Abstract
Long non-coding RNAs (lncRNAs) are a type of multifunctional endogenous RNA transcript. The dysregulation of lncRNAs is considered to play a role in the initiation and progression of cancer. One such lncRNA, long intergenic non-coding RNA-p21 (lincRNA-p21), was identified in 2010 as a regulator in the p53 pathway and is gradually being identified to play crucial roles in diverse cellular processes. In this review, we have summarised the diverse regulatory functions of lincRNA-p21. For example, lincRNA-p21 has been reported to function as a protein decoy, act as a competitive endogenous RNA, regulate the transcription, regulate the translation processes and exist in the secreted exosomes. Furthermore, we highlight the emerging roles of lincRNA-p21 in cancer cell regulation. Various types of cancers, including colorectal carcinoma, hepatocellular carcinoma and non-small cell lung carcinoma, aberrantly express lincRNA-p21. However, the current understanding of the roles of lincRNA-p21 in cancer remains limited. Therefore, considering its potential as a valuable therapeutic target or biomarker for cancer, more research should be conducted to understand the role of lincRNA-p21 in cancer and other diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long non-coding RNAs (lncRNAs) are cellular RNA transcripts that have more than 200 nucleotides (nt) and lack significant open reading frames [1]. The functions of lncRNAs have been extensively researched, revealing roles in differentiation, development, reprogramming and tumorigenesis through the regulation of transcription, splicing and translation [2]. To date, four subtypes of lncRNAs have been identified: intergenic, intronic, antisense and overlapping lncRNAs. Among them, long intergenic non-coding RNAs (lincRNAs) represent the majority of lncRNAs and have been confirmed to play multiple biological roles [3]. lincRNA-p21 was first identified in 2010 as a regulator in the p53 pathway. The name is derived from its location near the cell cycle regulator gene, Cdkn1a (p21) [4]. Recently, given the increasing focus on its roles, lincRNA-p21 has been shown to be involved in transcriptional and posttranscriptional regulatory processes. The emerging functions of lincRNA-p21 in cancers demonstrate its potential and warrant investigation.
Herein, we highlight lincRNA-p21 and review its diverse regulatory functions and the growing evidence regarding its vital roles in the development and progression of cancers.
The structure and biogenesis of lincRNA-p21
The lincRNA-p21 gene is located approximately 15 kb upstream of the cell cycle regulator gene p21/Cdkn1a on human chromosome 6p21.2, and its transcript is around 3.0 kb in humans. Chillon et al. [5]studied the structure of lincRNA-p21. They found that human lincRNA-p21 has a single exon without an intron and contains two inverted repeat Alu elements (IRAlus) that influence its nuclear localization and position of the paraspeckles. Both sense and antisense Alu elements of lincRNA-p21 are highly conserved among primates. A recent study reported the three-dimensional structures of sense and antisense lincRNA-p21 IRAlus, which helps to understand the functions of lincRNA-p21 better [6].
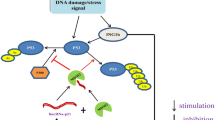
The expression of lincRNA-p21 can be regulated in different ways. First, the expression of lincRNA-p21 can be regulated by the transcription factor p53 (Fig. 1a). Baldassarre et al. reported that p53 significantly increased the expression of lincRNA-p21 [7]. Subsequently, Jin et al. [8] found that the expression of lincRNA-p21 was also regulated by the mutant p53 (mutp53)/nuclear transcription factor Y subunit alpha (NF-YA) complex in head and neck squamous cell carcinoma (HNSCC) (Fig. 1b). Meanwhile, the expression of lincRNA-p21 was strongly upregulated after DNA damage in human oral squamous cell carcinoma Cal27, HNSCC HN30 and breast cancer MCF7 cells with wild-type TP53 [8]. Recently, He et al. [9] found that lincRNA-p21 was upregulated by mutp53 by targeting the non-B structure to mediate apoptosis for chemosensitivity in oestrogen receptor (ER)-negative breast cancer cells. In ER-positive breast cancer cells, ERα combined with mutp53 to regulate the transcription of DDB2, thereby reducing the expression of lincRNA-p21 and resulting in chemoresistance (Fig. 1c). Second, lincRNA-p21 has been reported to be a hypoxia-induced lncRNA, and its expression is significantly increased in hypoxic conditions [10,11,12,13,14,15,16,17]. Further studies found that the transcription levels of lincRNA-p21 are upregulated by hypoxia-inducible factor-1α (HIF-1α) in response to a hypoxic environment [11, 17] (Fig. 1d). In addition, the X-radiation increased the expression of lincRNA-p21 in gastric cancer cells to increase the sensitivity to radiotherapy [18]. Third, the expression and stability of lincRNA-p21 were regulated by microRNAs (Fig. 1e). Yoon et al. [19] found that the association of HuR with lincRNA-p21 promoted the recruitment of let-7/Ago2 into lincRNA-p21, inducing the degradation of lincRNA-p21. HSF1 was shown to upregulate HuR to recruit the miR-320 family (a, b and c) to bind to a 5’ sequence of lincRNA-p21, leading to the lower stability of lincRNA-p21 in MDA-MB-231 cells [20]. In addition, the miR-181 family interacted with lincRNA-p21 to induce the degradation of lincRNA-p21 [21].
