Abstract
Aging is a major risk factor for venous thromboembolism. Compared to the general population, the elderly have a much higher 1-year mortality from venous thromboembolism (VTE). Clinical presentation of VTE in the elderly tends to be different, with atypical symptoms being more common than in the general population. Diagnostic work-up starts with establishing a VTE pretest probability followed by D-dimer testing for patients with low pretest probability and confirmatory testing for patients with high pretest probability of VTE. The age-adjusted D-dimer cutoffs are associated with a higher specificity without compromising the test’s sensitivity. Anticoagulation is the cornerstone of VTE therapy. The use of novel oral anticoagulants is safe in elderly patients and is associated with a decreased risk of bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
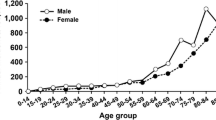
Venous thromboembolism (VTE) constitutes a significant disease burden in the geriatric patient population, and its management in this group presents unique challenges. Advanced age by itself is a major risk factor for VTE [1, 2]. The prevalence of pulmonary embolism (PE) increases progressively from <12 % in patients younger than 40 years to 44 % in patients older than 80 years [3]. Similarly, the risk of deep venous thrombosis (DVT) increases by nearly 90 % between 15 and 80 years of age. This roughly amounts to an added relative risk for VTE of 1.9 for each 10-year increase in age [4].
There are multiple mechanisms by which age increases the risk of VTE. Physical decline and limited mobility with increased age promotes venous stasis which in turn promotes thrombosis [5]. Decreased muscle tone and weakening of venous valves in elderly also promote venous stasis [6]. In addition, aging alters endothelium, platelets, coagulation cascade, and fibrinolytic pathways [7]. In particular, there is an increase in the levels of fibrinogen and factors 7 and 8, increased production of thromboxane A2 by the platelets, and increased levels of plasminogen activator inhibitor [8, 9], all of which result in a relatively more pro-thrombotic environment.
In this already pro-thrombotic milieu, secondary factors have been identified that further increase the risk of VTE. These factors include hospital or nursing home confinement, paresis, cancer, chemotherapy, and presence of central lines and varicose veins [10]. If one of these risk factors is present in a patient with VTE, VTE is called provoked. If no provoking factors are identified, VTE is considered an idiopathic event. Studies have found that with aging, the proportion of provoked VTEs increases [11]. Hospitalization increases the risk of developing VTE by more than 100 % [12]. The risk of VTE is 20-fold higher in cancer patients than in the general population [13], and in this patient population, it represents the second most common cause of death [14]. One study in elderly patients found that half of all of the patients diagnosed with DVT had an underlying malignancy. Even more interestingly, at a 2-year follow-up, elderly patients with DVT were 6.8 times more likely to be diagnosed with a neoplasm as compared to elderly patients without DVT [15]. The increased risk of thrombosis in cancer patients is likely mediated by elevated levels of microparticle-associated tissue factor and microparticle-associated epithelial mucin [16, 17]. While both cancer and aging are independent risk factors for VTE, it is, however, less clear if both of these have an additive affect [18]. Surgery in general and orthopedic surgery in particular are important risk factors for symptomatic VTE [19]. Liberman et al. found that the prevalence of proximal DVT after hip fracture surgery among elderly patients was as high as 6.1 %, despite thromboprophylaxis [20]. Other significant VTE risk factors in patients older than 80 years of age include chronic obstructive pulmonary disease (COPD), heart failure, and immobilization [21].
Not only is VTE more common in elderly patients, it is also associated with worse outcomes [22]. A study found that 52.5 % of patients older than 65 years as compared to 44 % of patients younger than 65 years had a severe PE defined as pulmonary vascular obstruction (PVO) of more than 50 % [23]. Mortality rates during VTE-related index hospitalization and at 3 months are 12 and 17.4 %, respectively [24, 25]. One-year mortality from PE in patients older than 65 years is 39 % [26], making it the third most common cardiovascular cause of death after myocardial infarction and stroke [27]. VTE represents immediate cause of death in 10–20 % of all deaths in geriatrics units [28, 29]. The most important risk factors for mortality from PE are age greater than 70 years, congestive heart failure, COPD, stroke, hypotension, tachypnea, and right ventricular hypokinesis on echocardiogram [24, 27].
