Abstract
Dillenia excelsa, a medicinal plant found in Brunei Darussalam, has been traditionally used by locals for treating various ailments. The present study aims to investigate the presence of phytochemicals, antioxidant properties, antibacterial activities and wound healing potential of methanolic extracts of the leaves, fruits, flowers and bark of Dillenia excelsa. Phytochemical screening using frothing test, Salkowski test, Dragendorff’s test and ferric chloride test revealed the presence of saponins, steroids, terpenoids, alkaloids and tannins. The total phenolic content (TPC) investigated using Folin-Ciocalteu reagent and total flavonoid contents (TFC) assessed using aluminium chloride colorimetric assay yielded the highest value of 106.3 ± 2.6 mg gallic acid equivalent per gram (GAE/g) of crude leaves extract and 23.1 ± 0.5 mg quercetin equivalent per gram (QE/g) of crude leaves extract, respectively. The antioxidant capacities of the extracts were assessed and showed the leaves extract has the highest 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity and ferric ion reducing antioxidant power with the value of 156.2 ± 59.3 mg GAE/g of crude extract and 2.77 ± 0.33 mg Trolox equivalent per gram (TE/g) of crude extract, respectively. Antibacterial activities against two gram-positive and two gram-negative strains using agar well diffusion method revealed only the flowers extract showed inhibition against Bacillus subtilis with minimum inhibitory concentration of 40 mg/mL. The in vivo wound healing potential was also carried out using male Wistar rats. The high dose leaves extract (50% w/w) showed the highest reduction in wound size at day 7, 10 and day 14 compared to the control groups and other treatment groups. Based on this present study, D. excelsa was shown to have the potential to promote wound healing and may serve as part of the armamentarium to traditional and complementary medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wounds have the intrinsic ability to negatively affect the quality of life of an individual with the cost being evaluated both financially and emotionally. Damage or disturbance to the normal anatomical structure and function is defined as a wound (Robson et al. 2001). A wound could be the outcome of an accident, an intended etiology, or a disease activity. It affects tissue and disturbs the local environment inside it, regardless of the source or forms it takes (Velnar et al. 2009). There are various types of wounds, including an incised wound, lacerated wound, abrasion, contusion, ulcer and burn wound (Somboonwong et al. 2012). Generally, the wound healing process includes four phases which are hemostasis, inflammation, proliferation, and wound remodeling (Machens 2012). Historically, as part of traditional practice, wounds have been treated topically with numerous medicinal plants or their extracts.
Dillenia, a term named after a German botanist Johann Jacob Dillenius, is a genus of about 100 species of flowering plants in tropical and subtropical trees of Southern Asia, Australasia, and the Indian Ocean Islands (Boulger and Mabberley 2004). Dillenia excelsa is known locally as “Simpor Laki” or “Simpoh Laki”. According to the Forest Research Institute of Malaysia, the plant is selectively distributed to Borneo Island, Peninsular Malaysia, and Indonesia especially in Sumatera and West Java (Tan and Latiff 2014). D. excelsa is usually found in lowland dipterocarp forest, most likely near a swampy area and can be described as an evergreen tree which grows up to more than 15 m tall (see Fig. 1a). The leaves of the plant can be oval (elliptic) or in an elongated form with parallel sides, almost similar to a rectangular shape (oblong) (see Fig. 1d). D. excelsa bears yellow flowers with purple colored stamen and pale pink or white carpel facing outwards (Fig. 1c). It also produces white, dehiscent star-shaped fruits (Fig. 1b). The features of its flowers and fruits are recognized as unique characteristics that separate D. excelsa from other species of Dillenia.
In Indonesia, D. excelsa fruits have been used for cooking, either in its unprocessed or dehydrated form to improve the taste of plant-based dishes (Sunariyati 2018; Lisdiani et al. 2022). It was employed as a holistic remedy with the purpose of alleviating and managing a diverse range of ailments. For example, the leaf buds, used by local tribes, were immersed in heated water and ingested to treat diarrhea (Lisdiani et al. 2022). Similarly, it was claimed that ingesting a decoction derived from the root of D. excelsa has the potential to alleviate symptoms of diarrhea (Quattrocchi 2012). Goh et al. (2017) also suggested that the D. excelsa bark could alleviate symptoms of diarrhea and stomach aches. The sap, leaves and bark of D. excelsa was also used for the treatment of malaria and toothache (Denny et al. 2021). The indigenous Dayak Tribes residing in Borneo Island, together with the local population of Brunei Darussalam, have long held the belief that the bark of D. excelsa had remarkable abilities in promoting the healing of wounds. (Santoso et al. 2019; Az-Zahra et al. 2021) However, these claims have lacked scientific verification.
To date, there are very few available scientific studies on the pharmacological reports of D. excelsa. Bate-Smith and Harborne (1971) claimed to have discovered many flavonoid chemicals in D. excelsa, including kaempferol, quercetin, rhamnetin, isorhamnetin, kaempferide, and leucocyanidin in an early investigation. A study by Thooptianrat et al. (2017), which analyzed the chemical constituents of D. excelsa leaves hexane extract using gas chromatography–mass spectrometry (GCMS), showed the presence of oleamide, vitamin E, palmitic acid, palmitamide, stearic acid and phenol. The study then went on to claim that D. excelsa extract has the potential to treat Alzheimer’s disease. A more recent study by Metussin et al. (2018) has reported that the methanolic extract of D. excelsa leaves exhibited antidiabetic effect on alloxan-induced diabetic rats and has the ability to facilitate the regeneration of islet tissue in the pancreas. There have been no additional reported investigations on D. excelsa since then. Despite the limited scientific studies on D. excelsa, there were various publications found on the other species of Dillenia on their phytochemistry and pharmacological activities such as on the antioxidant and wound healing activities (Abdille et al. 2005; Kviecinski et al. 2017; Alam et al. 2020; Muharni et al. 2022). Due to the lack of reports concerning D. excelsa, we have sought to determine the antioxidant activities and its potential in in vivo wound healing.
Materials and methods
Chemicals
All the chemicals used in this study were of analytical grade. These chemicals and reagents were used as received from the manufacturers without further purification.
Plant material
The leaves, fruits, flowers and bark of Dillenia excelsa were collected from Tutong District, Brunei Darussalam in July 2019 (Fig. 1). The species was authenticated by Hazlina Zaini in Universiti Brunei Darussalam Herbarium by comparison with a voucher specimen number S00757.
Preparation of extract
The leaves, fruits, flowers and bark of Dillenia excelsa were washed with distilled water to remove any foreign particles and dried using dehumidifier (Delonghi Tasciugo AriaDry Multi Dehumidifier DEXD216F) until constant mass was obtained. The dried samples were later ground and sieved. 15 g of ground powder of size less than 106 micron was then extracted with 300 mL of 80% (v/v) methanol using Soxhlet apparatus. The extract was then concentrated under reduced pressure at 40 °C in a vacuum rotary evaporator. The concentrated extract was then air dried to a constant weight at room temperature and stored in a desiccator until further use.
Preliminary phytochemical analysis
Extracts were subjected to various chemical tests in order to detect the presence of different phytochemicals such as alkaloids, tannins, saponins, steroids, and terpenoids. The qualitative tests were based on visual observation of color changes and formation of precipitate after the addition of chemical reagents to the extracts.
Alkaloids
The alkaloids were tested using the Dragendorff’s test, in accordance with the method stated by Jamila et al. (2016). 0.01 g of extract was stirred in 4.0 mL of 1% (v/v) HCl on a 25 °C water bath for 5 min and then filtered. The filtrate was added with 1 mL of Dragendorff’s reagent or potassium bismuth iodide solution. The formation of orange red precipitate indicates the presence of alkaloids in the sample.
