intervention in 56% of patients in one study (median age 82 years), most frequently in the domains of nutrition, cognition and mobility [6]. A geriatric assessment should therefore be considered in elderly patients with MPE.
Prognosis and Disease Burden
Retrospective analysis of 126,825 MPE admissions in the USA in 2012 showed the median age to be 68 years (interquartile range 58–77 years) [7]. MPE was associated with a hospital mortality rate of 11.6% and a median hospital stay of 5.5 days. Patients with lowest risk of inpatient mortality were females with history of gynaecological malignancy. This study also showed that hospitalised patients with MPE had a high prevalence of comorbidities including chronic pulmonary disease (29.5%), congestive heart failure (12.9%), weight loss (19.1%) and renal failure (12.6%). There are significant variations in reported survival of MPE reflecting the heterogeneity of this group, but in general, it is a sign of advanced disease. A study of three cohorts (UK, Australia and the Netherlands) showed multiple factors can affect survival in patients with MPE [8]. The highest median survival was observed in mesothelioma (339 days) and the lowest in melanoma (43 days) and urological malignancy (33 days). Lung cancer was associated with a median survival of 74 days and breast cancer 192 days. Based on multivariate analysis, five variables were found to independently affect survival (pleural lactate dehydrogenase LDH, Eastern Cooperative Oncology Group (ECOG) performance score (PS), serum pro-BNP, neutrophil-to-lymphocyte ratio and underlying malignancy type). Interestingly, age was associated with only a modest increased risk of death in the univariate analysis (hazard ratio 1.03, 95% CI 1.01–1.04) but that relationship was insignificant following the multivariate analysis.
Management
Management of MPE is essentially palliative in nature and is aimed at reducing symptoms such as dyspnoea, improving quality of life and reducing hospitalisations. Guidelines generally accept that treatment should be initiated when symptoms develop and/or persist after initial management [4]. It is unclear whether treating asymptomatic MPE is associated with any benefit. Treatment should aim for short-term symptom relief but also target the prevention of recurrence of MPE. Available options include repeated aspiration, chest drain insertion, talc pleurodesis through either slurry (using a chest drain) or poudrage (at thoracoscopy) and indwelling pleural catheter (IPC) insertion. Until recently, evidence was lacking regarding the best treatment options and patient selection for each procedure, but recent well-structured prospective studies have been conducted and the results of these will be reviewed.
Repeated Pleural Aspiration
The choice of repeated therapeutic thoracocentesis should only be employed in patients with poor expected short-term survival. The British Thoracic Society guidelines recommend this approach in patients with predicted survival of less than 1 month [4] although there are no validated tools to predict such outcome. The LENT score (pleural lactate dehydrogenase (LDH), ECOG performance status (PS), neutrophil-to-lymphocyte ratio and underlying malignancy type) was developed based on prospectively collected information from 789 patients in three international cohorts and was validated in a separate patient cohort [9]. In the validation cohort, the high-risk group was associated with 72% survival at 1 month, and therefore, the utility of the LENT score in predicting survival of less than 1 month is limited.
Chest Drain Insertion and Pleurodesis
Chemical pleurodesis involves the introduction of a sclerosing agent into the pleural space with the aim of inducing an inflammatory response creating adhesions between the visceral and parietal layers of the pleura. Different agents have historically been used including talc, bleomycin and tetracycline [10, 11]. Current guidelines recommend talc as the agent of choice for pleurodesis [4]. Successful pleurodesis requires the visceral and parietal pleura be apposed at the time of the procedure as detected by lung expansion and the lack of pleural effusion following tube drainage. Failure of lung re-expansion can be due to trapped lung (thick visceral pleural peel) or endobronchial obstruction, and in such cases, the chance of achieving pleurodesis is significantly diminished [12]. A small bore chest drain is generally preferred to a wide bore chest drain, being associated with less pain and having only a slightly high pleurodesis failure rate [13, 14]. Talc pleurodesis is often associated with mild chest pain and fever, but the most serious potential complication is the development of acute respiratory distress syndrome (ARDS) and rarely death [15,16,17]. Further research linked these serious complications to the use of non-graded talc [18, 19••] which is no longer in use in clinical practise. When performed with ultrasound guidance, chest tube insertion is a safe procedure that is generally well tolerated [20].