The biogenesis of lincRNA-p21. a P53 significantly increases the expression of lincRNA-p21 in response to DNA damage. b The expression of lincRNA-p21 is upregulated by the mutp53/NF-YA complex. c The expression of lincRNA-p21 is regulated by mutp53 depending on the ER status. d The expression of lincRNA-p21 is upregulated by HIF-1α in response to hypoxia. e MicroRNAs interact with lincRNA-p21 to induce the degradation of lincRNA-p21
Functions and mechanisms of lincRNA-p21
Recent studies have shown that lincRNA-p21 can affect apoptosis, cell cycle checkpoints, cell proliferation, reprogramming efficiency, the Warburg effect and tumorigenesis via the regulation of transcription, translation, chromatin state and energy metabolism. Moreover, lincRNA-p21 has been implicated in several signalling pathways, such as the p53, HIF-1α and exosome secretion pathways. Here, we summarise the functions and mechanisms of lincRNA-p21 to gain a better understanding of how it affects cellular functions. For example, lincRNA-p21 has been reported to function as a protein decoy (Fig. 2a), act as a competitive endogenous RNA (ceRNA) (Fig. 2b), regulate the transcription process (Fig. 2c), regulate the translation process (Fig. 2d) and exist in the secreted exosomes (Fig. 2e).
The functions and mechanisms of lincRNA-p21. a LincRNA-p21 acts as a protein decoy. LincRNA-p21 can bind with MDM2, HnRNP-K, STAT3 and HIF-1α to improve cell proliferation, glycolysis and inhibit cell apoptosis. b LincRNA-p21 functions as a ceRNA. LincRNA-p21 functions as an endogenous sponge by directly binding to miRNAs to regulate cell proliferation, migration and apoptosis. c LincRNA-p21 regulates the transcription process. LincRNA-p21 gene contains multiple enhancer elements which can directly regulate the transcription of p21 gene, and then modulate the chromatin state of a subset of PRC2 target genes via p21. d LincRNA-p21 regulates the translation process. LincRNA-p21 coordinated with the Rck/p54 RNA helicase leading to inhibit their target mRNAs translation, such as β-catenin. e. LincRNA-p21 exists in the secreted exosomes
lincRNA-p21 acts as a protein decoy
lincRNA-p21 was reported to function as a protein decoy or scaffold to affect the interaction between functional proteins. First, lincRNA-p21 has been reported to bind with MDM2, wherein the nt728–2057 fragment of lincRNA-p21 possibly binds with the RING domain of MDM2. MDM2 is a downstream target gene of p53 and accelerates the degradation of p53 by interacting with it [22,23,24]. Studies have reported that lincRNA-p21 knockdown can increase the binding capacity between p53 and MDM2 and decrease that between p53 and p300 [25, 26]. Therefore, the interaction between lincRNA-p21 and MDM2 may contribute to a decreased p53-MDM2 interaction. In other words, lincRNA-p21 and p53 competitively bind to MDM2, thereby promoting the acetylation of p53 via p300 and increasing the transcriptional activity of p53.
Huarte et al. [4]identified a 780 nt fragment at the 5’ end of lincRNA-p21, which could bind with heterogeneous nuclear ribonucleoprotein K (hnRNP-K). The p53 transcriptional response includes both activation and repression of numerous genes. In addition, hnRNP-K acts as a repressor in the p53 pathway [27, 28]. Silencing lincRNA-p21 reduces the association of hnRNP-K with the promoters of p53-repressed target genes. A study reported that lincRNA-p21 cooperates with hnRNP-K to repress many genes in a p53-dependent transcriptional response. Furthermore, the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signalling pathway plays an important role in regulating cancer progression [29]. Jin et al. [8] found that lincRNA-p21 could bind to STAT3 to inhibit the JAK2/STAT3 signalling pathway in HNSCC. Furthermore, Huarte et al. [4] found that lincRNA-p21 knockdown results in the de-repression of STAT3.
In cancer cells, the Warburg effect is an energy metabolism pattern associated with a high rate of lactic acid fermentation to substitute for the oxidation of pyruvate [30,31,32,33]. This respiratory mechanism fulfils the energy demand of tumour cells for adaption to a hypoxic tumour microenvironment [34, 35]. HIF-1α is considered a positive factor in mediating the Warburg effect, which functions via hydroxylation of the proline hydroxylase domain (PHD) proteins and is subsequently recognized by the von Hippel-Lindau (VHL) protein [10, 36, 37]. Yang et al. [11]reported that silencing lincRNA-p21 diminishes hypoxia-enhanced glycolysis, which includes glucose uptake, lactate production, LDHA enzymatic activity and Glucose transporter 1 (GLUT1) expression levels in HeLa cells. Further research has found that the mechanism by which lincRNA-p21 regulates HIF-1α expression is dependent on the interactions of lincRNA-p21 with HIF-1α and VHL protein. This results in a decrease in the interaction between HIF-1α and VHL protein to attenuate VHL-mediated HIF-1α ubiquitination and cause HIF-1α accumulation [11]. In summary, lincRNA-p21, as a hypoxia-responsive regulator, may act as a therapeutic target for cancer via the HIF-1α/lincRNA-p21 signalling pathway.
