Abstract
This study aimed to evaluate Chicory leaf extract for its potential activity on CKD model in STZ induced diabetic nephropathy in Wistar albino rats. Diabetic nephropathy was by administering STZ (35 mg/kg) for a period of seven days, which was confirmed by estimating the blood glucose levels (> 250 mg/dl). After the successful model establishment, the rats were allowed a progression period to CKD for 3 weeks and confirmed by estimating the serum creatinine levels. Thereafter, the rats were treated with chicory leaf extract for a period of 6 weeks at the doses 25 mg/kg, 50 mg/kg and 100 mg/kg. Changes in renal function related parameters in serum and urine were estimated along with histopathological studies. CKD rats exhibited renal dysfunction, as evidenced by reduced serum creatinine, increased blood glucose, blood urea nitrogen and proteinuria, along with alterations in the antioxidant parameters like SOD, CAT, GSH and MDA, that were reversed by Chicory leaf extract. Chicory leaf extract was also found to markedly improve the histopathological alteration sin the CKD effected kidneys. Chicory leaf extract was found to be beneficial in rats with chronic kidney disease seen by the reversion of the altered parameters to almost normal range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidneys are one of the most important organs of our body, whose main function is filtration. Kidneys filter about 200 L of fluid every day, allowing toxins, metabolic waste and excess ions to be eliminated while important constituents remain in the circulation (Ogobuiro and Tuma 2022). Kidney failure has become a global public health issue, with rising incidence and prevalence, high expenditure and poor results (Levey et al. 2005).
Chronic Kidney Disease (CKD) or chronic renal failure refers to the group of heterogenous disorders that alter the kidney structure and function (Levey and Coresh 2012). It is a silent, progressive illness that develops when left undiagnosed (Vanholder et al. 2021). CKD has become leading cause of mortality in the twenty-first century. The number of patients afflicted by CKD has also been rising, owing to the increase in risk factors such as obesity and Diabetes Mellitus. In 2017, around 843.6 million people worldwide were impacted by the disease (Kovesdy 2022). Renal damage or reduced kidney function are the indicators of CKD. Glomerular Filtration Rate (GFR) is a key factor in the pathophysiology of CKD. In addition to GFR, proteinuria and albuminuria have also been linked to the advancement of the disease (Levey and Coresh 2012; Kovesdy 2022).
There are several causes of CKD, among which diabetes and hypertension are in the lead in both developed and developing nations (Jha et al. 2013). According to the International Diabetic Federation (2015), the incidence of diabetes in adults aged 20–79 years is 8.8%, impacting roughly 440 Million people. By 2035, this is predicted to reach over 550 Million individuals (Sulaiman 2019).
One of the most serious complications of diabetes is diabetic nephropathy or diabetic kidney disease and is also a major cause of CKD (Amarasiri et al. 2018). Diabetic nephropathy is characterized by glomerular hyperfiltration, increasing albuminuria and a decrease in GFR, all of which eventually contribute to CKD (Alicic et al. 2017). Apart from these, other major pathological changes in diabetic nephropathy are expanded mesangial matrix in the glomerulus, thickening of the basement membrane and interstitial fibrosis which also are primary characteristics of CKD (Jia et al. 2019).
Chemical agents that preferentially destroy the insulin producing β-cells in the pancreas (resulting in hyperglycaemia) like Streptozotocin (STZ) and Alloxan, are significant tools for establishing animal model of diabetic complications (Tesch and Allen 2007a). Generally, STZ induces type 1 diabetes but when rats are fed with high fat diet (insulin resistance development) combined with a low dose of STZ, it leads to the development of type 2 diabetes (Zhang et al. 2008).
