Abstract
Purpose of Review
Laparoscopic radiofrequency ablation (Lap-RFA), which was FDA-cleared for the management of symptomatic uterine fibroids in 2012, offers a promising minimally invasive option for the treatment of fibroids. Although a relatively new technology, there is a substantial amount of literature that indicates it is a safe, effective option that has decreased operative time when compared to laparoscopic myomectomy, a low likelihood of complication, and a high chance of sustained reduction of fibroid burden for patients. We present the current literature that evaluates Lap-RFA in its current use, as well as present the future of the technology.
Recent Findings
The current literature demonstrates that Lap-RFA succeeds in treating a large amount of FIGO 2–6 fibroids with clinical improvements in fibroid volume, menstrual blood loss, dysmenorrhea, fibroid-related symptom scores, and overall quality of life. Some data indicates that the technology may be safe for patients who desire future fertility or have adenomyosis, though large trials have yet to be performed.
Summary
Lap-RFA offers promising long-term data that demonstrate treatment effectiveness is robust with relatively low complication rates. There are many possible future directions of research for this technology, including its use with patients who desire future fertility or have adenomyosis, as well as in combination with other treatments of fibroids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uterine leiomyomas, or fibroids, are the most common benign neoplasm in women [1]. By age 50, most women will have developed uterine fibroids, with an incidence of fibroids of approximately 80% in Black/African American women and 70% in white women with symptoms including pelvic pain and bloating, anemia, painful intercourse, infertility, and adverse pregnancy outcomes [2]. Traditionally, hysterectomy has been considered the gold standard definitive treatment for symptomatic fibroids. Uterine-sparing treatment options include myomectomy, uterine artery embolization, and more recently, radiofrequency ablation (RFA). Laparoscopic radiofrequency ablation (Lap-RFA) was FDA-cleared for the management of symptomatic uterine fibroids in 2012 and developed as a minimally invasive option for symptomatic fibroids [3]. Several prospective studies have demonstrated a significant decrease in menstrual blood loss, fibroid volume, and improvement in quality of life and fibroid symptom severity for up to 2 years from surgery [4•, 5]. This review aims to evaluate the existing literature of Lap-RFA.
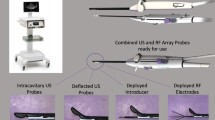
The impact of radiofrequency (RF) energy on tissue was described as early as the1890s and employed for the treatment of liver tumors beginning in the 1990s. The advent of bipolar RF needle electrodes was described by Goldfarb in 1995 without image guidance [6]. In 2002, Dr. Bruce Lee and colleagues first described the application of RF for use in the treatment of fibroids [7, 8]. RF energy is delivered through an electrode or an array to create a closed electrical circuit through the tissue to a dispersive electrode pad or pads. A high amount of energy is delivered to the tissue through small multi-pronged electrodes (or arrays), with energy decreasing rapidly as distance from the electrodes increases. The alternating current causes friction in water in the tissue, and in turn, generates heat, resulting in coagulative necrosis of the affected tissue. For the treatment of fibroids, temperatures are set to reach 95 to 105 °C for commercially available RFA devices, creating a “volumetric” ablation zone of tissue effects with each treatment. Currently, in the USA, the Food and Drug Administration (FDA) has cleared two devices specific for RFA of fibroids, Acessa® (Hologic, Inc., Marlborough, MA), in 2012, and Sonata System® (Gynesonics, Redwood City), in 2018 [3, 9].
Clinical Outcomes
Various prospective studies have demonstrated clinical improvements in fibroid volume, menstrual blood loss, dysmenorrhea, fibroid-related symptom scores, and overall quality of life after Lap RFA. Brucker et al. in a prospective single-center randomized study evaluated laparoscopic radiofrequency ablation in comparison to laparoscopic myomectomy and found a lower mean hospitalization time in the laparoscopic radiofrequency ablation group (10.0 h ± 5.5 vs 29.9 h ± 14.2), lower intraoperative blood loss (16 ± 9 mL vs 51 ± 57 mL) and a higher percentage of fibroids treated (98.6 vs 80.3%) [10]. The TRUST trial by Rattray et al., a randomized, prospective, multicenter, longitudinal trial, reported a mean hospitalization time of 6.7±3.0 h for laparoscopic radiofrequency ablation versus 9.9±10.7 h for myomectomy and a lower intraoperative blood loss (25.2±21.6 versus 82.4±62.5 mL) [11•]. It should be noted that these trials were not powered for clinical efficacy. The primary endpoint in the trial by Brucker et al. was the mean time to discharge from the hospital following the procedure, and the primary endpoint in the TRUST trial was hospitalization time. In a systematic review by Bradley et al. in 2019, clinical factors such as procedure time and time to normal work and activity were examined [12•]. In their study, the mean procedure time was 73 min, and the weighted mean time to discharge was 10.7 h. Additionally, the weighted mean time to return to normal activities was 9.0 days, and the weighted mean return to work was 6.5 days. In a meta-analysis by Lin et al., the overall procedure-related adverse events rate was 1.78% [13].