Tannins
The screening for the presence of tannins in the samples was done following the procedure by Aderotimi and Samuel (2006). 0.5 g of the crude extract was dissolved in distilled water followed by gravitational filtration. 5% (v/v) ferric chloride was then added drop by drop to the filtrate. The presence of tannin will result in a formation of black or blue-green precipitate.
Saponin
Saponin presence in the sample was tested using protocols laid out by Aderotimi and Samuel (2006). Briefly, 0.5 g of the crude extracts was vigorously shaken with 10 mL of distilled water in a test tube. Positive results for saponin will show formation of stable frothing even after the test tube was warmed in a water bath for 5 min.
Steroids and terpenoids
The Salkowski test was used for the screening of steroids and terpenoids, based on the procedure by Joshi et al. (2013). 5.0 mL of chloroform shaken with 0.1 g of crude extract. A few drops of acetic anhydride were then added to the test tube and left in a water bath for a few minutes. The test tube was then removed and left to cool in an ice-water bath. Then, 2.0 mL of concentrated sulfuric acid was added slowly. A formation of brown rings in between the two layers of the solvents and the upper layer turning green indicates the presence of steroids. However, if a deep red color was formed, it indicates the presence of triterpenoids.
Total phenolic content
The total phenolic content (TPC) of the crude extracts were determined using the Folin-Ciocalteu reagent in accordance with the procedure reported by Chlopicka et al. (2012) with minor modifications. Briefly, the crude extracts were dissolved in methanol and mixed with 2.5 mL of ten-fold diluted Folin-Ciocalteu phenol reagent. After 5 min, 2.0 mL of 7.5% (w/v) sodium carbonate was added to the mixture and left to stand for 1 h in the dark at room temperature. The absorbance was then measured at 760 nm using a single beam UV spectrophotometer (GENESYS™ 20S, Thermo Scientific Inc., USA). A standard calibration curve of gallic acid was obtained by plotting the concentrations of gallic acid ranging from 0 to 100 mg L−1 against their respective absorbance. The results were expressed as milligram gallic acid equivalent per gram (GAE/g) of crude extracts using Eq. 1.
Total flavonoid content
The total flavonoid content (TFC) of the crude extracts was determined using aluminium chloride colorimetric method following the procedure described by Lin and Tang (2007) with slight modifications. 0.5 mL of the standard quercetin was mixed with a solution containing 0.1 mL of 10% (w/v) aluminium chloride, 0.1 mL of 1 M potassium acetate, 1.5 mL of 80% (v/v) methanol, and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 min and the absorbance of the resultant mixture was recorded at 415 nm using a single beam UV spectrophotometer (GENESYS™ 20S, Thermo Scientific Inc., USA). A blank solution was prepared by using 0.1 mL potassium acetate, 1.5 mL 80% (v/v) methanol and 3.4 mL distilled water. A standard calibration curve was obtained using a quercetin solution with concentration ranging from 0 to 100 mg L−1, and results were expressed as milligram quercetin equivalent per gram (QE/g) of crude extract calculated using Eq. 2.
Antioxidant capacities
2,2-diphenyl-1-picrylhydrazyl radical (DPPH)
Free radical scavenging activity of the crude extracts was assayed using 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) following method described by Abdul Wahab et al. (2011) and Hashim et al. (2017) with slight modifications. A series of concentrations of plant extracts were prepared in methanol (10 mg L−1 to 500 mg L−1). 0.5 mL was taken from each concentration and mixed with 1 mL of 50 mg L−1 methanolic DPPH and incubated for 30 min in the dark at room temperature. The absorbance was measured at 517 nm using a single beam UV spectrophotometer (GENESYS™ 20S, Thermo Scientific Inc., USA) and compared with the standard gallic acid. The percentage inhibition of extracts was calculated using Eq. 3 for each concentration and plotted in graphs of percentage inhibition against concentration of the respective standard or sample extract.
where Ac = Absorbance of control (methanolic DPPH only) and As = Absorbance of standard or sample extract.
The free radical scavenging activity was expressed as IC50, that is, the concentration of standard gallic acid or sample extracts needed to inhibit 50% of the DPPH radicals. IC50 values can be determined from the plots of percentage inhibition against concentrations of gallic acid.
Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) assay of the crude extracts were determined according to the reported methods by Thaipong et al. (2006) with minor modifications. The FRAP reagent was freshly prepared by mixing 100 mL of 300 mM sodium acetate buffer, 10 mL of 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution and 10 mL of 20 mM FeCl3·6H2O solution. The FRAP reagent was kept warmed at 37 °C until ready to be used. 150 µL of extract was allowed to react with 2850 µL of FRAP solution for 30 min in the dark at room temperature. The absorbance was measured at 593 nm using a single-beam UV spectrophotometer (GENESYSTM 20S, Thermo Fisher Scientific Inc., USA) and compared with standard Trolox. A calibration graph was produced by plotting the concentrations of standard Trolox ranging between 0 to 250 mg/mL against their respective absorbances. The results were expressed as milligram Trolox equivalent per gram (TE/g) of crude extracts, which were derived from standard calibration graphs. FRAP were measured using Eq. 4.
Antibacterial assays
The antibacterial efficacy of the extracts was determined by agar well diffusion method as described by Chah et al. (2006) with slight modification using four bacterial strains. Two gram-positive strains (Staphylococcus aureus (ATCC 25923) and Bacillus subtilis (ATCC 23857)) and two gram-negative strains (Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853)) was prepared and cultured from commercially available bacterial stock (American Type Culture Collection, USA). Mueller–Hinton agar (MHA) was prepared according to the manufacturer's instructions. 1 L of MHA was sterilized by autoclaving at 121 °C then allowed to cool to a temperature of 45 °C. 20 mL of the MHA was poured into a sterile petri dish and allowed to solidify. Each MHA plate was inoculated with standardized culture (equivalent to 0.8 McFarland) and four holes of 6 mm diameter were punctured. The wells were filled with 50 µL of streptomycin (20 mg/m) sulfate (positive control), distilled water (negative control) and prepared extracts of leaves, fruits, flowers and bark of D. excelsa at different concentrations. The controls and extracts were allowed to diffuse and incubate for 24 h at 37 °C. After incubation, photographs were taken, and the zone of inhibition was measured using ImageJ software. Each screening was repeated 4 times.
Minimum inhibitory concentration (MIC)
In this study, the identification of MIC of the plant extracts was conducted using a broth-dilution technique on a 96-well plate according to the method described by Wilson et al. (2005) with slight modifications. The MIC analysis was carried out only for extract that showed positive antibacterial inhibition. Serial two-fold dilutions of the extracts were prepared in nutrient broth with concentrations ranging from 80 to 0.078 mg/mL. 50 µL of standardized bacterial culture of concentration 1 × 106 CFU/mL was inoculated into all wells containing extracts. The plate was then incubated for 24 h at 37 °C. Viable and non-viable bacteria were identified by adding 50 µL of 20 mg/mL of 2,3,5-triphenyltetrazolium chloride (TTC) solution to each well and further incubated at 37 °C for 30 min in the dark. Finally, the plate was monitored using a microplate reader (ELX800UV, Bio Tek, USA) to measure its absorbance at 625 nm.
Wound healing study
Animals
Male Albino Wistar rats aged between 8–12 weeks and weight ranging from 250 to 350 g were used throughout the experiment for the wound healing study. The animals were obtained and housed in the Universiti Brunei Darussalam animal facility.