Despite the enormous risk of developing and dying from VTE elderly patients face, VTE management presents unique challenges in this age group. In this article, we review pertinent literature and discuss the clinical presentation, diagnosis, prophylaxis, and management of VTE with specific focus on geriatric population.
Clinical Presentation
Elderly patients with PE tend to present with atypical and non-specific symptoms [30]. Dyspnea is the most common symptom, while chest pain and hemoptysis are much less common in patients older than 65 years [23]. In one study, chest pain was present in 61 % of young patients with PE [31] but was reported by only 27 % of PE patients older than 70 years [32]. Increased pain threshold in the elderly and decreased visceral pain sensation have been cited as possible explanations for decreased pain from PE in the elderly [33, 34]. Twenty-four percent of patients with PE older than 65 years present with a collapse as compared to only 3 % of patients younger than 65 years [34]. Another study found that older patients were three times more likely to present with syncope as compared to younger patients [23]. Cases of PE have also been reported in the elderly presenting with delirium [35, 36], while in some cases, PE was suspected based on sub-febrile temperatures and sensory disturbances [37]. PE patients older than 65 years are more likely to be hypoxic (oxygen saturation of <90 % on room air) and cyanosed as compared to younger patients [34]. Elderly patients with PE are also more likely to have ECG changes such as S1Q3T3, right bundle branch block, sinus tachycardia, atrial fibrillation, and anterior T wave inversions [34]. Given the atypical symptoms, it is hardly surprising that PE can be easily missed in the elderly, with one postmortem study reporting that a third of all PE cases in the elderly had been missed antemortem [38].
Age also alters the presentation of DVT in the elderly. Older patients with DVT are less likely to present with lower extremity discomfort and difficulty ambulating as compared to younger patients [39]. Many investigators have looked at whether age alters the risk of postthrombotic syndrome (PTS) after an acute episode of DVT. The results have been mixed with some studies reporting no change in the risk of PTS with age [40], while others reported a higher risk of PTS in patients older than 50 years [41].
Diagnosis
The work-up of suspected VTE starts with determining the VTE pretest probability based on one of the scoring systems, of which Wells Score and Revised Geneva Score are the most commonly used ones [42, 43]. For suspected PE, patients with low pretest probability get a D-dimer test as a screening tool with a positive test prompting computerized tomographic angiography (CTA) for confirmation. Patients with a high pretest probability of VTE directly proceed to CTA for confirmation [44–46]. No further testing is required in patients with low pretest probability and a negative D-dimer.
The diagnostic work-up for suspected DVT follows similar principles. Patients are risk-stratified based on Wells Score. Patients with low pretest probability are screened with a D-dimer test, while patients with intermediate or high pretest probability receive confirmatory testing with compression ultrasonography [47]. DVT can be ruled out in patients with low pretest probability of DVT and a negative D-dimer test, while patients with low pretest probability and a positive D-dimer require compression ultrasonography. Recently, PALLADIO study investigators proposed a new algorithm for work-up of suspected DVT [48••]. In this algorithm, a whole leg compression ultrasonography is suggested only for patients with both a high pretest probability of DVT and an elevated D-dimer test. For patients meeting only one of these two conditions (elevated D-dimer or a high pretest probability of DVT), a limited proximal leg compression ultrasonography was found to be sufficient to exclude DVT. However, this algorithm is yet to be validated in elderly patients.
A unique challenge in the elderly is that D-dimer levels increase with age; thereby, using the standard 500 ng/ml cutoff for D-dimer will give a large number of false-positive screening tests in the elderly [3, 6, 49]. Righini et al. reported a decrease in the specificity of D-dimer testing from 67 % for younger patients than 40 to 10 % for patients older than 80 years [3]. Therefore, investigators have proposed using age-adjusted D-dimer cutoffs when screening patients older than 50 years. This cutoff is calculated by multiplying age by 10 for patients older than 50 years [50]. ADJUST-PE was the landmark study evaluating the age-adjusted D-dimer cutoff [51••]. It was a prospective trial carried out at 19 centers with a total of 3346 patients evaluated. The study included 337 patients with low pretest probability of PE and D-dimer levels more than 500 ng/ml but less than their age-adjusted cutoffs. These patients did not get CTA and were followed clinically for 3 months. Only one out of these 337 patients was subsequently found to have a PE. This translates into a failure rate of 0.3 %. This failure rate is comparable to the 0.5 % failure rate that was reported by the Christopher Study using the traditional 500 ng/ml cutoff [46]. Of note, over 300 CTAs were avoided without significant added risk of missing clinically relevant PEs. Another study by Woller et al. reported a failure rate of 1.5 % at 3 months of follow-up using the age-adjusted D-dimer cutoffs [50]. Similar results were also obtained by Scouten et al. in a meta-analysis of 13 cohorts [52•]. They reported that using the age-adjusted D-dimer cutoff, the specificity of the test increased from 14.7 to 35.2 % for patients older than 80 years. Overall, they concluded that the age-adjusted cutoffs missed additional 1–4/1000 cases of PE but at the same time prevented 303–540/1000 additional CTAs that would have been performed if the traditional cutoff had been applied.
