Abstract
The ectopic expression of ubiquitin-specific peptidase 21 (USP21) is common in different types of cancer. However, its relationship with radio-sensitivity in cervical cancer (CC) remains unclear. In this study, we aimed to uncover the effect of USP21 on CC radio-resistance and its underlying mechanism. Our results showed that the expression of USP21 was markedly increased in CC tissues of radio-resistant patients and CC cells treated with radiation. Besides, knockdown of USP21 restrained the survival fractions, and facilitated apoptosis of CC cells in the absence or presence of radiation. Additionally, USP21 in combination with FOXM1 regulated the stability and ubiquitination of FOXM1. However, FOXM1 reversed the effects of USP21 knockdown on the radio-resistance of CC cells. Furthermore, FOXM1 knockdown activated the Hippo pathway by inhibiting the nuclear translocation of Yes-associated protein 1 (YAP1), and FOXM1 knockdown attenuated the radio-resistance of CC cells via inhibiting the Hippo–YAP1 pathway. USP21 activated the Hippo pathway by mediating FOXM1. Knockdown of USP21 enhanced the radio-sensitivity of CC cells in vivo. In summary, USP21 contributed to the radio-resistance of CC cells via FOXM1/Hippo signaling, and may serve as a promising target for radio-sensitizers in the radiotherapy of CC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer (CC) is the fourth-most common female malignant tumor in the world [1,2,3]. Surgery, radiotherapy, chemotherapy, and immunotherapy are the main treatment options for CC [3]. Although radiotherapy and chemotherapy have been shown to improve the prognosis of patients with locally advanced CC, radiation resistance of cancer cells is still considered to be a major reason for therapy failure [4]. An in-depth understanding of underlying mechanisms related to radio-resistance and exploring new therapeutic targets are essential to evaluate the prognosis and further improve the treatment of CC patients.
Ubiquitination, a prominent post-translational modification, is the process of addition of specific ubiquitin tags at appropriate locations [5]. Deubiquitinating enzymes (DUBs) are a family of proteases that remove ubiquitin tags from ubiquitinated proteins [6]. DUBs are considered to be key regulators of tumorigenesis and progression of cancers, including CC [7]. Ubiquitin-specific peptidase 21 (USP21) is identified as an efficient DUB that plays a vital role in signal transduction, apoptosis, and DNA repair due to the stability of its substrate [8, 9]. Studies have confirmed that USP21 is related to the occurrence and development of a variety of tumors, including bladder carcinoma [10], breast cancer [11], and non-small cell lung cancer [12]. However, the regulatory capacity and potential mechanism of USP21 in CC have not yet been reported.
FOXM1, a member of the Forkhead box transcription factor family, is ubiquitously expressed in cells undergoing proliferation [13]. Notably, the regulatory function of FOXM1 in the cell cycle has also been widely reported [14]. Targeting FOXM1 is considered to be a promising strategy to enhance the chemo-sensitivity of tumor cells [15]. Recent studies have shown that FOXM1 is associated with decreased radio-sensitivity for gastric cancer and glioma [16, 17]. More importantly, USP21 has been identified as a potential regulator of FOXM1 stability [11]. USP21 binds and removes the poly-ubiquitin chain from FOXM1 to protect it from proteasomal degradation. However, the roles of USP21 and FOXM1 in CC and CC radio-resistance remain unclear.
The Hippo pathway, named after the Drosophila Hpo kinase, plays major roles in multiple development and regenerative processes [18]. Studies have shown that dysregulation of the Hippo pathway is closely associated with the occurrence of several types of cancers, including cervical cancer, lung cancer, and ovarian cancer [19, 20]. Additionally, it was reported that Hippo signaling mediates radio-resistance by participating in DNA damage repair [21]. These data reveal that Hippo signaling exerts a vital function in tumorigenesis and radio-resistance. Yes-associated protein 1 (YAP1), the major transcriptional mediator of the Hippo pathway, plays a major role in the regulation of apoptosis and drug resistance in a variety of human malignancies [22, 23]. In the state of the Hippo signal inactivation, YAP1 enters the nucleus and fuses with the transcription factor TEAD, inducing the dysregulation of certain important genes related with cellular regulatory functions, including CYR61 and CTGF [24]. Inhibition of Hippo signaling leads to enhanced YAP1 activity, which can lead to the occurrence or recurrence of cancer [25, 26]. Notably, FOXM1 has been shown to be involved in regulating the activity of YAP1 [27]. Considering the potential role of USP21 in FOXM1 signaling, we hypothesized that USP21 regulated the activity of YAP1 by regulating the stability of FOXM1, thereby regulating the radio-sensitivity of CC.