Talc Slurry Versus Poudrage
Talc can be introduced into the pleural space either as a solution through a chest drain (talc slurry) or sprayed at thoracoscopy or video assisted thoracoscopic surgery (VATS) (talc poudrage). Stefani et al. conducted a randomised controlled trial comparing talc slurry and poudrage among patients with MPE with lung re-expansion after drainage [21]. One hundred and nine patients were included. Talc poudrage was superior to slurry for achieving a successful pleurodesis at 90 days (88.3 versus 69.6%, p value 0.047). In this study, talc poudrage was performed during VATS. Another study by Dresler et al. included 482 patients randomised 1:1 to either talc poudrage or slurry found no significant difference in success rates of pleurodesis (78 versus 71%, respectively) [22]. A subgroup analysis of the study showed that among patients with breast and lung cancer talc poudrage was more efficacious at achieving pleurodesis than talc slurry (82 versus 67%, respectively). Overall, however, there is lack of sufficient evidence to recommend thoracoscopic talc poudrage over slurry in all patients with malignant pleural effusions and decisions should be based on performance status, need to obtain pleural biopsies, patient preference and local expertise. An ongoing randomised controlled study comparing talc slurry and poudrage will likely present stronger evidence regarding the efficacy of these interventions [23].
Thoracoscopy and VATS
Medical (local anaesthetic) thoracoscopy is performed by physicians using local anaesthesia and moderate sedation. By contrast, video-assisted thorcoscopic surgery (VATS) requires general anaesthesia and single lung ventilation and is usually performed by surgeons. Thoracoscopic procedures allow pleural biopsies to be obtained, which might be required to confirm the diagnosis or to obtain additional material for molecular analysis which is often required for selection of systemic anticancer therapy. Medical thoracoscopy is generally a safe procedure with a low reported risk of mortality of 0.34% (including studies which used non-graded talc [24]). Other potential significant complications occur in 1.8% of cases and include pleural infection, bleeding, bronchopleural fistula and pneumothorax [24].
Indwelling Pleural Catheters
IPCs are silicone tubes that are tunnelled subcutaneously prior to entering the pleural space. These are long-term devices, associated with minimal risk of infection. Patients and their carers can undertake regular domiciliary drainage, often two or three times per week without requiring further hospital attendances. IPCs have a polyester cuff, located subcutaneously, which helps prevent dislodgement. They can be inserted as a day case procedure using local anaesthesia and occasionally conscious sedation [25]. It is widely accepted that IPCs are indicated in patients with MPE with trapped lung or those who have failed chemical pleurodesis. More recently, IPCs have been investigated as first-line treatment for MPE, with the focus on symptom control and quality of life (QoL) rather than necessarily achieving a successful pleurodesis [25].
The TIME2 study was a randomised controlled trial comparing IPC and talc pleurodesis as first-line treatment for patients with MPE in 106 patients followed up for 12 months [26••]. There was no significant difference in the primary outcome of mean daily dyspnoea as measured by visual analogue scale (VAS) in the first 42 days. IPC was associated with statistically significant fewer hospital days (3.5 days, 95% confidence interval 1.5–4.8). There was no difference between the two arms in the rate of serious adverse events. A more recent RCT, the AMPLE study, randomised 146 patients with MPE and no previous intervention to either talc pleurodesis or IPC insertion [27••]. The primary outcome for the study was the total number of hospitalisation days, and follow-up was for 12 months. The study also showed that IPC was associated with fewer hospital days compared to talc pleurodesis, but the estimated difference was only of 2.92 days. Dyspnoea scores and QoL improved in both treatment arms with no significant difference between patients that received talc pleurodesis compared to IPC. The TIME2 study population was subsequently analysed to assess the cost-effectiveness of IPC compared to talc pleurodesis [28]. There was no significant overall difference in cost effectiveness measures between the two interventions, but IPC appeared to be less cost-effective when survival was > 14 weeks or if weekly nursing requirements exceeded 2 h. The risk of pleural infection associated with IPC is 2.8% [29]. In 94% of cases with IPC-related pleural infection, the only treatment required is antibiotic therapy and drainage with the IPC left in place [30]. There is no increased risk of pleural infection in association with chemotherapy, and patients with an IPC can receive systemic treatment as planned [31].
