Abstract
To investigate the effect of the number of embryo cells on the clinical outcome of frozen-thawed embryo transfer and explore the optimal policy for decreases of multiple pregnancy rate, patients who experienced day 3 vitrified double frozen-thawed embryo transfer were retrospectively analyzed. According to the number of embryonic cells in each pre-frozen embryo, the patients were divided into six groups: 8C2 (two 8-cell embryos), 8C1– < 8C1 (one 8-cell embryo and one under-8-cell embryo), 8C1– > 8C1 (one 8-cell embryo and one over-8-cell embryo), < 8C2 (two under-8-cell embryos), < 8C1– > 8C1 (one under-8-cell embryo and one over-8-cell embryo), and > 8C2 (two over-8-cell embryos). The clinical data were analyzed. The classification decision tree was used to analyze the optimal transfer strategy. A total of 2184 cycles of day 3 vitrified double frozen-thawed embryo transfer were enrolled. In day 3 double frozen-thawed embryo cycles, the 8C2 group and 8C1– > 8C1 group had significantly (P < 0.05) higher pregnancy and multiple pregnancy rates than the other groups. No significant (P > 0.05) difference existed in the pregnancy rate and live birth rate between the 8C1– < 8C1 group, 8C2 group and 8C1– > 8C1 group, but the implantation rate and multiple pregnancy rate in the 8C1– < 8C1 group were significantly (P < 0.05) lower than in the other two groups. Compared with the multiple pregnancy rate of all cycles, the cycles in two branches showed significantly (P < 0.05) higher multiple pregnancy rates (≤ 29 years old: 8C2 / 8C1– > 8C1; 29 < age ≤ 36 years for the first transfer: 8C2 / 8C1– < 8C1 / 8C1– > 8C1, one branch showed similar rate (≤ 29 years old: 8C2 / 8C1– > 8C1) for the first transfer, and the remaining four branches demonstrated significantly (P < 0.05) lower rates. The clinical pregnancy rates before and after optimization were 51.0% vs 50.5%, and the multiple pregnancy rates were 38.5% vs 16.9%. In conclusion, the number of pre-frozen embryonic cells is an important factor affecting the clinical outcome of frozen-thawed embryo transfer in day 3 double good embryos frozen-thawed cycles. The age of patient, number of embryo cells, and the first time of transfer are the most valuable parameters for prediction. For women ≤ 29 years old, the single embryo transfer (SET) strategy was to choose an embryo ≥ 8 cells, and for women with < 29 age ≤ 36 years old, the SET strategy in the first transfer was to choose an embryo ≥ 8 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main challenge and goal of in vitro fertilization (IVF) is to cultivate and select the best embryo for achieving the best maternal–fetal outcome. In real-world practice, the number of embryos transferred is often increased in order to improve the pregnancy rate, but a subsequent problem is iatrogenic multiple pregnancies to affect both mothers and newborns. For example, from the maternal perspective, the incidence of obstetric complications, such as gestational diabetes, hypertensive disorders, postpartum hemorrhage, and risks during cesarean section, has significantly increased. From the perspective of fetus and newborns, the incidence of premature birth, low birth weight, cerebral palsy, neurological complications, and perinatal mortality has significantly increased [1, 2]. In order to reduce the rate of multiple pregnancies after assisted reproductive technology (ART), it is necessary to optimize the strategy of elective single embryo transfer (eSET). Single blastocyst transfer (SBT) has become the preferred strategy in many centers in the world because SBT can lead to a more satisfying pregnancy rate [3]. However, compared with cleavage stage embryo transfer, reports of preterm delivery, monozygotic twins, macrosomia, congenital malformations and sex ratio changes after blastocyst transfer have also attracted great attention. In addition, the epigenetic changes related to long-term embryo culture are another issue nonnegligible [4]. Extending the culture period to the blastocyst stage also increases the risk of cycle canceling due to lack of embryo transfer. Therefore, it is advisable to make an accurate choice between eSET and double embryo transfer (DET) during the cleavage stage [5]. The morphological criteria for evaluating embryo implanting potential in fresh cycles have developed from cell numbers, blastomeric symmetry, and cell fragmentation rates during the cleavage stage [6] to the quality of inner cell mass and nourishing ectoderm during the blastula stage [7]. Although the embryonic development and morphology helps to evaluate which embryos have the highest implantation potential and live birth rate, it may also affect the number of embryo transfer [8] or the best date for embryo transfer [9]. However, due to the spatiotemporal particularity of frozen-thawed cycles as well as freezing and resuscitation impact, the significance of morphological evaluation to frozen-thawed cycles may not be completely equal to that for fresh cycles. Moreover, frozen-thawed embryo transfer eliminates the effect of high estrogen levels or early progesterone elevation on endometrium in the fresh cycles, which is of great significance for evaluating the relationship between embryo morphology and clinical outcome. At present, in order to improve the utilization of embryos, some reproductive centers choose to freeze a small number of embryos on day 3 and to culture the remaining ones at the blastocyst stage. The patients who have already obtained Day 3 frozen embryos (double embryos in one Cryocarrier) and attempted to reproduce (for the first time or more) through frozen-thawed embryo transfer cycles are faced with the problem of clinical efficiency improvement. The number of embryonic cells is the most important and objective parameter of embryo morphology. Therefore, the purpose of this study was to investigate the relationship between the cell number of pre-frozen embryos and the clinical outcome of frozen–thawed cycles and to provide support for embryo freezing and transfer strategies in combination with classification decision tree.