Materials and methods
Tissues, cell lines, and treatment
CC tissues were obtained from Yantaishan Hospital. This study was reviewed and approved by the Ethics Committee of Yantaishan Hospital. Various human CC cell lines, including SiHa, HeLa, C33A, and Me-180 were obtained from Procell (Wuhan, China). The human cervical squamous cell line (Ect1/E6E7) was obtained from the American Tissue Culture Collection (Rockville, MD, USA). The CC cells (SiHa, HeLa, and C33A) were cultured in MEM medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA) and penicillin/streptomycin (100 units/0.1 mg/ml) in a 5% CO2 atmosphere at 37 °C. Me-180 cells were cultured in McCoy’s 5A medium supplemented with 10% FBS and penicillin/streptomycin (100 units/0.1 mg/ml) in a 5% CO2 atmosphere at 37 °C. Ect1/E6E7 cells were cultured in keratinocyte-serum free medium (Invitrogen, Carlsbad, CA, USA) containing penicillin/streptomycin (100 units/0.1 mg/ml), human recombinant epidermal growth factor (0.1 ng/ml), bovine pituitary extract (0.05 mg/ml), and CaCl2 (44.1 mg/l, final concentration 0.4 mM). Irradiation (IR) was performed using an AGO HS MP1 X-ray machine (AGO X-ray Limited, UK) at a dose rate of 2 Gy/min. Prior to harvesting, cells were cultivated in an incubator.
Cell transfection
The small interfering RNA (siRNA) targeting USP21 (si-USP21: 1#: 5′-GCUAGAAGAACCUGAGUUA-3′, 2#: 5′-GAGCUGUCUUCCAGAAAUA-3′, 3#: 5′-CUGUGAAGCCCUUUAAACA-3′) or negative control (si-NC: 5′-UUCUCCGAACGUGUCACGU-3′), si-FOXM1 (1#: 5′-GGACCACUUUCCCUACU-3′, 2#: 5′-CUCUUCUCCCUCAGAUAUA-3′), shRNA targeting USP21 (sh-USP21: 5′-GCCTTTCTACTCTGATGACAA-3′) or negative control (sh-NC: CGTACGCGGAATACTTCGA) was obtained from Songon Biotech (Shanghai, China). For the upregulation of FOXM1 expression, the full length of FOXM1 was amplified from human complementary DNA (cDNA) and cloned into pcDNA3.1 vector (oe-FOXM1; Invitrogen). The transfection was performed using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions.
RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the cultured CC cells with TRIzol reagent (Vazyme, Nanjing, China). Reverse transcription was performed using 1.0 µg total RNA and the PrimeScript RT Kit (Takara, Kusatsu, Japan). cDNA was used to perform qRT-PCR on the ABI7500 qPCR instrument (Applied Biosystems, Carlsbad, CA, USA) using Maxima SYBR Green/ROX qPCR Master Mix (2 ×) qPCR Kit (Thermo Fisher Scientific, Inc.). Results were expressed as fold differences relative to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2–ΔΔCT method. The primer sequences used were as follows: USP21 forward: 5′-AGGTGTCTCTGCGGGATTGTT-3′, USP21 reverse: 5′-CGATTCAGATGGAGCACGAGG-3′; FOXM1 forward: 5′-GGGCGCACGGCGGAAGATGAA-3′, FOXM1 reverse: 5′-CCACTCTTCCAAGGGAGGGCTC-3′; GAPDH forward: 5′-CGCTCTCTGCTCCTCCTGTTC-3′, GAPDH reverse: 5′-ATCCGTTGACTCCGACCTTCAC-3′.