In addition, the incorporation of the new technology by minimally invasive gynecologic surgeons has been shown to be positive. Braun et al. studied the incorporation of the Acessa procedure by 10 gynecologists and found that none of the surgeons found any problems with the device including technical issues or malfunctions, though two surgeons did offer enhancement recommendations for the ultrasound probe [14]. The mean length of the procedure was 1.9±1.0 h, and in the pivotal trial was 2.1±1.0 h [14]. Jacoby et al. found that 89% of gynecologists reported either being somewhat or very confident after just one case incorporating the new technology, and this rose to 100% after four procedures [15]. While these studies do indicate a positive response in terms of ease of implementation, given their limited sample size, more thorough evaluation is needed, as confidence does not directly correlate with safe outcomes without proctoring. This ease of implementation is complemented by the lower healthcare resource utilization of the procedure when compared to myomectomy [16].
Quality of Life
Much of the literature since the clearance of Lap-RFA suggests that patients appropriate for the procedure have improved quality of life and long-term sustained relief of symptoms [13]. The initial study by Chudnoff et al. reported a 94% satisfaction rate in their cohort of 135 patients [4•]. In this cohort, the total mean uterine volume reduction of was 15.7% at 3 months and 24.3% at 12 months, and reduction of myoma volume was 39.8% at 3 months, and 45.1% at 12 months. Lin et al. reported a significant improvement in quality of life at 36 months following the procedure in a meta-analysis of 581 patients, with maximum improvement noted at 12 months [13]. Long-term quality of life outcomes are not yet available although FDA clearance for laparoscopic radiofrequency ablation was obtained in 2012; however, early data does favor a sustained reduction in fibroid burden with low reintervention rates of 3.8 to 4.39% at 12 months [12•, 13].
Long-Term Data on Fertility, Pregnancy Outcomes
The pivotal trial for Lap-RFA excluded patients that desired future fertility; however, there has since been literature describing pregnancy outcomes following this treatment. Lap-RFA of fibroids is not specifically FDA-cleared in patients who desire future fertility; however, there are emerging data that have examined pregnancies after Lap-RFA.
A case series in 2020 by Berman et al. reported on 30 pregnancies in 28 women, resulting in 26 full-term live births and 4 spontaneous abortions. There were no cases of preterm delivery, uterine rupture, placental abruption, placenta accreta, or intrauterine growth restriction. Two complications were reported including 1 event of placenta previa and another of postpartum hemorrhage [17]. Polin and Hur followed with a meta-analysis of pregnancy outcomes following laparoscopic and transcervical RFA, inclusive of Berman’s case series. Fifty pregnancies were reported, 40 following 559 laparoscopic procedures and 10 following 364 transcervical procedures [18•]. The average age at the time of treatment amongst patients that ultimately conceived was 37 with a spontaneous abortion rate of 12%. Of note, an increased rate of cesarean delivery was observed. The most common indications for cesarean delivery included prior cesarean delivery and unknown safety of vaginal delivery after Lap-RFA procedure. Only 2 delivery complications were reported, placenta previa and postpartum hemorrhage, and occurred in the Lap-RFA group described in Berman’s case series. In a review of the Manufacturer and User Facility Device Experience (FDA MAUDE) data by Young et al. from 2012 to 2022, 5 cases of uterine rupture were reported, though there were no details related to regarding maternal morbidity in those cases [19].
Lap-RFA requires breaching the uterine serosa at least once, but often multiple times, to deliver the ablative energy within fibroids. Ultrasound guidance permits visualization of the endometrium and serosa as it relates to the ablative zone; however, the extent of thermal damage to the surrounding myometrium and endometrium is not measured during the procedure. To date, there are no studies evaluating the application of Lap-RFA for fibroids in patients seeking fertility-sparing treatment with the intention of future conception. Given the rarity of some delivery complications, it is unclear whether laparoscopic radiofrequency ablation of fibroids would increase the risk of these complications, such as uterine rupture.