lincRNA-p21 functions as a ceRNA
Recently, lincRNA-p21 was reported to function as an endogenous sponge by directly binding to miRNAs to regulate many biological processes and some diseases. LincRNA-p21 dampens miR-211 to regulate cell apoptosis in hippocampal neurons, miR-130b to regulate cell proliferation and apoptosis in vascular endothelial cells [38], miR-181b to regulate hepatic stellate cell (HSC) activation and liver fibrosis [39], and miR-1277-5p to regulate cell proliferation and apoptosis in SH-SY5Y cells treated with N-methyl-4-phenylpyridinium (MPP+) [40]. LincRNA-p21 can affect the progression of cancers by binding to miRNAs [41,42,43]. Ao et al. reported that lincRNA-p21 inhibits the proliferation and migration of lung cancer cells by directing the sponging of miR-17-5p to decrease the expression of miR-17-5p, affecting the expression of its associated genes [42]. Similarly, in human hepatocellular carcinoma (HCC) cells, lincRNA-p21 inhibits cell migration and invasion by sponging miR-9 to inhibit the expression of miR-9 and regulate the miR-9 mediated E-cadherin signalling pathway [43]. Furthermore, in glioma cells, lincRNA-p21 inhibits tumour progression by sponging miR-34c to decrease the expression of miR-34c and affecting the expression of CRFR1 [44]. Taken together, lincRNA-p21 may function as a molecular sponge by binding to miRNAs to play important roles in the regulation of tumour progression.
lincRNA-p21 regulates the transcription process
Cis-acting lncRNAs have been reported to regulate gene expression within their local sites [45]. Dimitrova et al. reported that lincRNA-p21 plays a positive role in modulating the transcriptional expression of p21 in cis, which is located near lincRNA-p21 [46]. They found that lincRNA-p21 could modulate the chromatin state of a subset of polycomb repressive complex 2 (PRC2) target genes via modulation of p21 expression in response to DNA damage. Further studies have revealed that there are multiple DNA enhancer elements in the lincRNA-p21 locus, which can regulate the transcription of nearby genes in cis, including p21 [47]. The conclusion is consistent that reported by Allen et al. that lincRNA-p21 is an enhancer RNA contributing to the binding between the transcription factor p53 and the p21 gene, thereby increasing the expression of p21 [48]. Subsequent research reported that the overexpression of lincRNA-p21 increases the expression of p21, which further influences the cell cycle of EC109 cells [49]. Additionally, a study reported that p53 is a regulator of lincRNA-p21 in HNSCC [12]. In summary, lincRNA-p21 regulates the expression of p21 in a cis-acting manner. The important role of lincRNA-p21 in the p53 tumour suppressor pathway should be explored.
lincRNA-p21 regulates the translation process
Recent studies provide evidence that lncRNAs have roles in posttranscriptional processes [50, 51]. For instance, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) plays a role in the alternative splicing process of mRNA [52]. LincRNA-p21 participates in posttranscriptional processes, acting as a translational regulator. In the cytoplasm, lincRNA-p21 represses mRNA translation of JUNB and CTNNB1 in coordination with the Rck/p54 RNA helicase [19]. In summary, lincRNA-p21 functions as a translational regulator by assisting Rck to their target mRNAs, leading to inhibition of their translation. However, the stability of lincRNA-p21 is regulated by HuR and let-7/Ago2, wherein HuR and let-7/Ago2 jointly promote the degradation of lincRNA-p21. Therefore, lincRNA-p21 plays a crucial role in HuR-dependent regulation of translation and is possibly involved in the regulation of other HuR-targeted mRNAs. Given that the target proteins of HuR participate in many pivotal regulatory processes, alteration in the expression of lincRNA-p21 might affect many disease processes, including cancer [20]. In addition to HuR, other RNA binding proteins (RBPs) may be associated with lincRNA-p21 to regulate the translation of mRNAs, which should be investigated further.
lincRNA-p21 exists in the secreted exosomes
Exosomes are important extracellular vesicles (EVs) for intercellular signal transfer [53, 54]. Diverse proteins and RNAs are presented in exosomes. The expression levels of lincRNA-p21 are much higher in the secreted exosomes of MCF7 and HeLa cells compared with total cell extracts [55]. The expression of lincRNA-p21 is increased both in cells and exosomes in response to DNA damage [55]. Thus, lincRNA-p21 packaged in exosomes can be transferred from one cell to another to perform its functions. Recently, Castellano et al. identified a higher expression of lincRNA-p21 in EVs from non-small cell lung carcinoma (NSCLC), which helps tumour cell adhesion to endothelial cells [10]. Moreover, the level of exosomal lincRNA-p21 in urine demonstrates potential as a prognostic marker of prostate cancer (PCa) [56]. However, the mechanism that influences its selective loading into exosomes requires further investigation, and the specific functions of lincRNA-p21 secreted with exosomes should be explored in the follow-up studies.
lincRNA-p21 in different cancers
Recent studies have reported aberrant expression of lincRNA-p21 in certain types of cancers, which suggests that lincRNA-p21 may act as a new player in the disease. Considering its implications in diverse cancer-related signalling pathways, lincRNA-p21 may play important roles in regulating the development and progression of cancers. However, current knowledge about the functions of lincRNA-p21 is superficial. Therefore, the role of lincRNA-p21 in cancer should be researched further. Here, we provide a summary of the altered expression of lincRNA-p21 in cancer and its effect on tumorigenesis and metastasis (Table 1).