Western blotting
Total proteins from CC cells were isolated and purified using RIPA assay buffer (Vazyme) in accordance with the manufacturer’s instructions. NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) were used to isolate the nuclear and cytoplasmic proteins, respectively, from cultured CC cells in accordance with the manufacturer’s protocol. Next, an equal amount of protein sample from each group was separated using 10% sodium dodecyl sulfate–polyacrylamide gel, and then transferred onto PVDF membranes. The membranes were blocked with 5% bovine serum albumin for 1 h. After blocking, the membranes were incubated with primary antibodies at 4 °C overnight, followed by treatment with secondary antibody for 1 h. Anti-USP21 (Catalog Number: MA5-34953; Thermo Fisher Scientific, Waltham, MA, USA), anti-GAPDH (Catalog Number: #AF0911; Affinity Biosciences, Changzhou, China), anti-FOXM1 (Catalog Number: ab207298; Abcam, Cambridge, MA, USA), anti-YAP1 (Catalog Number: ab52771; Abcam), anti-H3 (Catalog Number: #AF0863; Affinity Biosciences), anti-CYR61 (Catalog Number: #39382; Cell Signaling Technology, Beverly, MA, USA), and anti-CTGF antibodies (Catalog Number: #DF7091; Affinity Biosciences) were used in this study. Bands were visualized with ECL reagent (Vazyme).
Clonogenic formation
Cells (6 × 105 cells/well) were seeded in 60 mm dishes and irradiated alone using graded single doses of irradiation (0–8 Gy) for 24 h after indicated transfection. For rescue experiments, cells were pretreated with XMU-MP-1 (6 μM; the inhibitor of Hippo pathway to activate YAP1) for 6 h after indicated transfection, followed by irradiation treatment. After 12 days, the colonies were treated with methanol for 10 min and then subjected to staining procedures. Colonies containing at least 50 cells were counted. Results were presented as survival fractions depicted on a logarithmic scale and plotted against applied irradiation doses.
Flow cytometry
The Annexin V-FITC/Propidium lodide (PI) Apoptosis Kit (Beyotime) was applied to assess cell apoptosis in SiHa and HeLa cells. The collected SiHa and HeLa cells were stained with Annexin V-FITC and PI for 15 min according to the manufacturer’s instructions. The apoptotic cell percentage was analyzed and calculated using a BD FACS flow cytometer (BD Biosciences).
Co-immunoprecipitation (Co-IP) assay
The Co-IP Assay Kit was obtained from Cell Signaling Technology (Danvers, MA, USA). In brief, SiHa cells were lysed using RIPA lysis buffer (Beyotime). Genomic DNA was isolated and sheared into 200–600 bp fragments using sonication. Following centrifugation, the supernatants were collected and chromatin was precipitated with antibodies at 4 °C overnight. The immune complexes were precipitated with protein A/G-Sepharose beads for 4 h. The immune precipitates were eluted using elution buffer and reversal of cross-linking at 65 °C overnight. Next, the immuno-complexes were subjected to western blotting.
In vitro deubiquitination assay
A mixture 5 μg of FOXM1 overexpression construct and 2 μg of HA-ubiquitin overexpression construct was co-transfected with scrambled control or USP21 siRNA (50 nM) in SiHa cells. At 48 h after transfection, cells were subjected to MG132 (25 μM) treatment for 1 h. Cells were lysed using RIPA buffer. Cell lysates were pre-cleared, followed by incubation with FOXM1 antibody-conjugated agarose on a rotator at 4 °C for 3 h. Beads were washed with lysis buffer and proteins were eluted from the beads with the addition of Laemlli sample buffer, followed by boiling. The ubiquitination status of FOXM1 was evaluated using western blotting.
In vivo xenograft mouse model
A total of 20 Balb/c mice (5-week-old; female) were randomly divided into four groups, five in each group. HeLa cells were stably transfected with sh-NC or sh-USP21, and a total of 5 × 106 cells were subcutaneously injected into the flank region. Tumor length (L) and width (W) were recorded weekly, and tumor volume (V) was estimated using the formula: V = (L × W2)/2. When the average tumor volume reached about 100 mm3, the mice were randomized and treatment was started. A dosage of 15 Gy was used to irradiate the mice (once every 2 days for 5 treatments; 3 Gy/time). The tumor was subjected to radiation treatment with the rest of the animal shielded. In the end, nude mice were sacrificed by cervical dislocation four weeks after exposure to irradiation and the tumors were excised. The tumor samples were fixed with formalin and then prepared for immunohistochemical analysis of Ki67 and caspase-3. All animal experimental procedures were reviewed and approved by the Ethics committee of Yantaishan Hospital.
Statistical analysis
Data are presented as the mean ± standard (SD) of three independent experiments. A two-tailed unpaired Student’s t test was used for comparing two groups of data. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for multiple comparisons. Statistical analysis was performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was determined when p < 0.05.