All work involving animals was approved by the Universiti Brunei Darussalam University Research Ethics Committee (approval reference UBD/FOS/N1(I)). All procedures were carried out in accordance with the Universiti Brunei Darussalam guidelines on the care and use of animals for research and also internationally accepted guidelines.
Rats were kept under optimal conditions with controlled humidity under 12 h of light and dark cycles. They were kept inside white polycarbonate cages filled with standard bedding with not more than 6 adult rats per cage. Standard pellet diet (Altromin Spezialfutter, GmBH & Co., Germany) and access to clean tap water were provided ad libitum. Rats that showed abnormality were dismissed from the experiment and excluded from the results. Upon completion of the experiments, animals were euthanized by using carbon dioxide asphyxiation.
Preparation of wound healing ointment
Petroleum jelly (Vaseline) was used as the base to incorporate the methanolic extract of leaves, fruits, flowers and bark of D. excelsa for wound healing application. The formulation was made by melting 1 g of Vaseline and combining it with 1 g of extract to obtain 50% (w/w) high dose (HD) ointment. The ointment was stirred until homogeneous. The same procedure was also carried out in the preparation of 10% (w/w) low dose (LD) ointment using a 9 Vaseline: 1 extract ratio. The ointments were kept in airtight vials and stored inside a refrigerator prior to use.
Animal Groups
The animals were randomly grouped according to their respective treatments at n = 6 per group (Table 1). The untreated control group received no ointments at all while vehicle control group were treated with Vaseline only. Animals in the treated group received either 10% (w/w) LD or 50% (w/w) HD D. excelsa extracts mixed with Vaseline, which were topically applied on the wound surface for each designated group every alternate day.
Excision wound model
The dorsal thoracic region of rats was shaved using an electric razor while they were still under anesthesia using diethyl ether administered via inhalation anesthesia. The dorsal fur of the animals was shaved and an area of about 100 mm2 was excised. The excised rats were kept inside an individual cage and left to recover from anesthesia. Topical application of the prepared ointments was administered to the wound specifically at day 1, 3, 5, 7, 10, 12 and 14 for each treatment group.
Wound area determination
Photographs of the wound were taken using a digital camera (Sony Cyber-shot DSC-W810 camera). Every photograph includes an anesthetized rat which was kept flat on board with a plastic card labeled with experimental group name, day number and 10 mm scale bar placed near the wound site for measurement analysis (Fig. 2). The wound site was observed and analyzed via the digital images using ImageJ software (National Institute of Health, USA). Calibration of scale was carried out first by setting in the known distance of the scale bar from the photograph. Once calibrated, the wound area was outlined using the free hand selection option on the menu bar of the software followed by a measure which automatically evaluated the outlined wound area (Fig. 2).
The average wound area obtained from three readings for each rat in each group was calculated using Eq. 5.
Statistical analysis
The data in this study was subjected to statistical analysis using one-way ANOVA and the results were expressed as their mean ± standard deviation (SD). To assess the differences between two extracts, Student’s t-test was used and p value < 0.05 was considered statistically significant.
Results and discussion
Percentage yield of crude extracts
The percentage yield of the extracts is presented as a bar chart (Fig. 3). The average percentage yield values of the leaves, fruits, flowers and bark was 36.42% ± 1.35, 12.87% ± 1.31, 11.67% ± 0.80 and 7.53% ± 0.16 per gram of dried weight of sample, respectively. The values were expressed as mean ± standard deviation (SD) of repeats (n = 3).
The percentage yield of different plant species may vary owing to the variations in plant genetic composition, sample location, harvesting time, extraction type, and most importantly, the selection of solvent utilized in extraction. Methanol is one of the solvents that is frequently used for extraction and constantly produces higher percentage yield when compared to other solvents. For instance, Yakop et al. (2020) used methanol and water to extract the leaves of Dillenia suffruticosa and reported that the percentage yield was higher in methanolic extract (21.8%) than the aqueous extract (9.2%).
The extraction yields of the different plant parts used in this study were compared to other Dillenia species since D. excelsa have yet to be reported to the best of our knowledge. Research conducted by Dawood Shah et al. (2020) has reported that methanolic leaves extract of Dillenia suffruticosa has a percentage yield of 9.27% ± 0.75. Our result herein was approximately four times higher when compared to D. suffruticosa extract. Another study by Erin et al. (2013) found that methanolic stem bark and leaves extract of Dillenia ovata yielded 5.21% and 4.80%, respectively. Again, our bark extract showed higher percentage yield than their study. This might be due to the different extraction method and type of solvent used, in which they used n-hexane, ethyl acetate, methanol and water for sequential extraction via Soxhlet whereas our study only used methanol as a solvent. On the other hand, methanolic bark extract studied by Monirul Islam et al. (2013) showed 15.0% percentage yield which was approximately double our percentage yield. The discrepancy might be due to their extraction procedure – methanol reflux. Overall, the variations were also possibly due to the different chemical constituents extracted from each species and also the geographical location of the plant collected.
Preliminary phytochemical screening
The phytochemical screening revealed the presence of saponin, terpenoid, alkaloid, tannin and steroid (Table 2). All parts of the plant extracts contain saponins and terpenoids. Alkaloids and tannins were present in all extracts except the bark extract while steroids can only be found in leaves extract.
Terpenoids have been shown to have anti-tumor, antimicrobial, and anti-aging properties, while saponins are natural detergents with anti-inflammatory, phlegm-removing, immune-stimulating, anticancer, and blood cholesterol-lowering activities (Shi et al. 2004; Kokkaiah et al. 2016; Guimarães et al. 2019; Proshkina et al. 2020; Yang et al. 2020). Plant steroid hormones are a valuable source of medication because they have cardiotonic, antibacterial, and insecticidal qualities (Bagrov et al. 2009). Tannins are believed to be a potent anticancer agent with antioxidant effects (Sieniawska and Baj 2017).
There were several studies carried out for the screening of phytochemicals of D. excelsa using gas chromatography-mass spectrometry (GCMS). The phytochemicals found in the leaves include oleamide, Vitamin E, palmitic acid, palmitamide, stearic acid, phenol and kaempferide (Bate-Smith and Harborne 1971; Thooptianrat et al. 2017). Comparing to other species of Dillenia, Venkata Smitha et al. (2012) carried out a preliminary phytochemical screening of methanolic bark extract of D. pentagyna which shows the presence of alkaloids, flavonoids, saponins, tannins and phenols Another preliminary screening study by Patle et al. (2020) showed the presence of alkaloids, flavonoids, phenols, terpenoids, tannins, saponins and carbohydrates in the ethanolic, chloroform and n-hexane extracts of bark, leaves, sepals, fruits and seeds of D. pentagyna. Variations of phytochemical constituents in different parts of the plant can be explained by their respective biosynthesis. Meaning, from the biosynthesis, various secondary metabolites will be produced in different organs and tissues of the plant which is regulated by several factors such as deoxyribonucleic acid (DNA) methylation and histone modifications (Vriet et al. 2015). Therefore, different parts of a plant will have different phytochemicals and active compounds in them, hence showing different polyphenols content such as the total phenolic and flavonoid content.