Colorectal carcinoma (CRC)
CRC is one of the most common intestinal cancers, with an overall high mortality rate [57]. Many studies have investigated the role of lincRNA-p21 in CRC [58,59,60,61,62,63,64,65,66]. The expression of lincRNA-p21 was found to be lower in clinical samples of CRC compared with adjacent tissues [58, 59, 61]. Zhai et al. [58] investigated the clinical features of CRC and reported a higher expression of lincRNA-p21 in the rectum compared with the colon and in stage III tumours compared with stage I tumours. In addition, lincRNA-p21 was associated with higher pT and vascular invasion. Therefore, lincRNA-p21 may play a crucial role in the progression of CRC. In addition, studies have investigated the potential effect of lincRNA-p21 on the treatment of CRC. Wang et al. demonstrated that the level of lincRNA-p21 was upregulated in both SW1116 and LOVO cells exposed to X-ray treatment and indicated that lincRNA-p21 might increase radiosensitivity by inducing apoptosis of CRC cells [59]. Li et al. [66] reported that lincRNA-p21 might function as a prognostic marker for CRC, and a high expression of lincRNA-p21 may enhance the overall survival rate following postoperative chemoradiotherapy. The Ginkgo biloba extract EGb 761, a traditional Chinese medicine, has been reported to inhibit cell migration and invasion in colorectal/colon cancer mediated by lincRNA-p21 [62, 63]. Meanwhile, lincRNA-p21 inhibits the self-renewal and tumorigenicity of CRC stem cells [60]. The potential mechanisms of lincRNA-p21 in CRC are inhibition of the Wnt/β-catenin signalling pathway [59, 60], interaction with EZH2 to suppress the expression of fibronectin [62], and stabilization of E-cadherin via the suppression of BTRC-mediated E-cadherin ubiquitination [63].
In conclusion, these observations suggest that lincRNA-p21 may serve as a tumour suppressor due to its relationship with the p53 and Wnt/β-catenin signalling pathways in CRC. Its influence on the radiosensitivity of CRC indicates that lincRNA-p21 can serve as a potential target for CRC radiotherapy. However, clinically relevant data have implied that a high level of lincRNA-p21 promoted the progression and malignant transformation of CRC. The different functions of lincRNA-p21 may be associated with the mutation status of p53. Therefore, lincRNA-p21 may be a crucial factor in the development of CRC and affects the treatment of the disease. LincRNA-p21 could be considered a potential diagnostic, predictive and prognostic biomarker for CRC.
Hepatocellular carcinoma (HCC)
HCC is a highly aggressive cancer worldwide [67]. Recio et al. [68] reported that lincRNA-p21 is significantly upregulated in response to the carcinogen furan in the liver of furan-exposed mice, which suggested that lincRNA-p21 could be an epigenetic target or biomarker for carcinogenic exposures. However, the effects of changes in the expression of lincRNA-p21 in response to carcinogens and the regulatory mechanism of lincRNA-p21 in hepatotoxicity associated with carcinogenic exposures remain to be investigated further. To date, the role of lincRNA-p21 in HCC has been studied broadly. Previous studies have reported that the expression of lincRNA-p21 is downregulated in HCC tissue and cell lines [69,70,71,72]. LincRNA-p21 has been reported to inhibit cell proliferation, promote apoptosis, and inhibit sorafenib resistance partly by activating ER stress [71]. Subsequent studies reported that lincRNA-p21 inhibits the invasion and metastasis of HCC by regulating epithelial-mesenchymal transition by mediating the Notch signalling pathway [70] or regulating the miR-9-mediated E-cadherin signalling pathway [43]. Moreover, Shen et al. identified that the expression of lincRNA-p21 increased during irradiation or hypoxia and found that knockdown of lincRNA-p21 reduces the protein level of HIF-1αand GLUT1 to inhibit autophagy by activating the Akt/mTOR/p70S6K signalling pathway in hypoxic hepatoma and glioma cells [13]. In addition, lincRNA-p21 was found to reduce the expression of VEGF by targeting HIF-1α in liver cancer [16]. These studies indicate that lincRNA-p21 plays an important role in the development and progression of HCC and functions as a potential therapeutic target.