Materials and methods
Subjects
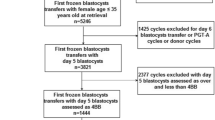
This study was approved by the ethics committee of our hospital, and all patients had given their signed informed consent to participate. The clinical data of patients who underwent day 3 vitrified double frozen-thawed embryo transfer (DFET) in our hospital from January 2018 to February 2019 were retrospectively analyzed. According to the number of embryonic cells in the pre-frozen embryos, the patients were divided into six groups: 8C2 (two 8-cell embryos), 8C1– < 8C1 (one 8-cell embryo and one under-8-cell embryo), 8C1– > 8C1 (one 8-cell embryo and one over-8-cell embryo), < 8C2 (two under-8-cell embryos), < 8C1– > 8C1 (one under-8-cell embryo and one over-8-cell embryo), and > 8C2 (two over-8-cell embryos). The inclusion criteria were (i) high-quality embryos derived from two pro-nucleus (2PN) fertilized embryos, with cell fragments less than 10% and cell heterogeneity below moderate, (ii) all survived after resuscitation, and (iii) double embryos being transferred on day 3.
The data of eSET selected from day 3 vitrified double frozen–thawed embryos through optimized transfer strategy from January 2020 to April 2020 were confirmed. The inclusion criteria were (i) high-quality embryos with fragments less than 10% and cell inequality below moderate, and (ii) all survived after resuscitation.
Methods of culture, score, freezingand thawing
Embryo culture
Conventional ovarian stimulation strategy was used. The oocytes were harvested 36 h after human chorionic gonadotropin (HCG) injection, and the oocytes were inseminated through conventional IVF/ICSI (intracytoplasmic sperm injection). The oocytes were placed in the cleavage embryo culture fluid (G1-plus Vitrolife, Sweden) 16–18 h after insemination, and the fertilization was observed. The morphology of Day 3 embryos was evaluated 48 h after culture, and whether to perform embryo transfer on Day 3 in the fresh cycle or to freeze the embryos was determined by the clinical conditions of the patients. According to the condition of embryos, the Day 3 embryos were frozen in 1–2 Cryocarriers (2 embryos per Cryocarrier), and the remaining embryos were cultured in medium droplets (G2-plus, Vitrolife, Sweden).
Embryo score and freezing standard
The cleavage stage embryos were evaluated according to the traditional morphological scores, including the number of blastomeres, degree of cell fragments, average degree of blastomeres, and appearance of mononucleated or multinucleated vacuoles. The Day 3 embryo freezing standard was (i) fertilized embryos derived from 2PN, (ii) the number of cells not less than 6, (iii) fragments less than 10%, (iv) heterogeneity less than moderate, and (v) no obvious morphological abnormality.
Embryo freezing and thawing
Day3 embryos were vitrified and thawed according to the Crytop-method reported by Kuwayam [10], and commercial kits (Kitazato, Japan) were used. After thawing, they were washed several times in the culture medium and finally transferred to the overnight balanced blastocyst culture medium (G-2plus Vitrolife, Sweden). Then they were put in the 37 °C, 6% CO2 incubator and scored for 2 h. Finally, the embryos were transferred about 6 h later.