Cichorium intybus.L, commonly known as Chicory, belongs to the Asteraceae family. It has been a viable source of essential elements like potassium, iron, calcium and Vitamins like A, B1, B2 and C and also other bioactive compounds like inulin, sesquiterpene lactones, coumarin derivatives, cichoric acid, phenolic acids, etc. all of which have potential effects on humans. It is also worth mentioning that chicory was one of the plants identified as having anti-SARS CoV-2 potential (Janda et al. 2021). Chicory has been traditionally used in the treatment of fever, jaundice, diarrhea and gallstones and is also known to possess hepatoprotective and anti-diabetic activities as well (Abbas et al. 2015).
The aim of the present study is to investigate the potential effect of chicory leaf extract (CLE) on CKD.
Materials and methods
Plant materials
Collection and authentication of plant
The aqueous extract of chicory leaves was received as a gift sample by Sami-Sabinsa group, Limited, Bengaluru.
Phytochemical screening
The aqueous extract of chicory leaves was subjected to phytochemical screening for determining the presence of constituents like tannins, saponins, flavonoids and alkaloids.
Tannins
The estimation of tannin content was carried out by ferric chloride test. A few drops of 10% ferric chloride solution were added to 2 ml of aqueous extract solution. The appearance of blackish blue or green-blackish colour confirmed the presence of tannins (Abbas et al. 2015).
Saponins
3 ml of aqueous extract was added to a test tube containing 10 ml of distilled water and the test tube was stoppered. The test tube was vigorously shaken for 5 min and allowed to stand for 30 min. The formation of a honeycomb froth indicated the presence of saponins (Auwal et al. 2014).
Flavanoids
The flavanoid content was determined by Pew’s test. In this method, 5 ml of the aqueous extract was added with 0.1 g of metallic zinc and mixed with 8 ml of sulphuric acid. The appearance of red colour indicated the presence of flavonoids (Auwal et al. 2014).
Alkaloids
The presence of alkaloids was confirmed by Dragendorff’s test. In this test, 1 ml of Dragendorff’s reagent was added to a test tube containing 2 ml of aqueuous extract along the sides of the test tube. The formation of orange reddish brown precipitate was indicative of presence of alkaloids (Das et al. 2020).
Animals
Wistar albino rats weighing 150–200 g were selected for in vivo studies. The animals were housed in standard environmental condition and provided with standard feed pellets and water ad libitum, under 12 h of light/dark cycle at a temperature of 25 ± 2 °C and controlled humidity.
The experiment was carried out according to guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Institutional Animal Ethical Committee of Acharya & BM Reddy College of Pharmacy.
(IAEC approval number: IAEC/ABMRCP/2020–2021/14-I)
Experimental design
The rats were randomly divided into five groups each consisting of six rats (n = 6):
-
Group 1: Negative control (Distilled water).
-
Group 2: Positive control [STZ (35 mg/kg; i.p.)].
-
Group 3: Treatment group-I [CLE (25 mg/kg; p.o.)].
-
Group 4: Treatment group-II [CLE (50 mg/kg; p.o.)].
-
Group 5: Treatment group-III [CLE (100 mg/kg; p.o.)].
The experimental group received high fat diet (70% standard laboratory chow, 15% carbohydrates, 10% lard, and 5% yolk powder) for a period of 28 days. After 28 days, diabetes was induced by a single intraperitoneal injection of STZ (35 mg/kg dissolved in 0.01 M Sodium citrate buffer, pH 4.4). A week after STZ injection, the blood glucose levels of rats were randomly measured for confirmation of successful establishment of diabetic nephropathy model. Blood glucose level more than 250 mg/dl were considered diabetic. For the next 3 weeks, the rats were given 10% dextrose solution once a week and allowed a progression period to CKD. After 3 weeks the serum creatinine levels estimation confirmed the presence of CKD. Then the rats were randomly divided into 4 groups (n = 6): Positive control, Treatment I, Treatment II and Treatment III where the treatment groups received the extract orally continuously for a period of 6 weeks. The body weight along with serum and urine parameters were estimated every week. The animals were sacrificed for the isolation of kidney and pancreas to carry out biochemical estimations and histopathological studies (Kshirsagar et al. 2017; Wang et al. 2014).