Non-small cell lung carcinoma (NSCLC)
NSCLC is the most common cancer in the world [73]. The expression of lincRNA-p21 is downregulated in NSCLC tissue and cell lines [12, 42], and it functions to inhibit cell proliferation, migration [42] and angiogenesis [12]. However, Yang et al. reported that the expression of lincRNA-p21 was upregulated in NSCLC tissues and cells, and overexpression of lincRNA-p21 was found to inhibit apoptosis by decreasing the expression of p53 upregulated modulator of apoptosis (PUMA) [74]. In line with other cancers, the expression of lincRNA-p21 is induced by hypoxic conditions in NSCLC cell lines [12] and EVs [10]. Castellano et al. found that lincRNA-p21 promotes the adhesion of NSCLC cells to endothelial cells via the miRNA cargo of EVs from NSCLC cells [10]. Further studies should explore the mechanism of lincRNA-p21 altering the EV cargo and the effects of EV lincRNA-p21 on relapse and drug resistance.
B-Cell malignancies
Chronic lymphocytic leukaemia (CLL) and multiple myeloma (MM) are two types of B-cell malignancies. Circulating levels of cell-free lincRNA-p21 were detected in blood by Mustafa et al. in patients with CLL and MM [75]. They found that the levels of circulating lincRNA-p21 were lower in patients with CLL compared with healthy people, while no significant changes were observed in patients with MM. This trend suggested that lincRNA-p21 displays a disease-specific expression pattern. Moreover, the expression of plasma lincRNA-p21 was decreased in patients with high clinical stages of CLL, which indicated that the plasma lincRNA-p21 level could reflect the degree of CLL progression. Furthermore, Elwafa et al. [76] reported downregulation of lincRNA-p21 in patients with CLL, and the low expression was associated with a poorer prognosis. Therefore, lincRNA-p21 can be considered a potential prognostic biomarker for CLL. Blume et al. reported that lincRNA-p21 is the p53-dependent lncRNA induced in response to DNA damage in CLL that causes cell death [77]. Follow-up studies are needed to elucidate the function of lincRNA-p21 in the development and progression of CLL and the mechanisms of action of cell-free lincRNA-p21 in cancers.
Prostate cancer (PCa)
PCa is one of the most common malignancies in men. A previous study demonstrated that the expression of exosomal lincRNA-p21 in urine might act as a novel diagnostic marker to predict the status of malignancy in patients with PCa [56]. Another clinical study revealed that the expression of lincRNA-p21 was lower in PCa and was associated with poor survival [78]. Physiological studies identified that lincRNA-p21 inhibits the proliferation and promotes apoptosis of PCa cells by inducing the expression of p53 downstream genes [78]. Moreover, these experiments found that lincRNA-p21 functions as a regulator of the Warburg effect by reducing the expression of pyruvate kinase M2 dependent on the PTEN/Akt/mTOR signalling pathway [79].
lincRNA-p21 also acts as a key player in the drug resistance of PCa [80, 81]. Enzalutamide (Enz) is an anti-androgen that is approved by the FDA to prevent the growth of castration-resistant PCa [82]; however, a significant proportion of patients have developed resistance to the drug [83]. Luo et al. reported that lincRNA-p21 is highly expressed in neuroendocrine PCa cells (NEPC), which is regulated by Enz. Additionally, lincRNA-p21 promotes EZH2 to enhance the methylation of STAT3, which consequently results in resistance to Enz [80]. Future studies should focus on the role of lincRNA-p21 in other drug-resistant PCa treatments.
Other cancers
The basal expression of lincRNA-p21 is extremely low in HeLa cells. Emre et al. [84] reported a substantial upregulation of lincRNA-p21 when HeLa cells were exposed to high DNA damage-induced apoptosis by BLM. Cisatracurium, an adjuvant anti-tumour drug, has been reported to inhibit cell proliferation, migration, invasion and induce apoptosis in ovarian cancer through p53/lincRNA21/miR-181b [85]. In breast cancer, HSF1 was shown to improve mammosphere formation through the upregulation of HuR to antagonize the effects of lincRNA-p21 on β-catenin translation [20]. A study reported that lincRNA-p21 was significantly upregulated in tumour-associated macrophages (TAMs) [86]. TAMs with lincRNA-p21 knockdown induced apoptosis of breast cancer cells and inhibited tumour cell migration and invasion. The results indicate that lincRNA-p21 is a key regulator of TAM function in the tumour milieu. Additionally, lincRNA-p21 has been shown to inhibit cell proliferation and induce apoptosis in HNSCC by binding to STAT3 to inhibit the activation of JAK2/STAT3 [8]. Furthermore, lincRNA-p21 inhibits the progression of glioma by binding with miR-34c to increase the expression of CRFR1 [44]. In summary, lincRNA-p21 is involved in the development of various cancers. However, the mechanisms and functions of lincRNA-p21 in the development and progression of different cancers should be elucidated further.
Conclusion
Recent studies provide evidence of the role of lincRNA-p21 in regulating gene expression, mRNA translation, chromatin modification, the Warburg effect and tumorigenesis. Studies have also revealed that lincRNA-p21 exerts these functions by the activation of several signalling pathways, such as the p53, Wnt/β-catenin, HIF-1α and exosome secretion pathways. Moreover, increasing evidence shows that lincRNA-p21 is aberrantly expressed and affects the efficacy of cancer treatments. Therefore, these findings suggest that lincRNA-p21 may serve as an attractive target for cancer treatment and can be a promising biomarker for cancer diagnosis and prognosis.