The age-adjusted D-dimer cutoffs have also been studied in suspected DVT cases and have been found to be able to safely exclude clinically significant DVT [53]. Zhang et al. reported that the specificity of D-dimer testing for the diagnosis of DVT in elderly increased by 20 % when age-adjusted cutoffs were used [54•].
Limiting the number of CTAs performed by using the age-adjusted D-dimer cutoffs is especially desirable since it reduces the incidence of contrast-induced nephropathy (CIN) associated with the intravenous contrast used in PE protocol. Mitchell et al. reported a 14 % risk of CIN after the CTA [55]. Age over 75 years is considered an independent risk factor for developing CIN [56]. Conversely, renal failure is also one of the contraindications for performing CTA. In one series of patients with suspected PE, Kreidy et al. reported that 36.8 % patients had an elevated creatinine that precluded the use of CTA, whereas 28 % patients had Alzheimer’s disease or some other condition that made it impossible for them to comply with the instructions for CT angiography [57]. In clinical practice, some of the patients who have a contraindication to confirmatory testing may be committed to empiric anticoagulation. However, using the age-adjusted D-dimer cutoffs can safely rule out VTE in a greater proportion of patients without undertaking CTA.
Since over 90 % of PEs originate in the lower extremity veins [58], some authors have recommended lower extremity ultrasounds as first-line tests for suspected PE [57, 59]. The yield of lower extremity ultrasound in suspected PE is higher in the presence of lower extremity edema. Eze at al. studied geriatric patients with suspected PE and reported a yield of 40 % for lower extremity ultrasound, in the presence of unilateral lower extremity symptoms [59]. This practice has not been widely adopted yet, but it may be a useful adjunct in evaluation of patients with suspected VTE, D-dimer levels higher than the age appropriate cutoff, and a contraindication to CTA.
Prophylaxis
Despite the overwhelming evidence of enormous VTE disease burden in geriatric patients, VTE prophylaxis even if clearly indicated is less than ideal. One study reported that 34.8 % of acutely ill, hospitalized elderly patients who should have been on VTE prophylaxis were not receiving it [60]. Bratzler et al. reported that only 39 % of high-risk surgical elderly patients were on VTE prophylaxis [61]. Perhaps, the main reason for the underuse of VTE prophylaxis in this patient population is the fear of bleeding [62]. Another reason for the underuse of prophylaxis seems to be the fact that physicians underestimate the risk of VTE in medical patients [63].
Low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), and fondaparinux have all been extensively studied for VTE prophylaxis in the elderly. Alikhan et al. and Dahan et al. studied enoxaparin for VTE prophylaxis in acutely ill, non-surgical elderly patients and reported VTE risk of 4.1 and 3.0 %, respectively. The VTE risk in these two studies, without prophylaxis, was 18.5 and 9.1 %, respectively [64, 65]. Bergmann et al. performed a randomized controlled trial comparing enoxaparin and UFH for VTE prophylaxis in elderly, acutely ill medical patients and reported a VTE risk of 4.8 and 4.6 % in the enoxaparin and UFH groups, respectively [66]. Major bleeding was reported in 0.9 and 1.8 % of the patients in the enoxaparin and UFH groups, respectively. Similarly, an approximately 50 % decrease in bleeding complications with LMWH as compared to UFH has been reported by other authors [67]. Dalteparin and bemiparin have also been studied for VTE prophylaxis, and the results have been similar to enoxaparin [68, 69]. Cohen et al. studied fondaparinux for VTE prophylaxis in acutely ill elderly patients and found that the VTE risk was almost halved as compared to placebo without any additional bleeding risk [70].