Chemotherapy and Other Systemic Treatments
The presence of malignant pleural effusion in lung cancer indicates stage IV disease [32] and metastatic disease in other malignancies, and therefore, treatment is given in most cases with palliative intent. Patients with MPE that are symptomatic will require some form of pleural intervention at least until a response to treatment is seen with only rare exceptions. MPEs due to lymphoma and small cell lung cancer can frequently respond to chemotherapy and might not require definitive pleural management as fluid could resolve with initial therapy [33]. In non-small lung cancer, some reports suggested adding bevacizumab, a vascular endothelial growth factor VEGF inhibitor, to chemotherapy as it was associated with better control of associated MPEs [34].
Deciding on Treatment
While more recent research suggests a paradigm shift in the management of MPE to considering IPC as first-line treatment, multiple factors need to be considered before deciding on the most appropriate intervention for an individual patient. The aim of treatment is to control symptoms, improve quality of life and reduce hospitalisation. Consideration should be given to expected survival, local expertise, available resources and most importantly patient preference. Managing an IPC in the community can prove challenging without the essential associated support. Figure 1 represents a proposed guide to treatment selection in patients suspected to have MPE. Patients with confirmed MPE who have poor predicted survival or would be poorly tolerant of invasive procedures, such as chest drain insertion, can be treated with recurrent therapeutic pleural aspiration if required. Patients who are of a good performance status who have negative pleural fluid cytology should be referred for thoracoscopy or VATS and have either talc poudrage or IPC insertion performed during the same procedure. When a chest drain is inserted and evidence of significant trapped lung is observed on chest X-ray, pleurodesis should not be attempted and IPC insertion will be the most appropriate next step. A large proportion of patients with MPE are elderly and consideration of a multimodal geriatric assessment is encouraged to improve symptomatology and determine appropriateness (or otherwise) of treatment with systemic anticancer treatment. Comorbidities such as arthritis, risk of falls and reduced mobility should be considered when making decisions regarding chest drain insertion and invasiveness of pleural interventions.
Future Directions
The approach of the insertion of indwelling pleural catheter once MPE is diagnosed and subsequent consideration of talc pleurodesis seems promising. This pathway was the subject of a recently published multicentre randomised controlled trial undertaken in the UK. Patients who underwent an IPC insertion had a chest X-ray performed at 10 days. Those without evidence of trapped lung (154 patients) were then randomised to outpatient injection of 4 g talc versus placebo through IPC. Talc administration was associated with successful pleurodesis in 43 versus 23% with placebo (P = 0.008) [35].
Conclusion
Patients with MPE should be managed by physicians with sufficient experience in pleural diseases in close liaison with geriatricians, medical oncologists and palliative medicine. Engagement with patients and families should be continuous during all stages of care. Treatment decisions should focus primarily on symptomatology, quality of life and patient preference, rather than striving to achieve hospital-based pleurodesis at all costs.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Gurung P, Goldblatt MR, Huggins JT, Doelken P, Sahn SA. Pleural fluid characteristics of paramalignant effusion. Chest. 2009;136(4):44S.
Hsu C. Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients. Diagn Cytopathol. 1987;3(1):8–12.
Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56(4):905–9.
Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40.
Marosi C, Köller M. Challenge of cancer in the elderly. ESMO Open. 2016;1(3):e000020.
Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, JPJ B, Pronk A, Hamaker M. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365–70.
Taghizadeh N, Fortin M, Tremblay AUS. Hospitalizations for malignant pleural effusions: data from the 2012 national inpatient sample. Chest. 2017;151(4):845–54.
Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69(12):1098–104.
Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016;5:CD010529.