Although lincRNA-p21 has drawn attention over the years, the findings raise several interesting questions. Does lincRNA-p21 cooperate with hnRNP-K as a corepressor or as a coactivator of p53-dependent gene transcription in response to DNA damage? Is there any interplay between lincRNA-p21-associated pathways? What function does lincRNA-p21 play in p53 mutant or missing cells? How does lincRNA-p21 guide its associated proteins, such as hnRNP-K or PRC2, to specific target gene loci? Further, since the expression of lincRNA-p21 is lower in CRC compared with normal cells but higher in aggravated CRC, there remains the question of whether lincRNA-p21 acts as a repressor or inducer of cancer development. Is lincRNA-p21 associated with tissue and disease-specific expression and precise spatio-temporal regulation? Finally, in addition to interacting with hnRNP-K, MDM2, PRC2, HuR and let-7, what other processes are hnRNP, chromatin-modifying complexes, RBP and microRNA involved in with lincRNA-p21? Answering these questions and understanding the roles of lincRNA-p21 in cancer development will not only provide a comprehensive picture regarding the mechanism of lincRNA-p21-regulated cellular biological functions and the molecular nature of lincRNA-p21-dependent cancer development but also explore a new direction in cancer treatment.
Abbreviations
- lncRNAs:
-
Long non-coding RNAs
- nt:
-
Nucleotides
- lincRNAs:
-
Long intergenic non-coding RNAs
- PRC2:
-
Polycomb repressive complex 2
- HNSCC:
-
Head and neck squamous cell carcinoma
- hnRNP-K:
-
Heterogeneous nuclear ribonucleoprotein K
- JAK2:
-
Janus kinase 2
- STAT3:
-
Signal transducer and activator of transcription 3
- NF-YA:
-
Nuclear transcription factor Y subunit alpha
- RBP:
-
RNA binding protein
- HIF-1α:
-
Hypoxia-inducible factor-1α
- PHD:
-
Proline hydroxylase domain
- VHL:
-
Von Hippel-Lindau
- NSCLC:
-
Non-small cell lung cancer
- HRE:
-
HIF-1α response element
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- miRNAs:
-
MicroRNAs
- MREs:
-
MiRNA response elements
- HSC:
-
Hepatic stellate cell
- HCC:
-
Hepatocellular carcinoma
- TDMD:
-
Target-directed miRNA degradation
- EV:
-
Extracellular vesicle
- CRC:
-
Colorectal carcinoma
- CLL:
-
Chronic lymphocytic leukaemia
- MM:
-
Multiple myeloma
- Enz:
-
Enzalutamide
- NEPC:
-
Neuroendocrine PCa cells
- TAMs:
-
Tumour-associated macrophages
References
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62.
Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–57.
Huarte M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142(3):409–19.
Chillon I, Pyle AM. Inverted repeat Alu elements in the human lincRNA-p21 adopt a conserved secondary structure that regulates RNA function. Nucleic Acids Res. 2016;44(19):9462–71.
D’Souza MH, et al. Biophysical characterisation of human LincRNA-p21 sense and antisense Alu inverted repeats. Nucleic Acids Res. 2022;50(10):5881–98.
Baldassarre A, Masotti A. Long non-coding RNAs and p53 regulation. Int J Mol Sci. 2012;13(12):16708–17.
Jin S, et al. p53-targeted lincRNA-p21 acts as a tumor suppressor by inhibiting JAK2/STAT3 signaling pathways in head and neck squamous cell carcinoma. Mol Cancer. 2019;18(1):38.
He YH, et al. ERalpha determines the chemo-resistant function of mutant p53 involving the switch between lincRNA-p21 and DDB2 expressions. Mol Ther Nucleic Acids. 2021;25:536–53.
Castellano, J.J., et al., Extracellular Vesicle lincRNA-p21 Expression in Tumor-Draining Pulmonary Vein Defines Prognosis in NSCLC and Modulates Endothelial Cell Behavior. Cancers (Basel), 2020. 12(3).
Yang F, et al. Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Mol Cell. 2014;53(1):88–100.
Castellano JJ, et al. LincRNA-p21 impacts prognosis in resected non-small cell lung cancer patients through angiogenesis regulation. J Thorac Oncol. 2016;11(12):2173–82.
Shen Y, et al. LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathway. Exp Cell Res. 2017;358(2):188–98.
Meng SS, et al. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res Ther. 2018;9(1):280.
Xia W, Zhuang L, Hou M. Role of lincRNAp21 in the protective effect of macrophage inhibition factor against hypoxia/serum deprivationinduced apoptosis in mesenchymal stem cells. Int J Mol Med. 2018;42(4):2175–84.
Ye Y, et al. Effect of lincRNA-p21 targeting HIF-1alpha on biological functions of liver cancer cells. Oncol Lett. 2019;17(6):4964–8.