Treatment
Anticoagulation poses various challenges in the elderly patients. Some of these challenges are general, and some are specific to certain drug classes. One of the general problems is the fact that many of the elderly patients with VTE might be on one or more antiplatelet agents for other indications, which increases the risk of bleeding and calls for closer monitoring [71]. In addition, with aging, there is a gradual decline in the renal function [72], which mandates dose adjustments and closer monitoring with most anticoagulants. Lastly, elderly patients are at an increased fall risk [73], which constitutes an important part of risk benefit equation when considering anticoagulation in these patients.
For many years, the mainstay of treatment for VTE has been warfarin therapy, initially accompanied by heparin until therapeutic international normalized ratio (INR) has been achieved [74]. Levine et al. compared LMWH and UFH for acute VTE and found that the rates of recurrence were 5.3 and 6.7 %, respectively. However, most strikingly, while patients treated with LMWH spent a mean of 1.1 (±SD2.9) days in the hospital, the patients treated with UFH spent a mean of 6.5 (±SD3.4) days as inpatients [75]. Almost half of the patients treated with LMWH in this study were believed to be stable enough to be treated in an ambulatory setting, without the need for hospital admission. Spyropoulos et al. compared the treatment with UFH and LMWH and concluded that treatment with LMWH was associated with a cost saving of $2583 per patient as compared to treatment with UFH [76]. This cost saving was primarily attributed to the outpatient-based management with LMWH.
Given its low cost, ease of administration, and physician familiarity, warfarin has been the standard anticoagulant for long-term VTE management [63]. However, warfarin use in the elderly may be challenging. Firstly, long-term warfarin use requires frequent INR checks and dose adjustments [77]; this is cumbersome for anyone but more so for the elderly. Secondly, warfarin has extensive food and drug interactions, which means that both patients and physicians have to exercise extreme caution to avoid potential interactions [78]. Thirdly, INRs are difficult to control in the elderly. Vogel et al. prospectively followed 110 patients older than 65 years taking vitamin K antagonists (VKA) and found that only 31 % patients had INR within the target range of 2–3 [79]. Fourthly, age by itself is an independent risk factor for bleeding with VKAs [80, 81]. Van der Meer et al. reported that for each 10-year increase in age, the risk of bleeding increases by 32 % and the risk of major bleeding increases by 46 % [82].
Given the above challenges, recently novel oral anticoagulants (NOACs) such as rivaroxaban, apixiban, and dabigatran have been studied in the very elderly (age >75 years) [83••]. Even though some initial reports suggested a heightened bleeding risk with dabigatran in the elderly [84–86], a recent meta-analysis showed that NOACs did not have a higher bleeding risk as compared to conventional therapy [83••]. For rivaroxaban, this meta-analysis included elderly patients from the EINSTEIN (2010), EINSTEIN-EXTENSION (2012), and EINSTEIN-PE and MAGELLAN (2013) trials [87–89]. The total number of events (VTE or VTE-related deaths) in the rivaroxaban group was 76 compared to 136 in the control group with an odds ratio (OR) of 0.55 (95 % CI 0.35–0.87) [83••]. Analysis of elderly patients from the AMPLIFY-EXT (2013) trial showed 99 events in the apixiban group as compared to 175 events in the control group with an OR of 0.49 (95 % CI 0.22–1.10) [83••, 90]. Lastly, analysis of elderly patients from the RE-MEDY (2013) trial revealed no events in both the dabigatran and the warfarin (control) groups [83••, 91]. Recently, the American College of Chest Physicians (ACCP) updated their antithrombotic guidelines as well and recommended the use of NOACs over VKAs for initial and long-term management of VTE patients without cancer [92••]. Data regarding the use of NOACs in cancer-related VTE is less convincing [93].
In the event of a massive PE complicated by hemodynamic instability with no significant improvement with routine anticoagulation, thrombolytic therapy may be considered [94]. However, the outcomes of treatment with thrombolytics for massive PE in the elderly patients have not been favorable. De Bonis et al. evaluated elderly patients (>65 years) who had massive PEs and were given thrombolysis. In-hospital mortality was 13.4 %, while 32.7 % patients died during follow-up [95]. They found elevated troponin levels, thrombocytopenia after thrombolysis, and history of cancer and cardiovascular disease to be significant predictors of mortality.