Results
USP21 expression was increased in CC cells after radiation treatment
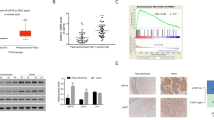
The expression level of USP21 in radio-resistant patients was higher than that in radio-sensitive patients (Fig. 1A). The expression of USP21 was remarkably increased in CC cells compared to that in Ect1/E6E7 cells (Fig. 1B). SiHa and HeLa cells with higher expression levels of USP21 were selected for follow-up experiments. SiHa and HeLa cells were radiated with different doses of radiation, and the expression of USP21 was assessed. The results showed that USP21 expression was markedly increased in SiHa and HeLa cells treated with different doses of radiation (Fig. 1C).
USP21 was upregulated in CC cells after radiation treatment. A The expression level of USP21 in radio-resistant patients was higher than that in radio-sensitive patients. B The expression of USP21 mRNA and protein in CC cells and normal cervical epithelial cell line (Ect1/E6E7). C The expression of USP21 mRNA and protein in SiHa and HeLa cells radiated with 2, 4, 6, and 8 Gy. *p < 0.05, **p < 0.01, and ***p < 0.001
USP21 knockdown enhanced the radio-sensitivity of CC cells
To analyze the effect of USP21 on radio-sensitivity in CC, si-USP21 was transfected into SiHa and HeLa cells. The expression of USP21 was successfully decreased following si-USP21 transfection (Fig. 2A, B). si-USP21-1# was used in subsequent experiments. SiHa and HeLa cells transfected with si-USP21 were exposed to graded doses of ionizing radiation, and clonogenic assay and flow cytometry analysis were performed to assess radio-sensitivity. Results confirmed that knockdown of USP21 markedly restrained the survival fractions of CC cells in the presence of radiation (Fig. 2C). Additionally, knockdown of USP21 facilitated apoptosis of CC cells in the absence or presence of radiation (6 Gy) (Fig. 2D). These findings demonstrated that the radio-sensitivity of CC cells was enhanced after USP21 knockdown.
USP21 knockdown enhanced the radio-sensitivity of CC cells. The expression of USP21 mRNA (A) and protein (B) in SiHa and HeLa cells transfected with USP21 siRNA. C Clonogenic survival of SiHa and HeLa cells transfected with USP21 siRNA. D Flow cytometric analysis of apoptosis in SiHa and HeLa cells transfected with USP21 siRNA. **p < 0.01 and ***p < 0.001
USP21 repressed the ubiquitination of FOXM1 in CC cells
TCGA datasets revealed a positive correlation between USP21 and FOXM1 expression in CC cells (Fig. 3A). To confirm the interaction between USP21 and FOXM1, Co-IP was performed in SiHa cells. As shown in Fig. 3B, endogenous USP21 and endogenous FOXM1 co-precipitated in SiHa cells, suggesting that they may interact with each other. Subsequently, the ubiquitination/deubiquitination assay was used to determine the effects of si-USP21 on the ubiquitination of FOXM1 in SiHa cells. Results confirmed that si-USP21 transfection promoted the ubiquitination of FOXM1 (Fig. 3C). Additionally, SiHa cells transfected with si-USP21 were treated with the proteasome inhibitor, MG132. Results revealed that MG132 treatment attenuated the si-USP21-induced decrease in FOXM1 expression (Fig. 3D), suggesting that USP21 knockdown led to a decrease in FOXM1 stability. Moreover, knockdown of USP21 had no effect on the level of FOXM1 mRNA, but reduced the level of FOXM1 protein (Fig. 3E, F). These data revealed that USP21 interacted with FOXM1 to regulate the stability and ubiquitination of FOXM1.
USP21 repressed the ubiquitination of FOXM1 in CC cells. A Correlation between USP21 and FOXM1 in TCGA-CESC database. B SiHa cells were lysed, immunoprecipitation was carried out with the indicated antibodies, and the immuno-complexes were analyzed by western blotting. C The effect of USP21 knockdown on FOXM1 ubiquitination in SiHa and HeLa cells. D The expression of USP21 and FOXM1 protein in SiHa and HeLa cells transfected with si-USP21 under MG132 treatment. E, F The expression of FOXM1 mRNA and protein, as well as USP21 protein in SiHa and HeLa cells transfected with si-USP21. **p < 0.01
FOXM1 reversed the effects of USP21 knockdown on the radio-resistance of CC cells
To determine whether the effect of USP21 on CC cell radio-sensitivity was mediated by FOXM1, si-USP21 and oe-FOXM1 were co-transfected into SiHa and HeLa cells. oe-FOXM1 transfection successfully upregulated the mRNA and protein expression of FOXM1 in SiHa and HeLa cells (Fig. 4A, B). Clonogenic assay showed that the survival fraction of SiHa and HeLa cells was markedly decreased by si-USP21 transfection after irradiation; however, this effect was reversed by co-transfection with oe-FOXM1 (Fig. 4C). Additionally, co-treatment with si-USP21 and oe-FOXM1 resulted in a decrease in apoptosis of SiHa and HeLa cells compared to treatment with si-USP21 alone in the presence of radiation (Fig. 4D).