Endometrial preparation and clinical pregnancy confirmation
The endometrium was not specially prepared for patients with regular menstrual cycle and normal ovulation, and the natural cycle was application. Transvaginal ultrasound was performed to monitor follicular development from the 9th to 10th day of menstrual cycle. Oral estradiol valerate (Progynova) was administrated at a dosage of 1–2 mg daily as appropriate when the endometrial thickness was insufficient. The standard of dominant follicle ≥ 16 mm and endometrial thickness ≥ 7 mm should be met during ovulation. Embryo transfer was performed on the third day after ovulation. All patients were given a daily intramuscular injection of 20 mg progesterone as luteal support starting from the day of ovulation.
In artificial cycle, all patients received oral estradiol valerate (Progynova) at a dosage of 2–4 mg twice daily beginning on the third day of their cycles. Transvaginal ultrasound was performed to monitor endometrial thickness. Progesterone supplementation was commenced at a dosage of 40–60 mg daily of intramuscular injection when the serum E2 was ≥ 200 pg/ml and the endometrial thickness was ≥ 7 mm. Embryo transfer was performed on the fourth day of progesterone administration. Intramuscular progesterone was continued with Crinone 90 mg daily supplemented as luteal support. The regular menstrual cycle of endometrial preparation in the natural cycle was used in patients with normal ovulation.
Clinical pregnancy was confirmed when the blood β-HCG was positive 14 days after embryo transfer, and B-ultrasound examination showed that pregnancy sac and primitive cardiac tube pulsation were present 4–5 weeks after embryo transfer.
Classification decision tree modeling
Chi-square automatic interaction detector (CHAID) was carried out by SPSS23.0 software (IBM, Chicago, USA). Singleton pregnancy, multiple pregnancies and non-pregnancy were used as dependent variables, whereas groups (8C2, 8C1– < 8C1, 8C1– > 8C1, < 8C2, < 8C1– > 8C1, and > 8C2), age, first transplant, infertility type, and years of infertility were treated as independent variables. The transformation day endometrial thickness was used as an influence variable. Using the growth method, CHAID selected the independent variables that had the strongest interaction with the dependent variables in each step and merged these categories to establish a classification decision tree model if the categories of the independent variables were not significantly different from the dependent variables.
Statistical analysis
The SPSS23.0 software was used for statistical analysis. Measurement data were expressed in mean ± SD and the counting data were expressed in %. One-Way ANOVA was used to analyze the mean difference among groups, Chi squre test was used to compare the classified data between groups, and binary Logistic regression (Enter) was used to calculate the OR value. The SPSS classification decision tree adopted the growth method CHAID. The statistically significant difference was set at P < 0.05.
Results
Baseline characteristics
A total of 2184 cycles met the inclusion criteria. According to the number of cells before freezing, the transferred embryos were divided into six groups, namely, 8C2, 8C1– < 8C1, 8C1– > 8C1, < 8C2, < 8C1– > 8C1, and > 8C2, and the number of cycles in each group was shown in Fig. 1. A significant (P < 0.001) difference existed in the ratio of first ET (%) and cycle rank order rather than in the female age, years of infertility, endometrial thickness on the day of transformation, endometrial preparation and type (Table 1). A significant (P < 0.05) difference also existed in female age between < 8C1– > 8C1 and 8C2 groups and in years of infertility between < 8C2 and 8C2, 8C1– > 8C1 or > 8C2 group.
No. of cycles and typical embryos of grouping. a No. of day 3 double frozen–thawed embryo cycles (one 8-cell embryo and one 8-cell embryo: 8–8, n = 837). b Typical embryos in different groups. 8C2 (two 8-cell embryos), 8C1– < 8C1 (one 8-cell embryo and one under-8-cell embryo), 8C1– > 8C1 (one-8-cell embryo and one over-8-cell embryo), < 8C2 (two under-8-cell embryos), < 8C1– > 8C1 (one under-8-cell embryo and one over-8-cell embryo), and > 8C2 (two over-8-cell embryos)
Clinical outcomes
The clinical outcome and birth status were compared among six groups (Tables 2 and 3 and Figs.1 and 2). The 8C2 and 8C1– > 8C1 groups had significantly (P < 0.05) higher live birth and multiple pregnancy rates compared with other groups; however, no significant significance (P > 0.05) was observed in the implantation rate or clinical pregnancy rate between these two groups. The implantation rate and multiple pregnancy rate were significantly (P < 0.001) lower in the 8C1– < 8C1 group than in the 8C2 or the 8C1– > 8C1 group.