While the medical management and anticoagulation recommendations are similar for both PE and DVT, one management aspect that is unique to DVT patients is the issue of compression stockings. The literature regarding the utility of compression stockings in DVT patients for the prevention of PTS is conflicting. While some trials have reported that compression stockings can reduce the risk of PTS after DVT by about 50 %, other trials have not been able to demonstrate any significant benefit [96]. Given this conflicting data, ACCP recently recommended against the use of compression stockings for the prevention of PTS [92••]. However, once the diagnosis of PTS has been established, compression stockings are the mainstay for its treatment [97, 98].
Inferior vena cava (IVC) filters have also been evaluated for long-term prevention of PE, but they have not been shown to reduce mortality or recurrent symptomatic VTEs [99•]. In addition, they have been associated with a complication rate of 29 % [100]. Therefore, 2012 ACCP guidelines recommended using the IVC filters only in patients with acute DVT who cannot tolerate anticoagulation because of active bleeding or increased risk of bleeding.
Conclusion
VTE constitutes a significant disease burden in the elderly. Not only is it more prevalent but also is associated with worse clinical outcomes in this patient population. Awareness of the atypical presenting features of VTE in the elderly patients can assist clinicians in prompt diagnosis and timely initiation of treatment. For patients with low pretest probability of VTE, the use of age-adjusted D-dimer cutoffs can decrease the use of confirmatory testing (CTA or compression ultrasonography), without significant adverse patient-related outcomes (i.e., increased risk of missing a true VTE). NOACs are safe and effective in elderly patients for treatment of non-cancer-related VTE. The data is less convincing for cancer-related VTE. Future trials are needed comparing NOACs to LMWH for the long-term treatment of cancer-related VTE. Given the high prevalence of malignancy in the elderly patients, this patient population should be well represented in such trials.
Abbreviations
- VTE:
-
Venous thromboembolism
- PE:
-
Pulmonary embolism
- DVT:
-
Deep venous thrombosis
- COPD:
-
Chronic obstructive pulmonary disease
- CTA:
-
Computerized tomographic angiography
- CIN:
-
Contrast-induced nephropathy
- PTS:
-
Postthrombotic syndrome
- VKA:
-
Vitamin K antagonists
- LMWH:
-
Low-molecular-weight heparin
- UFH:
-
Unfractionated heparin
- INR:
-
International normalized ratio
- NOAC:
-
Novel oral anticoagulant
- ACCP:
-
American College of Chest Physicians
- IVC:
-
Inferior vena cava
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sellier E, Labarere J, Sevestre M-A, Belmin J, Thiel H, Couturier P, et al. Risk factors for deep vein thrombosis in older patients: a multicenter study with systematic compression ultrasonography in postacute care facilities in France. J Am Geriatr Soc. 2008;56:224–30.
Weill-Engerer S, Meaume S, Lahlou A, Piette F, Saint-Jean O, Sachet A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc. 2004;52:1299–304.
Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109:357–61.
Montagnana M, Favaloro EJ, Franchini M, Guidi GC, Lippi G. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis. 2010;29:489–96.
Kim DY, Kobayashi L, Barmparas G, Fortlage D, Curry T, Coimbra R. Venous thromboembolism in the elderly: the result of comorbid conditions or a consequence of injury? J Trauma Acute Care Surg. 2012;72:1286–91.
Rosendaal FR, VAN Hylckama Vlieg A, Doggen CJM. Venous thrombosis in the elderly. J Thromb Haemost JTH. 2007;5 Suppl 1:310–7.
Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60:144–51.
Abbate R, Prisco D, Rostagno C, Boddi M, Gensini GF. Age-related changes in the hemostatic system. Int J Clin Lab Res. 1993;23:1–3.
Mari D, Mannucci PM, Coppola R, Bottasso B, Bauer KA, Rosenberg RD. Hypercoagulability in centenarians: the paradox of successful aging. Blood. 1995;85:3144–9.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15.
Spencer FA, Gurwitz JH, Schulman S, Linkins L-A, Crowther MA, Ginsberg JS, et al. Venous thromboembolism in older adults: a community-based study. Am J Med. 2014;127:530–7. e3.
Heit JA, Melton LJ, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76:1102–10.
Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost JTH. 2004;2:1760–5.
Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24.
Zanocchi M, Risso R, Maero B, Aimar T, Giona E, Francisetti F, et al. Deep venous thrombosis and cancer in the elderly. Minerva Med. 2001;92:307–13.
Furie B, Furie BC. Cancer-associated thrombosis. Blood Cells Mol Dis. 2006;36:177–81.