Complications
Concern with any emerging technology is complications previously not described and device-related issues. Complications related to laparoscopic myoma ablation have been reported as low, though the exact rate is not known [19]. In the principal study by Chudnoff et al., 5 of 135 patients had reported complications, including a pelvic abscess, bowel injury, and vaginal bleeding [4•]. In the FDA MAUDE review by Young et al. from 2012 to 2022, sixty unique adverse events were reported from both laparoscopic and transcervical radiofrequency ablation [19]. Of these reports, 58 occurred following the laparoscopic approach. Forty-three of the 60 adverse events were injury reports, the most common being infection and 35% of injuries required surgical intervention. Other complications included bowel injury, bladder injury, peripartum uterine rupture, and excessive post-operative pain. Currently, Lap-RFA is not recommended to be used for FIGO types 0, 1, given the risk of sloughing, or 7 and 8 fibroids given the risk of complication due to thermal spread [20].
Certain complications, such as post-operative uterine necrosis or necrosis of surrounding organs such as the rectum, can lead to rapid clinical deterioration [21, 22]. A case report by Lin et al. described post-operative necrosis of a 10×12-cm myoma. As reported in the literature, many of these complications can result in the need for reoperation. Lin et al. reported in their meta-analysis a procedure-related adverse event rate of 1.78%. A critical point of discussion with patients should be related to complications related to this emerging technology and the small but apparent possible need for reoperation.
Comparison to Myomectomy
Although drastically different surgical techniques, Lap-RFA and myomectomy both offer minimally invasive treatment of fibroids and require abdominal incisions, yielding a question of how their outcomes compare to each other. The first comparative randomized trial was performed by Brucker et al., which found that Lap-RFA of fibroids led to a shorter hospital stay, treatment of a greater number of fibroids, and less intraoperative blood loss (169± vs 51±57 mL, p < 0.001)when compared to laparoscopic myomectomy without statistically significant difference in symptomatology at 12 months postoperatively [10]. Rattray et al. performed a post-market prospective randomized trial that found less intraoperative blood loss (25.2±21.6 versus 82.4±62.5 mL, p=0.0002), shorter procedure and hospitalization times, and faster return to work through 3 months posttreatment with Lap-RFA of fibroids compared to myomectomy [11•]. In addition, there was decreased use of disposable and reusable surgery equipment and reduced healthcare resource utilization with Lap-RFA of fibroids. This was then followed up with a 12-month review of the same trial, which reported significant improvement in fibroid symptom burden at both 3 and 12 months, but those who underwent Lap-RFA of fibroids were hospitalized at a statistically significant lower rate [23]. In addition, the amount of time to return to work was significantly lower in the patients who underwent Lap-RFA.
Recurrence Rates
The risk of recurrence is inherent to all uterine-sparing procedures for fibroids. Understanding recurrence rates is important in counseling patients on expectations after surgery and comparing this procedure to other fibroid management options. The reported 5-year reintervention rate for other fibroid procedures includes 19% for myomectomy (including all modalities), 33% for endometrial ablation, and 24% for uterine artery embolization [24]. In a 3-year follow-up of 135 patients who underwent Lap-RFA, the cumulative repeat intervention rate was 11%. Of these reinterventions, two of the 14 were myomectomies, and 11 were hysterectomies [17]. In a meta-analysis by Bradley et al., the reintervention rate was 4.2%, 8.2%, and 11.5% at 1, 2, and 3 years [12•].
Treatment of Adenomyosis
Though not FDA-cleared for the treatment of adenomyosis, surgeons in other countries have been reporting on its use for this application. Findings from several small case series and a single randomized controlled trial have been positive, demonstrating significant decreases in uterine volume and focal adenomyosis volume as well as symptomatic improvements on validated pain scales after Lap-RFA treatment [25,26,27]. These case series did not involve Lap-RFA, but rather needle electrode RF. Most reported post-procedural complications in these studies were minor and self-limited. Minimal information is available regarding post-Lap-RFA fertility outcomes in the literature. Thus, preliminary data suggests both the safety and efficacy of RFA for the treatment of adenomyosis, and further research is needed to confirm and expand on these findings acknowledging the limitations of the literature as far as systematic reporting of outcomes and complications.
Future Opportunities for Research
Laparoscopic radiofrequency ablation is a minimally invasive technique with a growing utilization in the treatment of fibroids over the last decade. A summary of Lap-RFA characteristics as indicated in the current literature is displayed in Table 1. As a relatively new technology, there are numerous research opportunities. Information regarding optimal patient selection, device improvements, and understanding characterizing long-term outcomes need to be expanded upon. The initial FDA-cleared study by Chudnoff et al. was limited by the exclusion of small myomas >7cm, menstrual blood loss >500mL, and uterus greater than 14 weeks in size [4•]. Since there have been subsequent studies showing successful use in fibroids up to 10cm [23]. In actual use, we have seen Lap-RFA applied to fibroids and uteri greater than these initial clinical studies.