FOXM1 reversed the effects of USP21 knockdown on the radio-resistance of CC cells. The expression of FOXM1 mRNA (A) and protein (B) in SiHa and HeLa cells transfected with oe-FOXM1 or oe-Ctrl. C Clonogenic survival of SiHa and HeLa cells co-transfected with si-USP21 and oe-FOXM1 in the presence of radiation. D Flow cytometric analysis of apoptosis in SiHa and HeLa cells co-transfected with si-USP21 and oe-FOXM1 in the absence or presence of radiation (6 Gy). *p < 0.05, **p < 0.01, and ***p < 0.001
Knockdown of FOXM1 activated Hippo signaling by suppressing the nuclear translocation of YAP1
We further explored whether FOXM1 regulated the Hippo-YAP1 pathway. FOXM1 siRNAs were transfected into SiHa and HeLa cells, following which the mRNA and protein expression of FOXM1 was successfully downregulated (Fig. 5A, B). si-FOXM1#1 was selected for used in subsequent experiments. The nuclear and cytoplasmic proteins in SiHa and HeLa cells were separated to detect YAP1 subcellular expression. Our findings confirmed that the level of YAP1 was increased in the cytoplasm, but decreased in the nucleus (Fig. 5C), suggesting that FOXM1 knockdown activated the Hippo pathway by suppressing the nuclear translocation of YAP1.
Knockdown of FOXM1 enhanced the radio-sensitivity of CC cells via inhibiting YAP1
To verify whether the function of FOXM1 in the regulation of CC cell radio-sensitivity was achieved via the Hippo signaling pathway, SiHa and HeLa cells transfected with si-FOXM1 were treated with XMU-MP-1 for 6 h and then exposed to 6 Gy of radiation, followed by additional culture for 24 h. Results showed that si-FOXM1 transfection markedly decreased the survival fraction of SiHa and HeLa cells compared to si-NC transfection in the presence of irradiation (Fig. 6A). However, XMU-MP-1 treatment enhanced the survival fraction of SiHa and HeLa cells (Fig. 6A). Additionally, transfection with si-FOXM1 promoted the apoptosis of SiHa and HeLa cells in the presence or absence of irradiation, and this effect was reversed by XMU-MP-1 treatment (Fig. 6B).
Knockdown of FOXM1 enhanced the radio-sensitivity of CC cells via inhibiting the Hippo-YAP1 pathway. A Clonogenic survival of SiHa and HeLa cells transfected with si-FOXM1 and treated with or without XMU-MP-1, in the presence of radiation. B Flow cytometric analysis of apoptosis in SiHa and HeLa cells transfected with si-FOXM1 and treated with or without XMU-MP-1 in the absence or presence of radiation. *p < 0.05, **p < 0.01, and ***p < 0.001
Knockdown of USP21 activated the Hippo pathway by mediating FOXM1
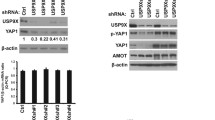
To determine the association between USP21 and FOXM1-induced Hippo pathway in CC cells, the protein levels of the YAP1 target genes, CYR61 and CTGF, were detected by qRT-PCR. Results suggested that the expression of CYR61 and CTGF was reduced in CC cells transfected with si-USP21. Additionally, co-transfection with si-USP21 and oe-FOXM1 resulted in an increase in the expression of CYR61 and CTGF in the presence of irradiation (Fig. 7A). Moreover, SiHa and HeLa cells transfected with si-USP21 alone displayed increased cytoplasmic YAP1 but decreased nuclear YAP1 levels in the presence of irradiation; however, this effect was reversed by co-transfection with oe-FOXM1 (Fig. 7B).