Tesselaar MET, Romijn FPHTM, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost JTH. 2007;5:520–7.
Fimognari FL, Repetto L, Moro L, Gianni W, Incalzi RA. Age, cancer and the risk of venous thromboembolism. Crit Rev Oncol Hematol. 2005;55:207–12.
Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet Lond Engl. 2001;358:9–15.
Lieberman DV, Lieberman D. Proximal deep vein thrombosis after hip fracture surgery in elderly patients despite thromboprophylaxis. Am J Phys Med Rehabil Assoc Acad Physiatr. 2002;81:745–50.
López-Jiménez L, Montero M, González-Fajardo JA, Arcelus JI, Suárez C, Lobo JL, et al. Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE). Haematologica. 2006;91:1046–51.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, Lohse CM, O’Fallon WM, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost. 2001;86:452–63.
Kokturk N, Oguzulgen IK, Demir N, Demirel K, Ekim N. Differences in clinical presentation of pulmonary embolism in older vs younger patients. Circ J Off J Jpn Circ Soc. 2005;69:981–6.
Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet Lond Engl. 1999;353:1386–9.
Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8.
Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994;154:861–6.
Weberová D, Weber P, Kubesová H, Meluzínová H, Polcarová V, Ambrosová P, et al. Occurrence of pulmonary embolism among 260 in-patients of acute geriatric department aged 65+ years in 2005-2010. Adv Gerontol Uspekhi Gerontol Ross Akad Nauk Gerontol Obshchestvo. 2012;25:506–12.
Kammoun S, Gold G, Bouras C, Giannakopoulos P, McGee W, Herrmann F, et al. Immediate causes of death of demented and non-demented elderly. Acta Neurol Scand Suppl. 2000;176:96–9.
Attems J, Arbes S, Böhm G, Böhmer F, Lintner F. The clinical diagnostic accuracy rate regarding the immediate cause of death in a hospitalized geriatric population; an autopsy study of 1594 patients. Wien Med Wochenschr 1946. 2004;154:159–62.
Ceccarelli E, Masotti L, Barabesi L, Forconi S, Cappelli R. Pulmonary embolism in very old patients. Aging Clin Exp Res. 2003;15:117–22.
Gisselbrecht M, Diehl JL, Meyer G, Collignon MA, Sors H. Clinical presentation and results of thrombolytic therapy in older patients with massive pulmonary embolism: a comparison with non-elderly patients. J Am Geriatr Soc. 1996;44:189–93.
Stein PD, Gottschalk A, Saltzman HA, Terrin ML. Diagnosis of acute pulmonary embolism in the elderly. J Am Coll Cardiol. 1991;18:1452–7.
Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272:G1–3.
Timmons S, Kingston M, Hussain M, Kelly H, Liston R. Pulmonary embolism: differences in presentation between older and younger patients. Age Ageing. 2003;32:601–5.
Ter Braak GI. Old, short of breath and confused; delirium as a manifestation of pulmonary embolism in geriatric patients. Ned Tijdschr Geneeskd. 1998;142:1046.
Soysal P, Isik AT. Hypoactive delirium caused by pulmonary embolus in an elderly adult. J Am Geriatr Soc. 2014;62:586–7.
Tan WL, Chel VG, Rooijers-Rebel M, Hennemann G. Multiple pulmonary embolisms in nursing home patients recognizable by alertness to certain aspecific symptoms. Ned Tijdschr Geneeskd. 1995;139:2487–91.
Taubman LB, Silverstone FA. Autopsy proven pulmonary embolism among the institutionalized elderly. J Am Geriatr Soc. 1986;34:752–6.
Piazza G, Seddighzadeh A, Goldhaber SZ. Deep-vein thrombosis in the elderly. Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2008;14:393–8.
Stain M, Schönauer V, Minar E, Bialonczyk C, Hirschl M, Weltermann A, et al. The post-thrombotic syndrome: risk factors and impact on the course of thrombotic disease. J Thromb Haemost JTH. 2005;3:2671–6.
Tick LW, Doggen CJM, Rosendaal FR, Faber WR, Bousema MT, Mackaay AJC, et al. Predictors of the post-thrombotic syndrome with non-invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J Thromb Haemost JTH. 2010;8:2685–92.
Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20.
Le Gal G, Righini M, Roy P-M, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165–71.
Perrier A, Roy P-M, Sanchez O, Le Gal G, Meyer G, Gourdier A-L, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760–8.