Data on long-term outcomes of fertility and pregnancy in patients who desire future conception have yet to be conducted. This data is vital to aid in patient counseling and delivery planning, given that in the small case series of pregnancy after RFA cites an unknown effect on the uterus as the indication for primary cesarean delivery. Bongers et al. recently reported on uterine wall integrity as assessed by magnetic resonance imaging before and after transcervical radiofrequency ablation of fibroids and showed a preservation of uterine wall integrity [28]. While ablative technology between laparoscopic and transcervical approaches is similar, extrapolating this data to inform counseling for any type of RFA would be short-sighted as the laparoscopic approach requires perforation of the uterine serosa.
A one-treatment-fits-all approach for fibroids within a single uterus is limiting and ignores concomitant disease processes likely influencing symptoms. Other procedures, such as myomectomy (hysteroscopic and laparoscopic), have been performed at the same time as RFA to address the various fibroid types in a single patient. Research evaluating outcomes of combination therapy may expand the patient population that is offered this minimally invasive treatment.
Multimodal fibroid therapy draws the question as to whether RFA could be used preventatively, for example, at the time of hysteroscopic myomectomy for the treatment of small intramural fibroids. This has been proposed as a means to intervene before clinical burden narrows treatment options, limiting further growth and morbidity. However, rigorous evaluation for the treatment of asymptomatic fibroids would be required before such a recommendation could be made. Prospective fertility data is also needed before RFA treatment can be recommended as a preventative treatment, along with improving our understanding regarding the impact that repeated RFA therapy may have on normal uterine myometrium.
Expanding treatment options for benign gynecologic conditions is a slow process with limited resources directed towards them, but as these new technologies emerge, it is necessary to compare them to the current standard of care. Randomized control trials comparing different modalities of fibroid treatment would further illuminate the optimal patient selection for this technology. Research on long term outcomes, procedural comparisons, and technological innovations can help elucidate the optimal role for this procedure in treating patients with fibroids.
Conclusion
Lap RFA, though relatively new compared to established fibroid treatments, is no longer “novel” since being FDA-cleared in 2012. Current experiences demonstrate that women are seeking uterine-preserving options that are minimally invasive and non-extirpative. Available longer-term data have demonstrated that treatment effectiveness is robust with relatively low complication rates. Future avenues for exploration include the application of RF technology for adenomyosis, in concurrent treatment with other fibroid procedures such as myomectomy, and for applications in patients seeking future fertility.
Data Availability
All data presented in the article to support the findings are included in the article and supplementary materials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194–9.
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7.
Halt Medical Inc. FDA 510(k) Summary and Letter for Acessa System. 2012 Nov.
• Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstetrics and Gynecology. 2013;121(5):1075–82. This is the initial large cohort trial examining the safety and efficacy of Lap-RFA which revealed the procedure to be well tolerated with effective symptom relief.
Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;13(11):139.
Goldfarb HA. Laparoscopic coagulation of myoma (myolysis). Obstet Gynecol Clin North Am. 1995;22(4):807–19.
Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstetrics & Gynecology. 2002;9(S):99.
Lee BB, Yu SP. Radiofrequency ablation of uterine fibroids: a review. Curr Obstet Gynecol Rep. 2016;5(4):318–24.
Gynesonics Inc. FDA 510(k) Summary and Letter for SONATA System. 2018 Aug.
Brucker SY, Hahn M, Kraemer D, Taran FA, Isaacson KB, Krämer B. Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet. 2014;125(3):261–5.
• Rattray DD, Weins L, Regush LC, Bowen JM, O’Reilly D, Thiel JA. Clinical outcomes and health care utilization pre- and post-laparoscopic radiofrequency ablation of symptomatic fibroids and laparoscopic myomectomy: a randomized trial of uterine-sparing techniques (TRUST) in Canada. Clinicoecon Outcomes Res. 2018;10:201–12. A study comparing Lap-RFA with laparoscopic myomectomy, found Lap-RFA to be associated with less intraoperative blood loss and shorter procedure and hospitalization times with comparable direct and indirect costs.
• Bradley LD, Pasic RP, Miller LE. Clinical performance of radiofrequency ablation for treatment of uterine fibroids: systematic review and meta-analysis of prospective studies. J Laparoendosc Adv Surg Tech A. 2019;29(12):1507–17. A systematic review and meta-analysis of radiofrequency ablation noted a significant reduction in fibroid volume, improvements in quality of life, and favorable reintervention rates.