Total phenolic and flavonoid contents
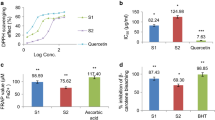
The total phenolic content (TPC) and total flavonoid content (TFC) for the extract of the leaves, fruits, flowers and bark of D. excelsa are tabulated in Table 3. The methanolic leaves extract of D. excelsa possessed the highest TPC value which is 106.3 ± 2.6 GAE/g of crude extract followed by bark (75.6 ± 2.5 GAE/g of crude extract), flowers (56.3 ± 1.5 GAE/g of crude extract) and fruits extract (50.8 ± 2.5 GAE/g of crude extract) (Fig. 4). Similarly, the leaves extract showed the highest TFC value of 23.1 ± 0.5 QE/g of crude extract followed by flowers, fruits and bark extracts with TFC values of 17.7 ± 1.0 QE/g of crude extract, 10.7 ± 0.6 QE/g of crude extract and 2.8 ± 0.6 QE/g of crude extract, respectively (Fig. 4).
a Total phenolic content (TPC) expressed as gallic acid equivalent (GAE) per gram of crude extract and b Total flavonoid content (TFC) expressed as quercetin equivalent (QE) per gram of crude extract for methanolic extracts of leaves, fruits, flowers and bark of D. excelsa. Error bars represent standard deviation (SD) of repeats (n = 3). ap < 0.05 compared to leaves extract, bp < 0.05 compared to fruits extract, cp < 0.05 compared to flowers extract
Phenolic compounds, which are a varied collection of secondary metabolites, display variation in their distribution and content across different plant parts and species (Robards et al. 1999; Mwamatope et al. 2020). This explains the variation of the TPC values of D. excelsa extracts in our study. The high TPC value reflects high content of phenols in the extracts such as gallic acid, ethyl gallate, protocatechuic acid and protocatechuic acid methyl ester (Sabandar et al. 2017). Another important aspect influencing the TPC values is the choice of solvents used for extraction. In this case, the methanol used for the Soxhlet extraction may increase the TPC values of the leaves, fruits, flowers and bark of D. excelsa. It could be explained by some of the phenolic compounds in the extract forming complexes with phenol groups or higher molecular weights, making them more soluble in methanol, compared to other type of solvents (Do et al. 2014). It complements the studies by Iloki-Assanga et al. (2015) and Padalia et al. (2018), whereby the methanolic extracts of their respective plants were found to have the highest amount of TPC in general. Conversely, a lower TPC value might be caused by the presence of polyphenol oxidases, an enzyme that speeds up the breakdown and oxidation of polyphenols into quinones, which then undergo sequential polymerization to generate the unwanted melanin pigments (Mayer and Harel 1979).
Similarly, the high TFC value in the extract of D. excelsa could be from the presence of flavanols, kaempferide and quercetin as reported by Thooptianrat et al. (2017) and Bate-Smith and Harborne (1971). There is no report yet on the TFC of D. excelsa but other Dillenia species have been studied for their TFC. For instance, a study by Ansari et al. (2021) showed the leaves of D. philippinensis contain TFC value of 23.07 ± 0.35, 20.13 ± 0.78, 44.44 ± 0.80 and 10.06 ± 0.43 QE/g of crude extracts in ethanol, hexane, ethyl acetate and butanol solvent respectively. This also suggests that the TFC values depend on the type of solvents used for extraction. The polarity of the flavonoids extracted is similar to the polarity of the solvent used (Benmeziane and Madani 2019). In contrast, the low TFC value could be due to the glycosylation of flavonoids such as rutin, myricitrin and astragalin (Rice-Evans et al. 1996; Sengul et al. 2009). From our finding, the leaves extract shared the same trend in the TPC and TFC value, that is the highest content. The statistical study demonstrated a positive correlation between each other where the correlation coefficient, r is relatively high (r = 0.922).
Antioxidant capacity
Many variables impact antioxidant capacity, which cannot be adequately characterized by a single approach. As a result, to account for the varied mechanisms of antioxidant activity, more than one sort of antioxidant capacity assay must be performed (Wong et al. 2006). In our approach, we used 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and Ferric Reducing Antioxidant Power (FRAP) assay.
2,2-diphenyl-1-picrylhydrazyl radical (DPPH)
Our results (Table 4) revealed that the leaves extract had the highest DPPH RSA followed by the fruits extract, flowers extract, and bark extracts with the value of 156.2 ± 59.3 GAE/g of crude extract, 71.7 ± 18.8 GAE/g of crude extract, 59.6 ± 6.7 GAE/g of crude extract and 25.3 ± 0.7 GAE/g of crude extract, respectively. There are no reported studies on the DPPH RSA of D. excelsa, thus our findings will be compared to other Dillenia species. A study by Saha et al. (2009) showed the DPPH IC50 value of 100.53 µg/mL for the ethanolic leaves extract of D. indica where ascorbic acid was used as their standard reference. Moreover, a study on D. indica was also carried out by Shendge et al. (2011), whereby the DPPH IC50 value was 38.40 µg/mL.
Generally, the antioxidant activities are frequently associated with active phenolic compounds due to their ability to scavenge DPPH radicals. Our findings of antioxidant activities of the leaves extract of D. excelsa by DPPH assay show uniformity with our TPC and TFC results which could suggest that polyphenol compounds are acting as the primary antioxidant compounds that are significant in scavenging the stable DPPH free radicals. To support this, correlation studies of the TPC versus DPPH and the TFC versus DPPH of the leaves extract were carried out which showed a strong positive correlation with the correlation coefficient, r value of 0.999 and 0.908 for TPC versus DPPH and the TFC versus DPPH, respectively.
The slightly higher correlation coefficient between the TPC and the DPPH RSA of the leaves extract suggests that the phenolic compounds may have been more involved and contributed more to the antioxidant activity compared to the flavonoids. In contrast to the high DPPH RSA, the bark extract herein fostered the lowest DPPH antioxidant activity even though the TPC value was relatively high. This might be owing to the Folin-Ciocalteu assay's low specificity (Singleton et al. 1999; Escarpa and González 2001). The stability of the extracted phenolic compounds is dependent on their bulky size, conformations, and the location of their side chain functional group. As a result, the bioactive compounds have more difficulty in donating protons and producing free radicals due to the steric hindrance (Ahmed et al. 2015; Bazylak and Gryn 2015; Ichikawa et al. 2019). The DPPH molecule's nitrogen radical site sits in the center of the molecule, surrounded by side chains, hence bigger antioxidants will struggle to reach the radical site to neutralize the free radical and create a stable product. The low amount of phytochemicals in the extracts may also have contributed to the low DPPH scavenging activity.
Ferric reducing antioxidant power (FRAP)
The results of our study (Table 4) revealed that the leaves extract shows the highest FRAP value which is 2.77 ± 0.33 TE/g of crude extract, followed by the fruits, flowers and bark with the FRAP value of 1.58 ± 0.05 TE/g of crude extract, 1.33 ± 0.22 TE/g of crude extract and 0.25 ± 0.10 TE/g of crude extract, respectively. The leaves extract was revealed to have a higher FRAP than the other parts of the plant (fruits, flowers, and bark extract of D. excelsa), which may be caused by the phytochemical compounds known to be present in the leaves. To compare, a study by Tene et al. (2021) demonstrated the methanolic leaves extract of D. pentagyna has a high FRAP value at approximately 180 mg ascorbic acid equivalent per gram (AAE/g) of crude extract. The FRAP of methanolic leaves extract of D. indica was also studied by Dutta et al., (2018), whereby the reducing power was 30.9 ± 1.3 GAE/g of crude extract. Their FRAP values are more than ten times greater than ours. The discrepancy in our result might be attributed to some of the phytochemicals in the leaves extract being inactive or inert towards Fe3+ ions due to steric inaccessibility of the antioxidant (Bazylak and Gryn 2015).
Antibacterial properties
Our study shows that the flowers extract of D. excelsa inhibits the growth of B. subtilis at concentrations of 40 mg/mL and 80 mg/mL (Table 5). The other extracts showed no inhibition on all of the bacterial strains at the concentrations tested. At concentrations of 40 mg/mL and 80 mg/mL, the zone of inhibition was found to be 8.59 ± 1.29 mm and 10.48 ± 0.13 mm, respectively. The inhibition zones demonstrated that the flowers extract revealed relatively low antibacterial properties when compared to the positive control which showed more than threefold of the value.