Estimation of serum parameters
Blood samples were withdrawn from retro orbital plexus with the help of light anesthesia and stored in 2–8 °C which were used within a week. The serum parameters like blood glucose, serum creatinine (Scr), serum triglcerides, serum cholesterol, total protein and Blood Urea Nitrogen (BUN) were estimated using ELISA kits.
Estimation of urine parameters
Rats were housed in individual metabolic cages for 24 h to collect urine samples which were centrifuged at 4000×g for 5 min. The urine parameters like urine creatinine and urine albumin levels were estimated using ELISA kits.
Estimation of antioxidant parameters
The animals were sacrificed at the end for the respective in vivo studies for the isolation of kidney and pancreas to carry out various biochemical estimations.
Superoxide Dismutase (SOD)
The superoxide dismutase enzyme inhibits the reduction of nitroblue tetrazolium to blue-colored tetrazolium in the presence of phenazine methosulphate and NADH in this form. The colour intensity was calculated at 560 nm (Kakkar et al. 1984).
Catalase (CAT)
CAT estimation was carried out its ability to oxidize hydrogen peroxide to water and oxygen. On decomposition of hydrogen peroxide by CAT, the absorption decreases with time. The UV light absorption of hydrogen peroxide solution can be easily measured between 230 and 250 nm (Catalase 1974).
Reduced glutathione (GSH)
Acid soluble sulfhydryl groups (non-protein thiols, of which reduced glutathione accounts for more than 93%) form a yellow coloured complex with DTNB. The coloured complex's absorbance was estimated at 412 nm (Ellman 1959).
Malondialdehyde (MDA)
MDA, the lipid peroxidation end product, was measured by the assay of lipid peroxide level in animal tissues by their reaction with thiobarbituric acid. 10% (w/v) tissue homogenate was mixed with sodium dodecyl sulfate, acetate buffer (pH 3.5), and aqueous solution of thiobarbituric acid. After heating at 95 °C for 60 min, the red pigment produced was extracted with n-butanol-pyridine mixture and estimated by the absorbance at 532 nm (Ohkawa et al. 1979)
Histopathological studies
After the experiment, rats were be sacrificed under anaesthesia and the kidneys and pancreas were immediately harvested and weighed. The kidney and pancreas were cleaned in ice–cold saline solution and fixed in 4% neutral formaldehyde solution. The tissue was examined with a photomicroscope (40 ×) after staining with haematoxylin and eosin (H–E) dye.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 5 and all the results were expressed as Mean ± SEM. Data was analysed using one-way ANOVA followed by Dunnett’s test. Values ranging from P < 0.05 (*), P < 0.01 (**) and P < 0.001(***) were considered to be statistically significant compared to positive control group.
Results
Phytochemical screening
The aqueous extract of Chicory leaf shows the presence of Tannins, Saponins, Flavonoids and Alkaloids.
Effect of CLE on serum and urine parameters
Various serum and urine parameters were evaluated in CKD rats which included blood glucose levels, serum creatinine, Blood urea nitrogen (BUN), serum total protein levels, cholesterol, triglycerides, urine creatinine and urine protein levels. The CKD rats showed alterations in all the above listed parameters including body weight.
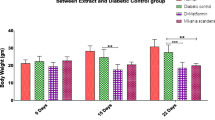
There was a marked increased in the body weight in positive control group (238.33 ± 13.76 g) when compared to the normal control group (126.66 ± 6.146 g). A significant decrease in body weights of CLE (25 mg/kg) (206.667 ± 4.21 g), CLE (50 mg/kg) (200 ± 7.30 g) and CLE (100 mg/kg) (188.33 ± 5.42 g) was seen after treatment (Figs. 1, 2).