Knockdown of USP21 enhanced the radio-sensitivity of CC cells in vivo
To confirm the role of USP21 in the radio-sensitivity of CC cells in vivo, HeLa cells stably transfected with sh-USP21 or sh-NC were inoculated into mice, and the mice were irradiated with 6 Gy irradiation. The tumor volume and weight of mice were markedly lower in the USP21 knockdown group than those in the sh-NC group (Fig. 8A, B). Additionally, we analyzed the expression of Ki67 and caspase-3 in the tumors. IHC revealed that, compared to the sh-NC group, the Ki67 positive rate was evidently decreased, while the caspase-3-positive rate was obviously enhanced in the sh-USP21 group (Fig. 8C), suggesting that interference of USP21 reduced the tumor growth of CC cells in vivo. Furthermore, radiation treatment also markedly suppressed tumor growth relative to vehicle-treated animals. More importantly, USP21 knockdown combined with radiation treatment exerted the most significant effects on tumor growth.
Discussion
In the current study, we demonstrated that USP21 level was significantly increased in CC tissues of radio-resistant patients and was related to the radio-sensitivity of CC cells. Additionally, our findings showed that USP21 is a deubiquitinating enzyme that regulates FOXM1 deubiquitination and protein stabilization. Importantly, we found that USP21 inhibited the activation of the Hippo signaling pathway by inducing the nuclear translocation of YAP1. Given that USP21 knockdown inhibited CC progression and radio-resistance, targeting the USP21/FOXM1/Hippo pathway may serve as a promising radio-sensitization strategy for the clinical treatment of CC.
The carcinogenic effect of USP21 has been extensively studied. Upregulation of USP21 expression in breast cancer and human renal cell carcinoma was shown to promote the malignant phenotype and stem-like properties of cancer cells [11, 28]. Moreover, USP21 has also been shown to play an important role in the regulation of cell paclitaxel sensitivity [11]. However, only a few studies have explored the effect of USP21 on the radio-resistance of CC cells. Here, our data indicated that USP21 is overexpressed in CC, suggesting that USP21 may be an oncoprotein involved in CC tumorigenesis. Subsequent functional studies also confirmed the hypothesis that depletion of USP21 enhances the radiotherapy sensitivity of CC cells in vivo and in vitro. For the first time, our findings revealed that USP21 had a previously unknown role in promoting CC radio-sensitivity.
It is well known that protein degradation can be regulated by E3 ubiquitin ligase and DUBs [29]. Previous studies have shown that USP21 promotes cancer genesis and development through inhibition of EZH2 ubiquitination in bladder cells [10]. It was further reported that DUBs regulate the stability of FOXM1 by removing ubiquitin, thereby protecting FOXM1 from degradation [30]. FOXM1 is considered to be one of the substrates of USP21, and USP21 regulates the FOXM1 transcription network by stabilizing FOXM1 [11]. Similarly, our results demonstrated that USP21 could bind to FOXM1 in CC cells and regulate its stability. FOXM1, a cell growth-specific transcription factor, plays an important role in controlling the transition from G1 to S phase and controlling G2/M phase [31, 32]. Upregulation of FOXM1 results in uncontrolled cell division and an unstable cell microenvironment, while silencing of FOXM1 can enhance cell apoptosis and increase sensitivity to chemotherapy [33, 34]. In the current study, we found that FOXM1 overexpression attenuated the inhibitory effect of USP21 knockdown on the radio-resistance of CC cells. Hence, we speculated that the regulatory effect of USP21 on CC radio-resistance might be achieved by regulating the stability of FOXM1.
The Hippo pathway has become a major obstacle for carcinogenic transformation [35]. Hippo signaling suppresses the activity of YAP/TAZ by activating LATs kinases, which directly phosphorylate YAP/TAZ, resulting in the cytoplasmic retention and subsequent degradation of YAP/TAZ [36]. The activation of YAP/TAZ is common in many human cancers, and YAP/TAZ have been shown to be essential for cancer initiation, progression, or metastasis [37,38,39]. Higher YAP1 activity in tumors tissues is usually correlated with poor prognosis [40, 41]. Additionally, the activation of YAP1 also enhances the resistance of tumor cells to anti-cancer drugs [23]. Notably, several evidences reveal that ubiquitin-mediated post-translational modifications regulate the Hippo pathway, which plays a vital role in human cancers [42, 43]. For instance, phosphorylated YAP1 is targeted by the bTrCP/SCF ubiquitin ligase system for degradation [43]. The renewal of YAP1 is regulated by the Ras pathway, through regulating the expression of SOCS5/6, which recruits YAP1 to the Elongin B/C-Cullin5 ubiquitin ligase compound [44]. Furthermore, evidence shows that USP21 indirectly controls YAP1/TAZ activity via modulating the stability of the members of the MARK family of protein kinases [45]. In the current study, knockdown of FOXM1 activated the Hippo pathway by inhibiting the nuclear translocation of YAP1. Simultaneously, the inhibitory effect of USP21 knockdown on the Hippo pathway was eliminated by FOXM1 overexpression. Hence, we speculated that USP21 deubiquitinated FOXM1 to control its stability, and FOXM1 further regulated the activity of YAP1. However, the regulatory effect of USP21/FOXM1 on the phosphorylation and stability of YAP1/TAZ remains to be explored in-depth.