The results indicate that the inhibitory activity of the flowers extract against B. subtilis exhibits a concentration dependent effect. The inhibition values demonstrated that the flowers extract revealed relatively low antibacterial properties when compared to the positive control which showed more than threefold of the value. The limited action of the flowers extracts on both of the gram-negative bacteria used in our study (E. coli and P. aeruginosa) supports the hypothesis that gram-negative bacteria are more resistant to antibacterial treatments than gram-positive bacteria. This is due to the unique outer membrane of gram-negative bacteria, which can keep hazardous chemicals out of the cell (Mai-Prochnow et al. 2016). Thus, the extract could not invade and inhibit the gram-negative bacterial strains, E. coli and P. aeruginosa. Moreover, the flowers extract of D. excelsa was also unable to inhibit S. aureus, the gram-positive bacterium. This might be owing to their ability to produce extremely resistant endospores that can survive hazardous chemicals (Remize 2017).
Plant-based polyphenols, such as flavonoids, were known to be effective sources of antimicrobial agents, therefore the antibacterial activity of D. excelsa flowers may be linked to their abundance of flavonoids (Cushnie and Lamb 2005). Flavonoids are naturally synthesized in plants in response to bacterial infections, and their capacity to damage bacterial cytoplasmic membranes, complex with cell walls and extracellular proteins, and agglutinate cells explains their ability to suppress bacteria (Yadav and Agarwala 2011; Babii et al. 2016). The simple mechanism of the antibacterial properties of the flowers extract of D. excelsa may be linked to synergistic interactions between the antibacterial compounds whereby each compound plays a different role to a targeted site which could enhance the overall antibacterial activities. For example, the presence of phenolic acids, which may damage bacterial cytoplasmic membranes, would make it easier for other antibacterial agents with intracellular target sites to enter the bacterial cell (Sanhueza et al. 2017). Other than that, tannins have been reported to be active against B. subtilis (Kumar and Pandey 2013). Since early screening of flower extracts indicated that tannin was present, it may have contributed to the antibacterial effects.
Excision wound healing application
The morphological observations of the wound were carried out to assess wound healing progression in all experimental groups at day 1, 3, 7, 10, 12 and 14 following wound excision. The wound area measurements were also obtained for all experimental groups using ImageJ software. Further, the percentage of wound contraction of all experimental groups was also determined (Eq. 5).
Wound evaluation
Photographs for all wounds of the experimental animals were taken in order to observe their wound healing progress specifically at day 1, 3, 5, 7, 10, 12 and 14 after excisions was made. Wound healing events of each experimental group are broken down in a time-dependent manner (Figs. 5 and 6).
From Figs. 5 and 6, it can be seen that all the groups including the control groups displayed noticeable reduction in the wound surface area from day 1 to day 14. There was minor reduction in wound surface areas on the first five days, but starting day 7 and day 10, it showed greater reduction in wound areas for the HD and LD extracts treated groups, respectively. The wound area of the HD leaves and bark extract were closed on day 14 when compared to the other groups. It is possible that the HD extracts accelerate the wound contraction when compared to the LD extracts as there will be more of the plant’s phytochemical constituents aiding the wound healing process. The healing of the untreated control group could be associated with the self-immunity of the individual animal as suggested in an early study by Dash et al. (2001). In contrast, there is a slight increase of wound area specifically for HD flowers and LD bark extract between day 1 and day 3 which may be due to the elastic nature of the skin (Everett and Sommers 2013). Another possible explanation is that an inflammation stage may occur during this time, whereby during this phase, the edge of the wound becomes red and swollen, thus increasing the wound area (Ih et al. 2018).
The scab formation of the HD and LD extracts treated groups can be seen to develop on day 3 and day 5, respectively. The scab eventually detached on day 10 for all the HD treated groups except for the HD flowers extract which detached earlier on day 7. The scabs of the LD treated groups detached on day 12 except for the LD bark extract which detached on day 7. Scab formation is significant in the wound healing process as they act as a temporary barrier between the wound and the atmosphere (Nagori and Solandi 2011). The surroundings may have pathogenic microorganisms, dust, and dirt specks, which can delay the healing process. As a result, an undesirable chronic wound may happen where the wound healing progress is stuck in one phase, generally inflammation, and does not advance to the next phase (Anderson and Hamm 2012). The scabs create a perfect environment under the surface for cell migration and wound edge displacement. Once the wound constricted and granulation tissue filled in the wound, then the scab will fall off and show smaller and red granulation tissue surface (Phillips 2000). Granulation tissue state is frequently a good predictor of how the wound is healing, therefore having granulation tissue that does not bleed easily and has a pink or reddish tinge is suggestive of healthy granulation tissue (Flanagan 2000).
Some groups still demonstrated a small wound area opening at the end of 14 days. However, compared to the control groups, the HD and LD extracts treated groups showed improved wound contraction during the final week of treatment. As a result, the wound healing integrity of the extracts treated groups based on the macroscopic observation such as formation of scab, detachment of scab and reduction in wound area, the HD leaves, HD and LD bark extracts showed the most promising results.
Reports on the wound healing effect on Dillenia species are limited. To the best of our knowledge, wound healing studies using D. excelsa have not been previously reported. Migliato et al. (2011) has found that there is a significant effect of wound healing on a lesion wound on Wistar rats using glycolic extract of D. indica. The wound healing effect reported by reported. Migliato et al. (2011) was linked to the anti-inflammatory characteristics of the D. indica fruits. This suggested link between wound healing effect and anti-inflammatory activity was also supported by Zakaria et al.(2006). Where the wound healing effect of M. malabathricum was directly related to anti-inflammatory activity. Interestingly, flavonoids, terpenoids and tannins have been reported to show anti-inflammatory activities, as these compounds briefly act as an inhibitor of the nuclear factor-κB (NF-κB) which controls the expression of growth factors involved in the wound healing process (Ramesh et al. 1998; Beirith et al. 1999; Karumi et al. 2003; Kim et al. 2004; Nam 2006). Since all D. excelsa extracts (except for bark extract) used in our study contain flavonoids, terpenoids and tannins, these chemicals may have contributed to the wound healing effect observed. Future works looking into the anti-inflammatory activity of D. excelsa should be done to further investigate and correlate with the wound healing properties.
The average percentage of wound contractions (n = 3) on day 3, 5, 7, 10, 12 and 14 after excision are tabulated on Table 6. Looking at this table, it shows that the average wound healing contraction at the end of the analysis for both the HD and LD groups eventually reached above 99% except for the HD flowers extract which only reached 98.3% for the time span of 14 days. In general, the untreated control group had better progression of wound contraction on day 5 to day 7, while the wound contraction of the experimental groups progressed better starting day 7 towards the end. Notably, on day 3, the untreated control group, HD fruits extract and LD bark extract displayed negative values which indicate expansion of wound. This may be due to the elastic nature of the skin (Everett and Sommers 2013). It often appears that the initial wound site in rats will be enlarged. Similar events were also observed in other studies (Shafie et al. 2020; Sofrona et al. 2020). The most apparent difference can be seen during day 5 of the experiment, whereby the wounds of the untreated control group are progressing significantly better compared to the experimental groups in general. Once reaching day 7, the experimental groups showed improved wound healing compared to the control groups. Consecutively, from day 5 to day 10 of the wound healing treatment, the HD groups show faster wound contraction than the LD groups in general. However, starting day 12, the LD groups keep up with the HD groups, thus showing similar wound contraction values. Focusing on the flowers extract, the wound contraction on the LD displayed better progress than the HD. The HD flowers extract has the potential to disrupt blood clotting or the function of platelets, as well as impact inflammatory responses and cell growth, which may influence the process of wound healing (Guo and DiPietro 2010). Similar activity was observed in some studies, both in vivo and in vitro (Wang et al. 2013; Gao et al. 2018).