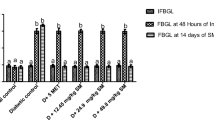
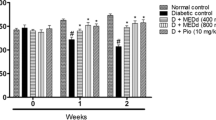
After the treatment period of 6 weeks, the blood glucose levels of the CKD rats were estimated and found to be higher when compared to the normal control group (346.5 ± 10.31 mg/dl), this was seen to significantly decrease after treatment with CLE i.e. CLE (25 mg/kg) (199.16 ± 2.32 mg/dl), CLE (50 mg/kg) (155.83 ± 1.13 mg/dl) and CLE (100 mg/kg) (132.5 ± 2.74 mg/dl).
Serum creatinine, BUN, Cholesterol and triglycerides levels were also seen to be markedly increased in the positive control animals (Table 1). After 6 weeks of treatment with CLE all these parameters have found to be improved, CLE 100 mg/kg showing the most significant effect (Figs. 3, 4, 5, 6, 7, 8, 9).
Effect of CLE on tissue antioxidants: SOD, CAT, GSH and MDA
A marked reduction was observed in the tissue antioxidants SOD, CAT and GSH in the positive control groups when compared to the normal control group. These antioxidants were significantly increased after the treatment with in CLE 25 mg/kg, 50 mg/kg and 100 mg/kg groups. Whereas, the MDA levels were found to be increased significantly in positive control group. These were reduced significantly in CLE 25 mg/kg, 50 mg/kg and 100 mg/kg groups after treatment (Figs. 10, 11, 12, 13, Table 2).
Histopathological studies
The histopathology of normal control group has shown normal architecture of kidney tissue with normal cellularity of the glomerulus along with tubules and interstitium within normal limits. The kidney tissue in the positive control group showed tubular degeneration, dilatation of tubules, inflammatory cell infiltration and congested glomerulus. These all changes were seen to be almost reversed in the treatment groups which have show high significant activity compared to the positive control group. The CLE (25 mg/kg) shows moderate congestion of the glomerulus and mild inflammation of tubules and interstitium when compared to the positive control group. Whereas, the CLE (50 mg/kg) and CLE (100 mg/kg) have shown mild congestion of the glomerulus along with mild to no inflammation of the interstitium and tubules (Figs. 14, 15).
The histopathology of normal control group shows that the pancreatic lobules consists of intact acinar cells and the centre of islet cells consists of Beta-cells (65%) while the periphery comprises of Alpha-cells (30%). The pancreatic tissue of the positive control shows atrophy of the islet cells with vacuolation of Beta-cells. It also shows quantitative decrease in the Beta-cells (38%) when compared to the normal control group. However, the treatment groups have shown significant improvement in the tissues. The CLE (25 mg/kg), CLE (50 mg/kg) and CLE (100 mg/kg) have shown moderate to mild degenerative changes and quantitative increase in the number of Beta-cells. The CLE (100 mg/kg) i.e. the high dose group have shown significant increase in the number of Beta-cells (50%) when compared to the positive control group.
Discussion
The potential effect of chicory leaf extract on CKD has been examined in the current investigation. Chicory, or Cichorium Intybus, is a member of the Asteraceae family. It is a very versatile plant that benefits both animals and people due to the substantial amount of proteins, carbohydrates, minerals, and phytobioactive components it contains. It is rich in polyphenol compounds like phenol, flavonoids, and coumarins, which are regarded as a natural source of probiotics and antioxidants. It has also been noted that a number of its phytoconstituents have properties that improve animal wellbeing when used in livestock production (Nwafor et al. 2017).
In the present study, Diabetic nephropathy model was established using STZ combined with high fat diet. STZ and Alloxan are important chemical compounds for creating animal models of diabetic problems because they selectively kill the insulin-producing beta-cells in the pancreas (resulting in hyperglycemia) (Tesch and Allen 2007b). In general, STZ induces type 1 diabetes, but when rats get a low dose of STZ along with a high-fat diet (which results in the development of insulin resistance), type 2 diabetes develops (Zhang et al. 2008).