Sun CY, et al. Orchestration of lincRNA-p21 and miR-155 in Modulating the Adaptive Dynamics of HIF-1alpha. Front Genet. 2020;11:871.
Chen L, et al. LincRNA-p21 enhances the sensitivity of radiotherapy for gastric cancer by targeting the beta-catenin signaling pathway. J Cell Biochem. 2019;120(4):6178–87.
Yoon JH, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47(4):648–55.
Chou SD, et al. HSF1 regulation of beta-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene. 2015;34(17):2178–88.
Ye Y, et al. A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuroinflammation. Cell Death Dis. 2018;9(8):803.
Wang, S., et al., Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb Perspect Med, 2017. 7(5).
Gupta A, et al. Reactivation of p53 gene by MDM2 inhibitors: a novel therapy for cancer treatment. Biomed Pharmacother. 2019;109:484–92.
Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20(8):471–80.
Wu G, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130(17):1452–65.
Tang SS, Zheng BY, Xiong XD. LincRNA-p21: implications in human diseases. Int J Mol Sci. 2015;16(8):18732–40.
Qin G, et al. Long noncoding RNA p53-stabilizing and activating RNA promotes p53 signaling by inhibiting heterogeneous nuclear ribonucleoprotein K deSUMOylation and suppresses hepatocellular carcinoma. Hepatology. 2020;71(1):112–29.
Moumen A, et al. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123(6):1065–78.
Chang R, et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat Commun. 2018;9(1):3486.
Vander Heiden, M.G., L.C. Cantley, and C.B. Thompson, Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 2009. 324(5930): p. 1029–33.
Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–7.
Li, L., et al., Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell, 2018. 33(3): p. 368–385 e7.
Hamanaka RB, Chandel NS. Cell biology. Warburg effect and redox balance Science. 2011;334(6060):1219–20.
Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95(7):912–9.
Leone RD, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–21.
Hoefflin R, et al. HIF-1alpha and HIF-2alpha differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat Commun. 2020;11(1):4111.
Palazon, A., et al., An HIF-1alpha/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell, 2017. 32(5): p. 669–683 e5.
Sun Q, et al. Long intergenic noncoding RNA p21 suppresses the apoptosis of hippocampus neurons in streptozotocin-diabetic mice by sponging microRNA-221 through upregulation of FOS. J Cell Physiol. 2019;234(11):21113–25.
He C, et al. The role of long intergenic noncoding RNA p21 in vascular endothelial cells. DNA Cell Biol. 2015;34(11):677–83.
Yu F, et al. Identification of a novel lincRNA-p21-miR-181b-PTEN signaling cascade in liver fibrosis. Mediators Inflamm. 2016;2016:9856538.
Xu X, et al. LincRNA-p21 inhibits cell viability and promotes cell apoptosis in Parkinson’s disease through activating alpha-synuclein expression. Biomed Res Int. 2018;2018:8181374.
Ao X, et al. lincRNAp21 inhibits the progression of nonsmall cell lung cancer via targeting miR175p. Oncol Rep. 2019;41(2):789–800.
Ding G, et al. LincRNA-p21 inhibits invasion and metastasis of hepatocellular carcinoma through miR-9/E-cadherin cascade signaling pathway molecular mechanism. Onco Targets Ther. 2017;10:3241–7.
Yang J, et al. Corticotropin-releasing factor suppresses glioma progression by upregulation of long non-coding RNA-p21. Life Sci. 2019;216:92–100.
Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020;21(2):102–17.
Dimitrova N, et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell. 2014;54(5):777–90.
Groff AF, et al. In Vivo characterization of linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 2016;16(8):2178–86.
Allen MA, et al. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife. 2014;3: e02200.
Zhang Y, et al. LincRNA-p21 leads to G1 arrest by p53 pathway in esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:6201–14.
Carrieri C, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491(7424):454–7.
Yoon JH, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939.
Tripathi V, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39(6):925–38.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Kalluri, R. and V.S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478).
Gezer U, et al. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38(9):1076–9.
Isin M, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168.
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Zhai H, et al. Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer. 2013;12(4):261–6.
Wang G, et al. LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/beta-catenin signaling pathway. Oncol Rep. 2014;31(4):1839–45.
Wang J, et al. miRNA-regulated delivery of lincRNA-p21 suppresses beta-catenin signaling and tumorigenicity of colorectal cancer stem cells. Oncotarget. 2015;6(35):37852–70.
Zhao W, et al. Combined identification of long non-coding RNA CCAT1 and HOTAIR in serum as an effective screening for colorectal carcinoma. Int J Clin Exp Pathol. 2015;8(11):14131–40.
Liu T, et al. Ginkgo biloba extract EGb 761-induced upregulation of LincRNA-p21 inhibits colorectal cancer metastasis by associating with EZH2. Oncotarget. 2017;8(53):91614–27.
Chang L, et al. lincRNA-p21 mediates the anti-cancer effect of ginkgo biloba extract EGb 761 by stabilizing e-cadherin protein in colon cancer. Med Sci Monit. 2018;24:9488–96.