The implantation rate was significantly (P < 0.05) lower in the 8C1– < 8C1 (OR 0.699, 95% CI 0.576, 0.849), < 8C2 (OR 0.655, 95% CI 0.487, 0.881), < 8C1– > 8C1 (OR 0.619, 95% CI 0.482, 0.794), and > 8C2 (OR 0.618, 95% CI 0.495, 0.771) than in the 8C2 reference group. The clinical pregnancy rate was significantly (P < 0.05) lower in the < 8C1– > 8C1 (OR 0.623, 95% CI 0.482, 0.864) and > 8C2 (OR 0.697, 95% CI 0.522, 0.932) groups than in the 8C2 reference group. The multiple birth rate was significantly (P < 0.05) lower in the 8C1– < 8C1 (OR 0.531, 95% CI 0.361, 0.781), < 8C2 (OR 0.389, 95% CI 0.207, 0.732), and > 8C2 (OR 0.527, 95% CI 0.388, 0.822) groups than in the 8C2 reference group. The live birth rate was significantly (P < 0.05) lower in the < 8C1– > 8C1 (OR 0.694, 95% CI 0.499, 0.966) and the > 8C2 (OR 0.726, 95% CI 0.542, 0.973) group than that in the 8C2 group.
Classification decision tree analysis
A total of seven branches were generated, and the multiple pregnancy rate in two branches in the decision tree was higher than that in the total transplantation cycle (age ≤ 29 years old: 8C2 / 8C1– > 8C1; 29 < age ≤ 36 years old for the first embryo transfer: 8C2 /8C1– < 8C1 / 8C1– > 8C1). The multiple pregnancy ratio in one branch was about equal to the proportion of multiple pregnancy in the total transplantation cycle (age ≤ 29 years old: 8C1– < 8C1 / < 8C2 / < 8C1– > 8C1 / > 8C2 for the first embryo transfer) (Figs. 3 and 4).
Decision tree analysis. I, singleton pregnancy; II, multiple pregnancy; III, non-pregnancy; 1, 8C2; 2, 8C1– < 8C1; 3, 8C1– > 8C1; 4, < 8C2; 5, < 8C1– > 8C1; and 6. > 8C2. Red, the proportion of branched multiple pregnancy was higher than that of total multiple pregnancy; Yellow, the proportion of branched multiple pregnancy was about equal to that of total multiple pregnancy
8cell-8cell/8cell– > 8cell:8C2 or 8C1– > 8C1. A selective SET strategy was established based on the decision tree analysis to reduce the multiple birth rate but ensure the pregnancy rate. For women with the age ≤ 29 years old, the SET strategy was selected in the groups of 8C2 / 8C1– > 8C1, and for women with the age > 29 years old but ≤ 36 years old, the SET strategy was in the first transfer 8C2 / 8C1– < 8C1 / 8C1– > 8C1. SET, single embryo transfer
Confirmatory analysis
The baseline values of clinical data before and after implementation of the selective SET strategy were analyzed using the decision tree for comparing the implantation rate, clinical pregnancy rate, multiple pregnancy rate, and birth rate before and after the improved transfer strategy (Tables 4 and 5). A significant (P < 0.05) difference existed in the implantation rate and multiple pregnancy rate rather than in the implantation rate and birth rate before and after implementation of selective SET.
Discussion
This study investigated the effect of the number of pre-frozen embryo cells on the clinical outcome in day 3 twin embryo frozen–thawed cycle. By combining age, type of infertility, years of infertility, cycle rank order, and transformation-day endometrial thickness, a decision tree was established to ensure the clinical pregnancy rate while reducing the multiple pregnancy rate, which had been proved by the confirmatory analysis. All the following information was included in order to establish the transfer strategy suitable for most day-3 double-embryo frozen–thawed cycles: the age, regimen of periodic medication, cause of infertility, mode of periodic fertilization, and number of eggs obtained in the cycle. The final analysis included a total of 2184 cycles, and the confirmatory analysis included a total of 586 cycles. It was found that both the 8C2 and 8C1– > 8C1 groups had significantly higher rates of implantation, clinical pregnancy, multiple pregnancy, and live birth, but without significant differences in these parameters between these two groups. A significant difference existed in the implantation rate between 8C1– < 8C1 group and 8C1– > 8C1 group. The classification decision tree was established by taking singleton pregnancy, twin pregnancy, and non-pregnancy as dependent variables, demonstrating that the age of the woman, the number of embryo cells before freezing, and being the first embryo transfer are the most predictive parameters.