The progression of diabetic nephropathy to CKD (3 weeks) was confirmed by estimating the serum creatinine levels. Traditionally, creatinine levels have been used to evaluate renal function. The blood level of creatinine will be increased if the kidneys become impaired for any reason because the kidneys are unable to adequately remove creatinine from the body. Thus, abnormally high creatinine levels serve as a warning sign for potential renal damage or failure (Asif et al. 2020). Chicory leaf extract has been found capable of reducing the elevated creatinine levels, normalize body weight and reduce the blood glucose levels, BUN levels along with urine creatinine and protein levels.
Several studies have shown that CKD is associated with hypercholesterolemia and hyperglyceridemia. Numerous lipid abnormalities are seen in CKD and aberrant lipid metabolism is linked to the progression of renal disease (Kim et al. 2020). In the present study, levels of cholesterol and triglycerides were seen to be increased in CKD rats which were significantly reduced after treatment with chicory leaf extract. We have found that treatment group 3 has shown significant decrease when compared with positive control group and treatment groups 1 and 2.
Multiple antioxidant systems tend to protect the renal tissues and cells from oxidative stress caused by ROS/RNS. These include endogenous antioxidant enzymes such as SOD, CAT and GSH. SOD is an oxidoreductase that serves to dismutase the superoxide anion, CAT acts by disproportionating hydrogen peroxide and GSH is a marker of defense against lipid peroxidation. While the levels of these antioxidant enzymes reduces in case of oxidative stress, the generation of MDA increases which serves as a marker of oxidative stress (Ratliff et al. 2016).
The treatment groups have shown significant increase in the antioxidant parameters like SOD, CAT and GSH while there is a significant reduction in the MDA levels when compared to the positive control group.
On histopathological examination, the kidney and pancreatic sections showed progressive damage. Several studies have suggested that the majority of kidney with severe hyperglycemia displayed abnormalities resembling human glomerulosclerosis, thickening of the glomerular membrane and extensive tubular necrosis. The main symptom of diabetic nephropathy is progressive glomerulosclerosis, which is accompanied by decreasing kidney function and leads to CKD (Zafar et al. 2009).
Additional investigations have demonstrated gradual, widespread tubular necrosis with tubular epithelial alterations, loss of the brush border, expansion of the tubule lining cells, and build up of glycogen in the kidney tubules (Ramesh and Pugalendi 2006; Ramesh et al. 2007).
In the present study, histopathological evaluation of the kidney tissues in the treatment groups has shown significant reduction in the tubular degeneration and congestion of glomerulus when compared to the positive control group. The histopathological evaluation of pancreatic tissues also have shown significant improvement in the number of Beta-cells and reduced degenerative changes.
From this histopathological evaluation, it can be concluded that the chicory leaf extract showed renal protective effect by reversing the tissue damage close to normal condition.
Conclusion
Chicory leaf extract has shown anti-diabetic as well as nephroprotective effect on CKD model in STZ induced diabetic nephropathy.