Diacon AH, Wyser C, Bolliger CT, Tamm M, Pless M, Perruchoud AP, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusions. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1445–9.
Bilaceroglu S, Guo Y, Hawthorne ML, Zhu Z, Stathopoulos GT, Lane KB, et al. Oral forms of tetracycline and doxycycline are effective in producing pleurodesis. Chest. 2005;128(5):3750–6.
Kennedy L, Rusch VW, Strange C, Ginsberg RJ, Sahn SA. Pleurodesis using talc slurry. Chest. 1994;106(2):342–6.
Caglayan B, Torun E, Turan D, Fidan A, Gemici C, Sarac G, et al. Efficacy of iodopovidone pleurodesis and comparison of small-bore catheter versus large-bore chest tube. Ann Surg Oncol. 2008;15(9):2594–9.
Clementsen P, Evald T, Grode G, Hansen M, Krag Jacobsen G, Faurschou P. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med. 1998;92(3):593–6.
Bouchama A, Chastre J, Gaudichet A, Soler P, Gibert C. Acute pneumonitis with bilateral pleural effusion after talc pleurodesis. Chest. 1984;86(5):795–7.
Rinaldo JE, Owens GR, Rogers RM. Adult respiratory distress syndrome following intrapleural instillation of talc. J Thorac Cardiovasc Surg. 1983;85(4):523–6.
Brant A, Eaton T. Serious complications with talc slurry pleurodesis. Respirol Carlton Vic. 2001;6(3):181–5.
Maskell NA, Lee YCG, Gleeson FV, Hedley EL, Pengelly G, Davies RJO. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med. 2004;170(4):377–82.
•• Janssen JP, Collier G, Astoul P, Tassi GF, Noppen M, Rodriguez-Panadero F, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet Lond Engl. 2007;369(9572):1535–9. This study revealed the serious adverse events rate associated with talc pleurodesis is significantly lower than previously reported in studies from the United States.
Hooper C, Lee YCG, Maskell N. BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii4–17.
Stefani A, Natali P, Casali C, Morandi U. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2006;30(6):827–32.
Dresler CM, Olak J, Herndon JE, Richards WG, Scalzetti E, Fleishman SB, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127(3):909–15.
Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HEG, Kahan BC, Hooper CE, Davies HE, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open. 2014;4(11):e007045.
Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJO, Downer NJ, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii54–60.
Bhatnagar R, Maskell NA. Indwelling pleural catheters. Respiration. 2014;88(1):74–85.
•• Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–9. This large randomised controlled study showed no difference between IPC insertion and talc pleurodesis in the control of dyspnoea symptoms in patients with MPE.
•• Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318(19):1903–12. This study revealed IPC insertion was associated with less days spent in hospital when compared to talc pleurodesis.
Olfert JAP, Penz ED, Manns BJ, Mishra EK, Davies HE, Miller RF, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirol Carlton Vic. 2017;22(4):764–70.
Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26(1):70–6.
Fysh ETH, Tremblay A, Feller-Kopman D, Mishra EK, Slade M, Garske L, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144(5):1597–602.
Mekhaiel E, Kashyap R, Mullon JJ, Maldonado F. Infections associated with tunnelled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchol Interv Pulmonol. 2013;20(4):299–303.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203.
Egan AM, McPhillips D, Sarkar S, Breen DP. Malignant pleural effusion. QJM Mon J Assoc Physicians. 2014;107(3):179–84.
Tao H, Meng Q, Li M, Shi L, Tang J, Liu Z. Outcomes of bevacizumab combined with chemotherapy in lung adenocarcinoma-induced malignant pleural effusion. Thorac Cancer. 2018;9(2):298–304.
Bhatnagar R, Keenan EK, Morley AJ, Kahan BC, Stanton AE, Haris M, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378(14):1313–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mohammed Ahmed and John Wrightson declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pulmonology and Respiratory Care
Rights and permissions
About this article
Cite this article
Ahmed, M., Wrightson, J.M. Malignant Pleural Effusions—Personalised Management. Curr Geri Rep 7, 154–159 (2018). https://doi.org/10.1007/s13670-018-0246-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-018-0246-0