Conclusion
Our study provided evidence that high USP21 expression was associated with CC radio-resistance. Moreover, our findings demonstrated that knockdown of USP21 could enhance the radio-sensitivity of CC cells by inhibiting cell proliferation and inducing cell apoptosis. Mechanistically, USP21 may activate YAP1 by regulating the stability of FOXM1, thereby inhibiting Hippo signaling. Hence, our data provide new insights into the molecular mechanism of USP21 involved in CC occurrence and radio-resistance.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD. Cancer statistics. CA Cancer J Clin. 2020;70:7–30.
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR. The global burden of women’s cancers: a grand challenge in global health. Lancet (London). 2017;389:847–60.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (London). 2019;393:169–82.
Prosser SL, O’Regan L, Fry AM. Novel insights into the mechanisms of mitotic spindle assembly by NEK kinases. Mol Cell Oncol. 2016;3:1062952.
Lopata A, Kniss A, Löhr F. Ubiquitination in the ERAD Process. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155369.
Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawawaki J. Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. J Cell Biol. 2020. https://doi.org/10.1083/jcb.201912144.
Das S, Chandrasekaran AP, Suresh B, Haq S, Kang JH, Lee SJ. Genome-scale screening of deubiquitinase subfamily identifies USP3 as a stabilizer of Cdc25A regulating cell cycle in cancer. Cell Death Differ. 2020;27:3004–20.
Bonacci T, Emanuele MJ. Dissenting degradation: Deubiquitinases in cell cycle and cancer. Semin Cancer Biol. 2020;67:145–58.
Liu J, Kruswick A, Dang H, Tran AD, Kwon SM, Wang XW. Ubiquitin-specific protease 21 stabilizes BRCA2 to control DNA repair and tumor growth. Nat Commun. 2017;8:137.
Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther. 2017;10:681–9.
Arceci A, Bonacci T, Wang X, Stewart K, Damrauer JS, Hoadley KA. FOXM1 deubiquitination by usp21 regulates cell cycle progression and paclitaxel sensitivity in basal-like breast cancer. Cell Rep. 2019;26:3076-3086.e6.
Xu P, Xiao H, Yang Q, Hu R, Jiang L, Bi R. The USP21/YY1/SNHG16 axis contributes to tumor proliferation, migration, and invasion of non-small-cell lung cancer. Exp Mol Med. 2020;52:41–55.
Korver W, Roose J, Clevers H. The winged-helix transcription factor Trident is expressed in cycling cells. Nucleic Acids Res. 1997;25:1715–9.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59.
Liu C, Shi J, Li Q, Li Z, Lou C, Zhao Q. STAT1-mediated inhibition of FOXM1 enhances gemcitabine sensitivity in pancreatic cancer. Clin Sci. 2019;133:645–63.
Xiu G, Sui X, Wang Y, Zhang Z. FOXM1 regulates radiosensitivity of lung cancer cell partly by upregulating KIF20A. Eur J Pharmacol. 2018;833:79–85.
Li T, Ma J, Han X, Jia Y, Yuan H, Shui S. MicroRNA-320 enhances radiosensitivity of glioma through down-regulation of sirtuin type 1 by directly targeting forkhead box protein M1. Transl Oncol. 2018;11:205–12.
Taha Z, Janse van Rensburg HJ, Yang X. The Hippo pathway: immunity and cancer. Cancers. 2018;10:94.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Dey A, Varelas X. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19:480–94.
Zeng Y, Liu Q, Wang Y, Tian C, Yang Q, Zhao Y. CDK5 activates hippo signaling to confer resistance to radiation therapy via upregulating TAZ in lung cancer. Int J Radiat Oncol Biol Phys. 2020;108:758–69.