Quiroz R, Kucher N, Zou KH, Kipfmueller F, Costello P, Goldhaber SZ, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293:2012–7.
Van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–9.
Hirsh J, Lee AYY. How we diagnose and treat deep vein thrombosis. Blood. 2002;99:3102–10.
Ageno W, Camporese G, Riva N, Iotti M, Bucherini E, Righini M, et al. Analysis of an algorithm incorporating limited and whole-leg assessment of the deep venous system in symptomatic outpatients with suspected deep-vein thrombosis (PALLADIO): a prospective, multicentre, cohort study. Lancet Haematol. 2015;2:e474–80. Study has proposed a new algorithm using which some of the patients with suspected DVT can be safely worked up just by limited lower extremity ultrasounds.
Righini M, Nendaz M, Le Gal G, Bounameaux H, Perrier A. Influence of age on the cost-effectiveness of diagnostic strategies for suspected pulmonary embolism. J Thromb Haemost JTH. 2007;5:1869–77.
Woller SC, Stevens SM, Adams DM, Evans RS, Lloyd JF, Snow GL, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest. 2014;146:1444–51.
Righini M, Van Es J, Den Exter PL, Roy P-M, Verschuren F, Ghuysen A, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–24. Largest trial evaluating the utility of age adjusted D-dimer levels for suspected PE.
Schouten HJ, Geersing GJ, Koek HL, Zuithoff NPA, Janssen KJM, Douma RA, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492. Meta-analysis which further substantiates the findings of ADJUST-PE study.
Taylor C, Sultan L. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 2: the use of age-related D-dimers to rule out deep vein thrombosis. Emerg Med J EMJ. 2015;32:747–9.
Zhang S, Li J, Liu C, Tan G, Cao X, Wang J. The value of age-adjusted D-dimer cut-off value in diagnosing deep vein thrombosis in elderly patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:945–9. A large trial that validated use of age adjusted D-dimer levels for suspected DVTs.
Mitchell AM, Jones AE, Tumlin JA, Kline JA. Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med Off J Soc Acad Emerg Med. 2012;19:618–25.
Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–5.
Kreidy R, Stephan E, Salameh P, Waked M. Value of venous color flow duplex scan as initial screening test for geriatric inpatients with clinically suspected pulmonary embolism. Vasc Health Risk Manag. 2011;7:585–9.
Matzdorff AC, Green D. Deep vein thrombosis and pulmonary embolism: prevention, diagnosis, and treatment. Geriatrics. 1992;47:48–52. 55–7, 62–3.
Eze AR, Comerota AJ, Kerr RP, Harada RN, Domeracki F. Is venous duplex imaging an appropriate initial screening test for patients with suspected pulmonary embolism? Ann Vasc Surg. 1996;10:220–3.
Chopard P, Dörffler-Melly J, Hess U, Wuillemin WA, Hayoz D, Gallino A, et al. Venous thromboembolism prophylaxis in acutely ill medical patients: definite need for improvement. J Intern Med. 2005;257:352–7.
Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med. 1998;158:1909–12.
Di Minno G, Tufano A. Challenges in the prevention of venous thromboembolism in the elderly. J Thromb Haemost JTH. 2004;2:1292–8.
Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging. 2006;23:651–71.
Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2003;14:341–6.
Dahan R, Houlbert D, Caulin C, Cuzin E, Viltart C, Woler M, et al. Prevention of deep vein thrombosis in elderly medical in-patients by a low molecular weight heparin: a randomized double-blind trial. Haemostasis. 1986;16:159–64.
Bergmann JF, Neuhart E. A multicenter randomized double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group. Thromb Haemost. 1996;76:529–34.
Mismetti P, Laporte-Simitsidis S, Tardy B, Cucherat M, Buchmüller A, Juillard-Delsart D, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost. 2000;83:14–9.
Rodríguez-Mañas L, Gómez-Huelgas R, Veiga-Fernández F, Ruiz GM, González JM, ANCIANOS Investigators. Thromboprophylaxis with the low-molecular-weight heparin bemiparin sodium in elderly medical patients in usual clinical practice: the ANCIANOS study. Clin Drug Investig. 2010;30:337–45.
Kucher N, Leizorovicz A, Vaitkus PT, Cohen AT, Turpie AGG, Olsson C-G, et al. Efficacy and safety of fixed low-dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165:341–5.
Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–9.
Buresly K, Eisenberg MJ, Zhang X, Pilote L. Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction. Arch Intern Med. 2005;165:784–9.
Fehrman-Ekholm I, Skeppholm L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol. 2004;38:73–7.
Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–7.
Di Minno G, Tufano A, Cerbone AM. Antithrombotic drugs for older subjects. Guidelines formulated jointly by the Italian Societies of Haemostasis and Thrombosis (SISET) and of Gerontology and Geriatrics (SIGG). Nutr Metab Cardiovasc Dis NMCD. 2001;11:41–62.
Levine M, Gent M, Hirsh J, Leclerc J, Anderson D, Weitz J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996;334:677–81.
Spyropoulos AC, Hurley JS, Ciesla GN, de Lissovoy G. Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin. Chest. 2002;122:108–14.
Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:204S–33.
Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–106.
Vogel T, Coriol V, Kaltenbach G, Kiesmann M, Berthel M. Prospective study of oral anticoagulation control in 110 very elderly hospitalized patients and of risk factors for poor control. Presse Médicale Paris Fr 1983. 2008;37:1723–30.
Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87:144–52.
Launbjerg J, Egeblad H, Heaf J, Nielsen NH, Fugleholm AM, Ladefoged K. Bleeding complications to oral anticoagulant therapy: multivariate analysis of 1010 treatment years in 551 outpatients. J Intern Med. 1991;229:351–5.
Van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briët E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med. 1993;153:1557–62.
Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–64. First study that evaluated the safety of NOACs in elderly patients.
Legrand M, Mateo J, Aribaud A, Ginisty S, Eftekhari P, Huy PTB, et al. The use of dabigatran in elderly patients. Arch Intern Med. 2011;171:1285–6.
Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med. 2012;366:864–6.
Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother. 2012;46:e10.
EINSTEIN Investigators, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510.
EINSTEIN–PE Investigators, Büller HR, Prins MH, Lensin AWA, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97.
Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–23.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708.
Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18.
CHEST issues new antithrombotic guideline update for treatment of VTE disease [Internet]. Am Coll Chest Physicians. [cited 2016 Jan 11]. Available from: https://www.chestnet.org/News/Press-Releases/2016/01/AT10-VTE. Recent update issued by ACCP, recommending the use of NOACs over VKAs for the management of non-cancer related VTE. It also recommended against the routine use of compression stockings for the prevention of PTS.
Sardar P, Chatterjee S, Herzog E, Pekler G, Mushiyev S, Pastori LJ, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460–8.
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94.
De Bonis S, Rendina D, Vargas G, Di Minno D, Piedimonte V, Gallotta G, et al. Predictors of in-hospital and long-term clinical outcome in elderly patients with massive pulmonary embolism receiving thrombolytic therapy. J Am Geriatr Soc. 2008;56:2273–7.
Prandoni P, Lensing AWA, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–56.
Kahn SR. How I treat postthrombotic syndrome. Blood. 2009;114:4624–31.
Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308–20.
Mismetti P, Laporte S, Pellerin O, Ennezat P-V, Couturaud F, Elias A, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313:1627–35. Recent trial that demonstrated no added benefit of using IVC filters along with anticoagulation for the prevention of recurrent PEs.
Arnold TE, Karabinis VD, Mehta V, Dupont EL, Matsumoto T, Kerstein MD. Potential of overuse of the inferior vena cava filter. Surg Gynecol Obstet. 1993;177:463–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Muhammad Sajawal Ali and Kasia Czarnecka-Kujawa declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pulmonology and Respiratory Care
Rights and permissions
About this article
Cite this article
Ali, M.S., Czarnecka-Kujawa, K. Venous Thromboembolism in the Elderly. Curr Geri Rep 5, 132–139 (2016). https://doi.org/10.1007/s13670-016-0163-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-016-0163-z
Keywords
- Geriatrics
- Venous thromboembolism
- Pulmonary embolism
- Deep venous thrombosis
- D-dimer
- Novel oral anticoagulants
- Warfarin
- Low-molecular-weight heparin
- Unfractionated heparin
- Postthrombotic syndrome
- Cancer
- Computerized tomographic angiography
- Contrast-induced nephropathy
- Wells Score
- Ultrasonography
- Rivaroxaban
- Apixiban
- Dabigatran
- Thrombolysis
- Inferior vena cava filter
- Compression stockings