To better see the rate of wound contraction for the individual parts of the D. excelsa used in our study, the percentage wound contraction was compared between the untreated wound, Vaseline only and extracts in high and LD (Fig. 7a–d). It can be seen that the leaves extract (see Fig. 7a) showed dose dependent performance as the healing of wound was better in HD compared to LD for 14 days. The bark extract also showed similar results for the first week. However, after 7 days, the LD bark extract performed better towards the end (see Fig. 7b). This trend was also alternately observed for the fruits extract in Fig. 7c whereby LD extracts were showing better performance in healing the wound up to day 5 but once reaching day 7, the HD extract showed more prominent healing. In contrast, the LD flowers extract showed a higher percentage of wound contraction than its HD extract throughout the 14 days of the experiment (see Fig. 7d).
Percentage of wound contraction (%) for low dose and high dose leaves extract of D. excelsa including the control groups at day 3, day 5, day 7, day 10, day 12, and day 14 for a fruits extract, b bark extract, c flowers extract and d leaves extract. The error bars represent SD of the mean values (n = 3)
Overall, based on Fig. 7, all of the extracts showed similar effects on the doses as their performance at each day indicated in the graphs fluctuated throughout the experimental period. The wound permeability may vary throughout the transition of wound healing phases, thus giving out different effects across the individual rats used (Dorsett-Martin and Wysocki 2008).
The change in average wound area between each assessment day (day 1, 3, 5, 7, 10,12 and 14) was calculated for the leaves, fruits, flowers and bark extracts of D. excelsa, both in high and LD including the control groups. The values were obtained by subtracting the average wound area between day 1 versus day 3, day 3 versus day 5, day 5 versus day 7, day 7 versus day 10 and so on. The progress on the wound healing phases can generally be observed including the inflammatory phase and proliferation phase based on these values.
The variation on the change in average wound area across the days (see Fig. 8) could be attributed to the different phases of wound healing. During the first three to five days of the wound healing process, the inflammatory phase occurs after the hemostasis phase where inflammation reacts to local injury and aids the immune system in fighting infections by eliminating foreign bodies or toxic chemicals (Devasvaran and Yong 2016). It is usually characterized by pain, heat, redness, swelling, and incapacity to function (Hadagali and Chua 2014). This may explain why the average wound area for all groups on days 1–3 showed modest changes when compared to other days, since it concentrates on combating infection and boosting the immune system. The wound healing process reached the proliferation phase on day 5 up to 3 weeks, which is characterized by a decrease in wound size induced by a mix of physiological processes such as granulation, contraction, and epithelialization (Flanagan 2000). This observation accurately explained why the graphs showed the greatest change in the average wound area on day 5–7. At this stage, new capillaries grow to produce connective tissue via angiogenesis, which is driven by macrophage activity and tissue hypoxia caused by blood flow interruption after injury (Flanagan 2000). After the wound has healed, the remodeling phase begins, during which the development of granulation tissue ends and macrophages encourage collagen fiber restructuring into a stronger collagen resulting in the increase of tensile strength of the recovered tissue (Reinke and Sorg 2012).
Change in average wound area (n = 3) for high dose and low dose of a leaves, b flowers, c bark and d fruits extract, including the control groups. The change in wound area is obtained by subtracting the average wound area between day 1 versus day 3, day 3 versus day 5, day 5 versus day 7, day 7 versus day 10 and so on
Conclusion
Medicinal herbs have long been recognized as a rich source of antioxidant chemicals with health-promoting properties. Dillenia excelsa, a plant found in Brunei Darussalam has been claimed to possess wound-healing properties, however, there are limited scientific studies on this plant. In this present study, the methanolic leaves, fruits, flowers and bark extract of D. excelsa were evaluated for the presence of phytochemicals, phenolics, flavonoids, antioxidant activity, antibacterial and wound healing properties. Our findings provided insights into the therapeutic efficacy of D. excelsa as well as support for their claimed traditional use. Based on the antioxidant activity of D. excelsa, it may offer additional health advantages that require further investigation, since our study revealed it to be an antioxidant source. D. excelsa may be further explored in wound healing applications. The precise phytochemicals and bioactive constituents that may have contributed to our findings should be investigated further in order to fully utilize the health benefits of D. excelsa.
References
Abdille MH et al (2005) Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem 90(4):891–896. https://doi.org/10.1016/j.foodchem.2004.09.002
Abdul Wahab NZ et al (2011) Antioxidant, antibacterial and antiviral properties of Goniothalamus umbrosus leaves methanolic extract. Afr J Microbiol Res 5(20):3138–3143. https://doi.org/10.5897/ajmr10.758
Aderotimi B, Samuel A (2006) Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri 18(1):39–44
Ahmed D, Khan MM, Saeed R (2015) Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants 4(2):394–409. https://doi.org/10.3390/antiox4020394
Alam MB et al (2020) Phytochemical characterization of Dillenia indica l. Bark by paper spray ionization-mass spectrometry and evaluation of its antioxidant potential against t-bhp-induced oxidative stress in raw 264.7 cells. Antioxidants 9(11):1–15. https://doi.org/10.3390/antiox9111099
Anderson K, Hamm RL (2012) Factors that impair wound healing. J Am Coll Clin Wound Spec 4(4):84–91. https://doi.org/10.1016/j.jccw.2014.03.001
Ansari SS et al (2021) Antioxidant activity, xanthine oxidase inhibition and acute oral toxicity of Dillenia philippinensis Rolfe (Dilleniaceae) leaf extract. J Pharm Pharmacogn Res 9(6):846–858
Az-Zahra FR et al (2021) Review: Traditional knowledge of the dayak tribe (borneo) in the use of medicinal plants. Biodiversitas 22(10):4633–4647. https://doi.org/10.13057/biodiv/d221057
Babii C et al (2016) Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J Appl Microbiol 120(3):630–637. https://doi.org/10.1111/jam.13048
Bagrov AY, Shapiro JI, Fedorova OV (2009) Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61(1):9–38. https://doi.org/10.1124/pr.108.000711
Bate-Smith EC, Harborne JB (1971) Differences in flavonoid content between fresh and Herbarium leaf tissue in Dillenia. Phytochemistry 10(1952):1967–1970
Bazylak G, Gryn A (2015) Antioxidant activity and total flavonoid content in variable phyto-stem cells extracts obtained by high-pressure homogenization method and assigned for use in biocosmetics. Planta Med. https://doi.org/10.1055/s-0035-1565835
Beirith A et al (1999) Study of the antinociceptive action of the ethanolic extract and the triterpene 24-hydroxytormentic acid isolated from the stem bark of Ocotea suaveolens. Planta Med 65(1):50–55. https://doi.org/10.1055/s-1999-13962