References
Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA (2015) Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Bio Sci. 22(3):322–326
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12(12):2032–2045
Amarasiri SS, Attanayake AP, Jayatilaka KA, Mudduwa LK (2018) Animal models of chronic kidney disease: screening tool to investigate nephroprotective effects of natural products. Int J Pharm Chem Anal 5(2):52–58
Asif AA, Hussain H, Chatterjee T (2020) Extraordinary creatinine level: a case report. Cureus 12(7):1–7
Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A (2014) Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum 5(2):95–100
Catalase AH (1974) InMethods of enzymatic analysis. Academic Press, Cambridge, pp 673–684
Das PE, Abu-Yousef IA, Majdalawieh AF, Narasimhan S, Poltronieri P (2020) Green synthesis of encapsulated copper nanoparticles using a hydroalcoholic extract of Moringa oleifera leaves and assessment of their antioxidant and antimicrobial activities. Mol 25(3):555–558
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Janda K, Gutowska I, Geszke-Moritz M, Jakubczyk K (2021) The common cichory (Cichorium intybus L.) as a source of extracts with health-promoting properties—a review. Molecules 26(6):1814–1827
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B et al (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382(9888):260–272
Jia Q, Yang R, Liu XF, Ma SF, Wang L (2019) Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol Med Rep 19(1):423–431
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophy 21(1):130–132
Kim YJ, Oh SH, Ahn JS, Yook JM, Kim CD, Park SH et al (2020) The crucial role of xanthine oxidase in CKD progression associated with hypercholesterolemia. Int J Mol Sci 21(20):7444–7462
Kovesdy CP (2022) Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl 12(1):7–11
Kshirsagar RP, Chouthe RS, Reddy GB, Kumar BD (2017) Geraniol ameliorates endothelial dysfunction in streptozotocin-induced diabetic rats. J Pharm Res 9(11):1159–1165
Levey AS, Coresh J (2012) Chronic kidney disease. Lancet 379(9811):165–180
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J et al (2005) Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 67(6):2089–2100
Nwafor IC, Shale K, Achilonu MC (2017) Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci World J 1(1):1–11
Ogobuiro I, Tuma F (2022) Physiology, renal. In: StatPearls [Internet].StatPearls Publishing, Treasure Island [Updated 2021 Jul 26]
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ramesh B, Pugalendi KV (2006) Impact of umbelliferone (7-hydroxycoumarin) on hepatic marker enzymes in streptozotocin diabetic rats. Ind J Pharmacol 38(3):209–218
Ramesh B, Viswanathan P, Pugalendi KV (2007) Protective effect of Umbelliferone on membranous fatty acid composition in streptozotocin-induced diabetic rats. Eur J Pharmacol 566(1–3):231–239
Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS (2016) Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25(3):119–146
Sulaiman MK (2019) Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol Metabol Synd 11(1):1–5
Tesch GH, Allen TJ (2007a) Rodent models of streptozotocin-induced diabetic nephropathy (Methods in Renal Research). Nephrology 12(3):261–266
Tesch GH, Allen TJ (2007b) Rodent models of streptozotocin-induced diabetic nephropathy (Methods in Renal Research). Nephrol 12(3):261–266
Vanholder R, Annemans L, Bello AK, Bikbov B, Gallego D, Gansevoort RT, Lameire N et al (2021) Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin Kid J 14(7):1719–1730
Wang J, Liu H, Li N, Zhang Q, Zhang H (2014) The protective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar Drugs 6:3292–3306
Zafar M, Naeem-Ul-Hassan Naqvi S, Ahmed M, Kaimkhani ZA (2009) Altered kidney morphology and enzymes in streptozotocin-induced diabetic rats. Int J Morphol 27(3):783–790
Zhang M, Lv XY, Li J, Xu ZG, Chen L (2008) The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 2008(704045):1–9
Acknowledgements
We are thankful to Sami-Sabinsa group, Limited, Bengaluru for providing us the gift sample. We are thankful to the management and principal of Acharya & BM Reddy College of Pharmacy, Bengaluru for providing us necessary support throughout our research.
Funding
The authors declare that this study has no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies involving animals performed by any of the authors. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
Simran Sultana has no conflict of interest. Uday Raj Sharma has no conflict of interest. Reddibathina Leela Haripriya has no conflict of interest. Athira Ramachandran has no conflict of interest. Runashree Borah has no conflict of interest, V. Surendra has no conflict of interest. T. Haribabu has no conflict of interest. S. V. Gayathri has no conflict of interest. Suresh Janadri has no conflict of interest. P. M. Manjunatha has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sultana, S., Sharma, U.R., Haripriya, R.L. et al. Evaluation of chicory leaf extract on chronic kidney disease model in streptozotocin-induced diabetic nephropathy. ADV TRADIT MED (ADTM) 24, 765–775 (2024). https://doi.org/10.1007/s13596-023-00724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-023-00724-z