Lin L, Ma H, Wang J, Guan H, Yang M, Tong X, et al. Quality of life, adverse events, and reintervention outcomes after laparoscopic radiofrequency ablation for symptomatic uterine fibroids: a meta-analysis. J Minim Invasive Gynecol. 2019;26(3):409–16.
Braun KM, Sheridan M, Latif EZ, Regush L, Maksymowicz A, Weins L, et al. Surgeons’ early experience with the AcessaTM procedure: gaining proficiency with new technology. Int J Womens Health. 2016;8:669–75.
Jacoby VL, Parvataneni R, Oberman E, Saberi NS, Varon S, Schembri M, et al. Laparoscopic radiofrequency ablation of uterine leiomyomas: clinical outcomes during early adoption into surgical practice. J Minim Invasive Gynecol. 2020;27(4):915–25.
Yu S, Silverberg K, Bhagavath B, Shobeiri SA, Propst A, Eisenstein D. Post-market safety of laparoscopic ultrasound-guided radiofrequency ablation. JSLS. 2020;24(4).
Berman JM, Guido RS, Garza Leal JG, Pemueller RR, Whaley FS, Chudnoff SG, et al. Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21(5):767–74.
• Polin M, Hur HC. Radiofrequency ablation of uterine myomas and pregnancy outcomes: an updated review of the literature. J Minim Invasive Gynecol. 2022;29(6):709–15. A systematic review on 50 pregnancy outcomes after laparoscopic and transcervical radiofrequency ablation.
Young RJ, Puma L, Latham M, Kho KA. Radiofrequency ablation for treatment of leiomyomas: review of the Manufacturer and User Facility Device Experience (MAUDE) database. Obstetrics and Gynecology. 2023;142(1):147–50.
Baxter BL, Seaman SJ, Arora C, Kim JH. Radiofrequency ablation methods for uterine sparing fibroid treatment. Curr Opin Obstet Gynecol. 2022;34(4):262–9.
Lin E, Sendukas E, Kho KA. Postoperative uterine necrosis and peritonitis following laparoscopic radiofrequency myoma ablation. J Minim Invasive Gynecol. 2022;29(10):1123–4.
Niddam R, Netter A, Gauthier A, Calderon L, Agostini A, Miquel L. postoperative rectal necrosis after laparoscopic multibipolar radiofrequency myoma ablation. J Minim Invasive Gynecol. 2023;30(6):433–5.
Yu S, Bhagavath B, Shobeiri SA, Eisenstein D, Levy B. Clinical and patient reported outcomes of pre- and postsurgical treatment of symptomatic uterine leiomyomas: a 12-month follow-up review of TRUST, a surgical randomized clinical trial comparing laparoscopic radiofrequency ablation and myomectomy. J Minim Invasive Gynecol. 2022;29(6):726–37.
Davis MR, Soliman AM, Castelli-Haley J, Snabes MC, Surrey ES. Reintervention rates after myomectomy, endometrial ablation, and uterine artery embolization for patients with uterine fibroids. J Womens Health (Larchmt). 2018;27(10):1204–14.
Scarperi S, Pontrelli G, Campana C, Steinkasserer M, Ercoli A, Minelli L, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19(4).
Lin XL, Hai N, Zhang J, Han ZY, Yu J, Liu FY, et al. Comparison between microwave ablation and radiofrequency ablation for treating symptomatic uterine adenomyosis. Int J Hyperthermia. 2020;37(1):151–6.
Abdel-Fattah H, El-Lakkany N, Helal AS, Mosbah A, Abdel-Hady ES, Abdel-Shaheed M. Conservative laparoscopic electrocoagulation adenomyolysis for the management of symptomatic adenomyosis. Gynecol Surg. 2015;12(3):139–47.
Bongers M, Gupta J, Garza-Leal JG, Brown M, Felberbaum R. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35(5):299–303.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript text and reviewed the manuscript. Author NC created the mansucript table.
Corresponding author
Ethics declarations
Conflict of Interest
Authors 1, 2, and 3 report not conflict of interest. Author 4 has received a grant from Hologic for a PI investigator-initiated study, and serves on the Medical Advisory Board for Channel Med Systems.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cohen, N.D., Lin, E., Shields, J.K. et al. Laparoscopic Radiofrequency Fibroid Ablation: A Review of Current Use and Future Applications. Curr Obstet Gynecol Rep 13, 66–71 (2024). https://doi.org/10.1007/s13669-024-00382-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-024-00382-1