Benmeziane F, Madani K (2019) Optimization of phenolic compounds recovery and in vitro antioxidant activity of Algerian eggplant (Solanum melongena L.). Adv Hortic Sci 33(4):567–580. https://doi.org/10.13128/ahsc-8214
Boulger GS, Mabberley DJ (2004) Dillenius, Johann Jakob (1687–1747), botanist. Oxford University Press, Oxford. https://doi.org/10.1093/ref:odnb/7648
Chah KF et al (2006) Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol 104(1–2):164–167. https://doi.org/10.1016/j.jep.2005.08.070
Chlopicka J et al (2012) Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci Technol 46(2):548–555. https://doi.org/10.1016/j.lwt.2011.11.009
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26(5):343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002
Dash GK, Suresh P, Ganapaty S (2001) Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J Nat Remed 1(2):105–110
Dawood Shah M, Seelan Sathiya Seelan J, Iqbal M (2020) Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab J Chem 13(9):7170–7182. https://doi.org/10.1016/j.arabjc.2020.07.022
Denny, Wardani M, Susilo A (2021) Diversity and potential utilization of medicinal plants in Way Kambas National Park. IOP Conf Ser: Earth Environ Sci. https://doi.org/10.1088/1755-1315/914/1/012001
Devasvaran K, Yong YK (2016) Anti-inflammatory and wound healing properties of Malaysia Tualang honey. Curr Sci 110(1):47–51. https://doi.org/10.18520/cs/v110/i1/48-52
Do QD et al (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3):296–302. https://doi.org/10.1016/j.jfda.2013.11.001
Dorsett-Martin WA, Wysocki AB (2008) Rat models of skin wound healing. In: Source book of models for biomedical research, pp 631–638. https://doi.org/10.1007/978-1-59745-285-4_65
Dutta SK et al (2018) Bioactivity and traditional uses of 26 underutilized ethno-medicinal fruit species of North-East Himalaya, India. J Food Meas Charact 12(4):2503–2514. https://doi.org/10.1007/s11694-018-9867-4
Erin LSH et al (2013) Evaluation of four extracts from Dillenia ovata stem bark and leaves for antibacterial and antifungal activity. Int J Pharm Pharm Sci 5(SUPPL 3):471–474
Escarpa A, González MC (2001) Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal Chim Acta 427(1):119–127. https://doi.org/10.1016/S0003-2670(00)01188-0
Everett JS, Sommers MS (2013) Skin viscoelasticity: physiologic mechanisms, measurement issues, and application to nursing science. Biol Res Nurs 15(3):338–346. https://doi.org/10.1177/1099800411434151
Flanagan M (2000) The physiology of wound healing. J Wound Care 9(6):299–300. https://doi.org/10.12968/jowc.2000.9.6.25994
Gao SQ et al (2018) Topical application of Hydroxysafflor Yellow A accelerates the wound healing in streptozotocin induced T1DM rats’. Eur J Pharmacol 823:72–78. https://doi.org/10.1016/j.ejphar.2018.01.018
Goh MPY et al (2017) Ethnobotanical review and pharmacological properties of selected medicinal plants in Brunei Darussalam: Litsea elliptica, Dillenia suffruticosa, Dillenia excelsa, Aidia racemosa, Vitex pinnata and Senna alata. Asian Pac J Trop Biomed 7(2):173–180. https://doi.org/10.1016/j.apjtb.2016.11.026
Guimarães AC et al (2019) Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 24(13):1–12. https://doi.org/10.3390/molecules24132471
Günter CI, Machens H-G (2012) New strategies in clinical care of skin wound healing. Eur Surg Res 49:16–23. https://doi.org/10.1159/000339860
Guo S, DiPietro LA (2010) Critical review in oral biology & medicine: factors affecting wound healing. J Dent Res 89(3):219–229. https://doi.org/10.1177/0022034509359125
Hadagali MD, Chua LS (2014) The anti-inflammatory and wound healing properties of honey. Eur Food Res Technol 239(6):1003–1014. https://doi.org/10.1007/s00217-014-2297-6
Hashim NA et al (2017) In vitro antioxidant, antityrosinase, antibacterial and cytotoxicity activities of the leaf and stem essential oil from Piper magnibaccum C. DC. J Essent Oil-Bearing Plants 20(1):223–232. https://doi.org/10.1080/0972060X.2017.1282839
Ichikawa K et al (2019) Effect of side chain functional groups on the DPPH radical scavenging activity of Bisabolane-type phenols. Antioxidants 8(3):65. https://doi.org/10.3390/antiox8030065
Ih H et al (2018) The potential ethnomedicine plant of Impatiens balsamina leaves from Pontianak, West Kalimantan, Indonesia for wound healing. Nusantara Biosci 10(1):58–64. https://doi.org/10.13057/nusbiosci/n100109
Iloki-Assanga SB et al (2015) Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum Complementary and Alternative Medicine. BMC Res Notes 8(1):1–14. https://doi.org/10.1186/s13104-015-1388-1
Jamila N et al (2016) Phytochemical analysis, antioxidant, anti-hyperglycemic and antituberculosis activities of phylogenetically related Garcinia mangostana (mangosteen) and Garcinia hombroniana (seashore mangosteen). J Chem Soc Pak 38(6):1181–1189
Joshi A, Bhobe M, Sattarkar A (2013) Phytochemical investigation of the roots of Grewia microcosm Linn. J Chem Pharm Res 5(7):80–87
Karumi Y, Onyeyili P, Ogugbuaja V (2003) Anti-inflammatory and antinociceptive (analgesic) properties of Momordical balsamina Linn. (balsam apple) leaves in rats. Pak J Biol Sci 6:1515–1518. https://doi.org/10.3923/pjbs.2003.1515.1518
Kim HP et al (2004) Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 96(3):229–245. https://doi.org/10.1254/jphs.CRJ04003X
Kokkaiah I, Sethupandian G, Palanichamy M (2016) Investigation on antimicrobial activity and phytochemical screening of Randia spinosa (Thunb.) Poir. and Dillenia pentagyna Roxb. J Coast Life Med 4(11):879–883. https://doi.org/10.2980/jclm.4.2016J6-169
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J Flavones 58(4):145–148. https://doi.org/10.2307/j.ctt1w0ddx8.35
Kviecinski MR et al (2017) Healing effect of Dillenia indica fruit extracts standardized to betulinic acid on ultraviolet radiation-induced psoriasis-like wounds in rats. Pharm Biol 55(1):641–648. https://doi.org/10.1080/13880209.2016.1266672
Lin JY, Tang CY (2007) Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 101(1):140–147. https://doi.org/10.1016/j.foodchem.2006.01.014
Lisdiani L, Susanto D, Manurung H (2022) Phytochemical screening and antioxidant activity of methanol extract of Dillenia excelsa leaf. Biodiversitas 23(7):3827–3835. https://doi.org/10.13057/biodiv/d230760
Mai-Prochnow A et al (2016) Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep 6:610. https://doi.org/10.1038/srep38610
Mayer AM, Harel E (1979) Polyphenol oxidases in plants. Phytochemistry 18(2):193–215. https://doi.org/10.1016/0031-9422(79)80057-6
Metussin N et al (2018) Evaluation of antioxidant capacity of aidia borneensis leaf infusion, an endemic plant in Brunei Darussalam. Food Res 2(1):12–19. https://doi.org/10.26656/fr.2017.2(1).109
Migliato, K. F. et al. (2011) ‘Effect of Glycolic Extract of Dillenia indica L. Combined With Microcurrent Stimulation on Experimental Lesions in Wistar Rats.’, Wounds : a compendium of clinical research and practice, 23(5):111–120
MonirulIslam M et al (2013) Antidiarrheal activity of Dillenia indica bark extract. IJPSR 4(2):682–688
Muharni M et al (2022) Wound healing activity of Dillenia ochreata leaves ethanol extract in Wistar rats. J Pharm Pharmacogn Res 10(5):896–904. https://doi.org/10.56499/jppres22.1444_10.5.896
Mwamatope B et al (2020) Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci African 9:e00481. https://doi.org/10.1016/j.sciaf.2020.e00481
Nagori BP, Solandi R (2011) Role of medicinal plants in wound healing. Res J Med Plant 5(4):392–405