Zhang S, Zhang X, Sun Q, Zhuang C, Li G, Sun L. LncRNA NR2F2-AS1 promotes tumourigenesis through modulating BMI1 expression by targeting miR-320b in non-small cell lung cancer. J Cell Mol Med. 2019;23:2001–11.
Shibata M, Ham K, Hoque MO. A time for YAP1: tumorigenesis, immunosuppression and targeted therapy. Int J Cancer. 2018;143:2133–44.
Chang Y, Fu XR, Cui M, Li WM, Zhang L, Li X. Activated hippo signal pathway inhibits cell proliferation and promotes apoptosis in NK/T cell lymphoma cells. Cancer Med. 2019;8:3892–904.
Luo J, Yu FX. GPCR-hippo signaling in cancer. Cells. 2019;8:426.
Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–56.
Sun HL, Men JR, Liu HY, Liu MY, Zhang HS. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch Biochem Biophys. 2020;685:108349.
Peng L, Hu Y, Chen D, Jiao S, Sun S. Ubiquitin specific peptidase 21 regulates interleukin-8 expression, stem-cell like property of human renal cell carcinoma. Oncotarget. 2016;7:42007–16.
Mevissen TET, Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem. 2017;86:159–92.
Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016;35:668–84.
Yue M, Li S, Yan G, Li C, Kang Z. Paeoniflorin inhibits cell growth and induces cell cycle arrest through inhibition of FoxM1 in colorectal cancer cells. Cell Cycle (Georgetown). 2018;17:240–9.
Nakamura S, Hirano I, Okinaka K, Takemura T, Yokota D, Ono T. The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia. Carcinogenesis. 2010;31:2012–21.
Varghese V, Magnani L. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9:1505.
Peng WX, Han X, Zhang CL, Ge L, Du FY, Jin J. FoxM1-mediated RFC5 expression promotes temozolomide resistance. Cell Biol Toxicol. 2017;33:527–37.
Nguyen HT, Hong X, Tan S, Chen Q, Chan L, Fivaz M. Viral small T oncoproteins transform cells by alleviating hippo-pathway-mediated inhibition of the YAP proto-oncogene. Cell Rep. 2014;8:707–13.
Kim W, Khan SK, Liu Y, Xu R, Park O, He Y. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut. 2018;67:1692–703.
Byrne JJ, Soh MS, Chandhok G, Vijayaraghavan T, Teoh JS, Crawford S. Disruption of mitochondrial dynamics affects behaviour and lifespan in Caenorhabditis elegans. Cell Mol Life. 2019;76:1967–85.
Tocci P, Cianfrocca R, Di Castro V, Rosanò L, Sacconi A, Donzelli S. β-arrestin1/YAP/mutant p53 complexes orchestrate the endothelin A receptor signaling in high-grade serous ovarian cancer. Nat Commun. 2019;10:3196.
Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–10.
Fullenkamp CA, Hall SL, Jaber OI, Pakalniskis BL, Savage EC, Savage JM. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget. 2016;7:30094–108.
Bae SJ, Kim M, Kim SH, Kwon YE, Lee JH, Kim J. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat Commun. 2015;6:6314.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72–85.
Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–69.
Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, Voorhoeve PM. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. EMBO J. 2014;33:2447–57.
Nguyen HT, Kugler JM, Loya AC, Cohen SM. USP21 regulates Hippo pathway activity by mediating MARK protein turnover. Oncotarget. 2017;8:64095–105.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
ZLL, XJL, and GXJ made crucial contribution to the conception of this study. ZLL, HZY, and SPW carried out all of the experiments. SLZ prepared the first draft of this manuscript. GXJ agreed to the final design of this work and revised the manuscript critically. XJL performed cell culture and western blotting. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The use of human CC tissues was approved by the Ethics Committee of Yantaishan Hospital (approval No.: 2020016), and the experimental procedures were performed in accordance with the guidelines stipulated in the World Medical Association Declaration of Helsinki. Signed informed consent was obtained from all patients before research initiation. All animal experimental procedures were reviewed and approved by the Ethics Committee of Yantaishan Hospital, and the experimental procedures were performed in accordance with the Guidelines of the National Regulation of China for Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Liu, X., Yu, H. et al. USP21 regulates Hippo signaling to promote radioresistance by deubiquitinating FOXM1 in cervical cancer. Human Cell 35, 333–347 (2022). https://doi.org/10.1007/s13577-021-00650-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00650-9