Zhao, M., et al., Expression of long non-coding RNA H19 in colorectal cancer patients with type 2 diabetes. Arch Physiol Biochem, 2019: p. 1–7.
Chaleshi V, et al. Association of lncRNA-p53 regulatory network (lincRNA-p21, lincRNA-ROR and MALAT1) and p53 with the clinicopathological features of colorectal primary lesions and tumors. Oncol Lett. 2020;19(6):3937–49.
Li, Y., et al., LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients. Life (Basel), 2020. 10(9).
Liu, J., et al., A Viral Exposure Signature Defines Early Onset of Hepatocellular Carcinoma. Cell, 2020. 182(2): p. 317–328 e10.
Recio L, et al. Differential expression of long noncoding RNAs in the livers of female B6C3F1 mice exposed to the carcinogen furan. Toxicol Sci. 2013;135(2):369–79.
Yang A, et al. AGO-bound mature miRNAs are oligouridylated by TUTs and subsequently degraded by DIS3L2. Nat Commun. 2020;11(1):2765.
Jia M, et al. lincRNA-p21 inhibits invasion and metastasis of hepatocellular carcinoma through Notch signaling-induced epithelial-mesenchymal transition. Hepatol Res. 2016;46(11):1137–44.
Yang N, et al. LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget. 2015;6(29):28151–63.
Wang S, et al. Serum lincRNA-p21 expression in primary liver diseases and liver metastatic diseases. Pathol Res Pract. 2019;215(4):779–83.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54.
Yang T, et al. Long intergenic noncoding RNA-p21 inhibits apoptosis by decreasing PUMA expression in non-small cell lung cancer. J Int Med Res. 2019;47(1):481–93.
Isin M, et al. Investigation of circulating lncRNAs in B-cell neoplasms. Clin Chim Acta. 2014;431:255–9.
Abo Elwafa, R., A. Abd Elrahman, and O. Ghallab, Long intergenic non-coding RNA-p21 is associated with poor prognosis in chronic lymphocytic leukemia. Clin Transl Oncol, 2021. 23(1): p. 92–99.
Blume CJ, et al. p53-dependent non-coding RNA networks in chronic lymphocytic leukemia. Leukemia. 2015;29(10):2015–23.
Wang, X., et al., Long intragenic non-coding RNA lincRNA-p21 suppresses development of human prostate cancer. Cell Prolif, 2017. 50(2).
Wang, X., et al., LincRNA-p21 suppresses development of human prostate cancer through inhibition of PKM2. Cell Prolif, 2017. 50(6).
Luo J, et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun. 2019;10(1):2571.
Hu L, et al. Targeting TR4 nuclear receptor with antagonist bexarotene increases docetaxel sensitivity to better suppress the metastatic castration-resistant prostate cancer progression. Oncogene. 2020;39(9):1891–903.
Hussain M, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–74.
Boudadi K, Antonarakis ES. Resistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate Cancer. Clin Med Insights Oncol. 2016;10(Suppl 1):1–9.
Ozgur E, et al. Differential expression of long non-coding RNAs during genotoxic stress-induced apoptosis in HeLa and MCF-7 cells. Clin Exp Med. 2013;13(2):119–26.
Zhu D, et al. Cisatracurium inhibits the growth and induces apoptosis of ovarian cancer cells by promoting lincRNA-p21. Bioengineered. 2021;12(1):1505–16.
Zhou L, et al. LincRNA-p21 knockdown reversed tumor-associated macrophages function by promoting MDM2 to antagonize* p53 activation and alleviate breast cancer development. Cancer Immunol Immunother. 2020;69(5):835–46.
Jiang, Y.J. and D.D. Bikle, LncRNA profiling reveals new mechanism for VDR protection against skin cancer formation. J Steroid Biochem Mol Biol, 2014. 144 Pt A: p. 87–90.
Hall JR, et al. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6: e1700.
Spurlock CF 3rd, et al. Methotrexate inhibits NF-kappaB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol. 2014;66(11):2947–57.
Wang H, et al. p53-dependent LincRNA-p21 protects against proliferation and anti-apoptosis of vascular smooth muscle cells in atherosclerosis by upregulating SIRT7 via MicroRNA-17-5p. J Cardiovasc Transl Res. 2021;14(3):426–40.
Cekin N, et al. Decreased FENDRR and LincRNA-p21 expression in atherosclerotic plaque. Anatol J Cardiol. 2018;19(2):131–6.
Funding
This research was supported by the Natural Science Foundation of China (No. 82003126); Luzhou Science and Technology Program (No. 2021-JYJ-71); Scientific Research Foundation of Southwest Medical University (No. 2021ZKMS009); Shenzhen Science and Technology Projects (No. JCYJ20190807102601647, NO. JCYJ20210324103604013); Suining First People’s Hospital and Southwest Medical University Project (No. 2020SNXNYD01).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. References preparation was performed by WX. The first draft of the manuscript was written by WS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Yi, Q., Feng, J. et al. The role of lincRNA-p21 in regulating the biology of cancer cells. Human Cell 35, 1640–1649 (2022). https://doi.org/10.1007/s13577-022-00768-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00768-4