Nam N-H (2006) Naturally occurring NF-κB inhibitors. Mini-Rev Med Chem 6(8):945–951. https://doi.org/10.2174/138955706777934937
Padalia H, Poptani R, Chanda S (2018) Evaluation of in vitro antioxidant properties of solvent extracts of selected medicinal plants and their synergistic efficacy. J Herbs Spices Med Plants 24(1):15–27. https://doi.org/10.1080/10496475.2017.1357159
Patle TK et al (2020) Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 242:118717. https://doi.org/10.1016/j.saa.2020.118717
Phillips SJ (2000) Physiology of wound healing and surgical wound care. ASAIO J 46(6):2–5
Proshkina E et al (2020) Terpenoids as potential geroprotectors. Antioxidants 9(6):1–51. https://doi.org/10.3390/antiox9060529
Quattrocchi U (2012) CRC world dictionary of medicinal and poisonous plants. Common names, scientific names, eponyms, synonyms, and etymology. CRC Press, New York
Ramesh M et al (1998) Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol 62(1):63–66. https://doi.org/10.1016/S0378-8741(98)00048-8
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49:35–43. https://doi.org/10.1159/000339613
Remize F (2017) Spore-forming bacteria, the microbiological quality of food: foodborne spoilers. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-08-100502-6.00007-8
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20(7):933–956. https://doi.org/10.1016/0891-5849(95)02227-9
Robards K et al (1999) Phenolic compounds and their role in oxidative processes in fruits. Food Chem 66(4):401–436. https://doi.org/10.1016/S0308-8146(99)00093-X
Robson MC, Steed DL, Franz MG (2001) Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg 38(2):72–140. https://doi.org/10.1201/b18196
Sabandar CW et al (2017) Medicinal uses, chemistry and pharmacology of Dillenia species (Dilleniaceae). Phytochemistry 134:6–25. https://doi.org/10.1016/j.phytochem.2016.11.010
Saha MR et al (2009) In vitro anti-oxidant activity of the leaves of Dillenia indica. Orient Pharm Exp Med 9(4):277–284. https://doi.org/10.3742/opem.2009.9.4.277
Sanhueza L et al (2017) Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 12(2):1–15. https://doi.org/10.1371/journal.pone.0172273
Santoso EA, Jumari, Utami S (2019) Inventory and biodiversity medicinal plants of dayak tomun society in lopus village Lamandau regency central Kalimantan. J Phys: Conf Ser 1217(1):012171. https://doi.org/10.1088/1742-6596/1217/1/012171
Sengul M et al (2009) Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci 22(1):102–106
Shafie NA et al (2020) Evaluation of antioxidant, antibacterial and wound healing activities of Vitex pinnata. F1000Research 9:1–17. https://doi.org/10.2688/f1000research.21310.2
Shendge P, Patil L, Kadam V (2011) In vitro evaluation of antioxidant activity of Dillenia indica Linn. leaf extracts. Int J Pharm Sci Res 2(7):1814–1818
Shi J et al (2004) Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food 7(1):67–78. https://doi.org/10.1089/109662004322984734
Sieniawska E, Baj T (2017) Tannins, pharmacognosy: fundamentals, applications and strategy. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-802104-0.00010-X
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299(25):152–178. https://doi.org/10.1016/j.scienta.2016.11.004
Sofrona E et al (2020) In vivo evaluation of the wound healing activity of extracts and bioactive constituents of the marine isopod ceratothoa oestroides. Mar Drugs 18(4):4–7. https://doi.org/10.3390/md18040219
Somboonwong J et al (2012) Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med 12(1):1. https://doi.org/10.1186/1472-6882-12-103
Sunariyati S (2018) Ethnobotanical studies of plants utilization in the gold mining region in central Kapuas, Indonesia. Biodiversitas 19(1):215–221. https://doi.org/10.13057/biodiv/d190129
Tan AL, Latiff A (2014) A taxonomic study of Dillenia in Peninsular Malaysia. J Gen Microbiol 66(3):338–353
Tene K et al (2021) Polyphenolic-rich compounds from Dillenia pentagyna (Roxb.) attenuates the doxorubicin-induced cardiotoxicity: a high-frequency ultrasonography assisted approach. Front Pharmacol 12:1–15. https://doi.org/10.3389/fphar.2021.624706
Thaipong K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Thooptianrat T et al (2017) Screening of phytochemicals and toxicity of medicinal plants, Dillenia species, reveals potential natural product resources. J Food Biochem 41:1236. https://doi.org/10.1111/jfbc.12363
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37(5):1528–1542. https://doi.org/10.1177/147323000903700531
Venkata Smitha P et al (2012) Screening of antimicrobial and antioxidant potentials of Acacia caesia, Dillenia pentagyna and Buchanania lanzan from Maredumilli Forest of India. J Pharm Res 5(3):1734–1738
Vriet C, Hennig L, Laloi C (2015) Stress-induced chromatin changes in plants: of memories, metabolites and crop improvement. Cell Mol Life Sci 72(7):1261–1273. https://doi.org/10.1007/s00018-014-1792-z
Wang R et al (2013) Wound-healing plants from TCM: in vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia 84(1):308–317. https://doi.org/10.1016/j.fitote.2012.12.020
Wilson B et al (2005) Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J Ethnopharmacol 99(1):147–151. https://doi.org/10.1016/j.jep.2005.02.004
Wong SP, Leong LP, William Koh JH (2006) Antioxidant activities of aqueous extracts of selected plants. Food Chem 99(4):775–783. https://doi.org/10.1016/j.foodchem.2005.07.058
Yadav R, Agarwala M (2011) Phytochemical analysis of some medicinal plants. Liaquat Med Res J 3(12):10–14. https://doi.org/10.38106/lmrj.2021.36
Yakop F et al (2020) Phytochemical screening, antioxidant and antibacterial activities of extracts and fractions of Dillenia suffruticosa leaves. Malays Appl Biol 49(1):121–130
Yang W et al (2020) Advances in pharmacological activities of terpenoids. Nat Prod Commun 15(3):555. https://doi.org/10.1177/1934578X20903555
Zakaria ZA et al (2006) Antinociceptive, anti-inflammatory and antipyretic properties of Melastoma malabathricum leaves aqueous extract in experimental animals. Can J Physiol Pharmacol 84(12):1291–1299. https://doi.org/10.1139/Y06-083
Acknowledgements
Thank you to Universiti Brunei Darussalam for the opportunity given to carry out my research under the Government of His Majesty the Sultan and Yang Di-Pertuan of Negara Brunei Darussalam Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
Animal studies was approved by Universiti Brunei Darussalam Research Ethics Committee (Approval number: UBD/FOS/N1(I)). The care and use of animals for research at Universiti Brunei Darussalam was carried out in conformity with all applicable local and international regulations. According to the Universiti Brunei Darussalam policy on the care and use of animals for research, all researchers affirm that every effort was made to minimize harm to the animals by close monitoring for symptoms of pain and suffering and responding in cases where such signals were noticed.
Conflict of interest
Fatin Sauli has no conflict of interest. Hartini M. Yasin has no conflict of interest. Norhayati Ahmad has no conflict of interest. Fairuzeta Ja’afar has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sauli, F., Yasin, H.M., Ahmad, N. et al. An evaluation of the antioxidant capacities of Dillenia excelsa extracts and its wound healing activity in Wistar rats. ADV TRADIT MED (ADTM) 24, 891–908 (2024). https://doi.org/10.1007/s13596-024-00746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-024-00746-1