At present, the value of the time-lapse system has been further confirmed in the prediction of embryo potential by embryo morphodynamics. The determination of embryo morphodynamics parameters for in vitro development has a predictive value for clinical selection of transferred embryos [11, 12]. But classical morphology is still the most important parameter. Carrasco et al. [13] performed an analysis of morphokinetic parameters in embryos cultured in an incubator with the time-lapse system in combination with the embryo morphology assessment on D3 to develop a hierarchical model which set the classical morphological score, the t4 and t8 morphokinetic values as the variables with the best prognosis of implantation. This study confirmed that the classical morphological score was the most predictive parameter for implantation through the decision tree analysis. The number of embryo cells is the primary observation index of embryo morphology, which can reflect the speed of embryo development, and the subjective influence of uniformity is less than that of fragments. Moreover, the selection criteria of frozen embryos were mainly high-quality embryos, with the homogeneity of fragments being below moderate. Therefore, our study evaluated the clinical outcome of frozen embryos from the number of embryo cells, providing data support for transplantation strategy. Our study showed that in the frozen–thawed transfer cycle of day-3 double embryos with one embryo of 8 cells, significantly higher rates of implantation, clinical pregnancy, and live birth were obtained. However, the rates of implantation, clinical pregnancy, multiple birth, and live birth were significantly lower in the 8C1– < 8C1 group than those in the 8C2 group and 8C1– > 8C1 group. It is possible that the number of blastomeres represents the rate of embryo development, the implantation potential of slow development is poor, and the implantation potential of the fast development is similar to that of the normal growth. This is in line with some reports on the effect of the number of embryonic cells in the fresh cycle on the developmental potential of embryos [14], and this study [14] had the end point of blastocyst formation and showed that dilated or expanded blastocysts from 7-cell or 8-cell embryos were superior to the blastocysts from other mitotic stages, regardless of division or asymmetry. The studies by Zhao et al. [15] and Stylianou et al. [16] also reached similar outcomes. Embryos with normal growth have higher implantation rates [16] than those with slow growth or fast growth. The study by Zhao et al. [15] showed that high-quality embryos with age ≤ 35 years, > 10 cell and 8 cell on day 3 have similar clinical results. However, in our study, although the average age in the > 8C2 group was small and the proportion of first transplantation was high, the implantation rate was lower than those of the first three groups of 8C2, 8C1– < 8C1, and 8C1– > 8C1, which may be due to the small number of cases in the > 10 cell- > 10 cell group (a total of 40 cycles).
Inhomogeneity is an important parameter in morphological evaluation and can be caused by one-third abnormal cleavage [17]. This study used frozen–thawed embryos, most of which are high-quality embryos; however, the bias caused by abnormal cleavage cannot be completely excluded. In our study, we did not compare the number of eggs obtained in the cycle, number of available embryos and dosage of drug regimens, which may be related to abnormal cleavage. Early abnormal cleavage of embryos is related to blastocyst formation and implantation rate [18]. In a word, our study did not fully show that the implanting rate of > 8-cell embryo is similar to or higher than that of the 8-cell embryo. The implantation rate and multiple pregnancy rate in the 8C2 group and 8C1– < 8C1 group were similar, but significantly higher than those in the other groups, although there was no significant difference in the multiple pregnancy rate between the < 8C1– > 8C1 group and other groups. Because the number of cases in the < 8C1– > 8C1 group was smaller and the clinical pregnancy rate was the lowest, the multiple pregnancy rate was higher since the multiple pregnancy rate was calculated as the number of pregnancies. In order to better optimize the clinical application, based on the results of inter-group statistics and Logistic regression, our study further used the classification decision tree to establish a model conducive to clinical transplantation strategy, and this is to ensure the pregnancy rate while reducing the multiple pregnancy rate for providing a clear and accurate transplantation strategy.
At present, with the development of IVF technology, the increased multiple pregnancy rate is a widespread problem in all major reproduction centers even though single blastocyst transfer in some centers has achieved good results. There are a large number of day-3 frozen tubes of two embryos in each center, and with the permission of the second child in one family in China, many patients who have given birth to the first child through assisted reproduction are prepared to have a second child through the frozen–thawed cycle. Under these circumstances, it is necessary not only to enable pregnancy quickly and effectively, but also to avoid multiple births. The conditions under which embryos are cultured in vitro can themselves affect the morphology of developing embryos. Therefore, this leads to the problem of data differences between standardized grading systems and different centers, and it is necessary to establish different laboratory evaluation standards.
In daily clinical work, the selection of the best embryo is never a problem, but the selection and ranking of available embryos is. In addition, in the frozen-thawed cycle, there are updates of culture system and laboratory equipment during freezing and resuscitation, so it is of great clinical significance to establish the current frozen–thawed transplantation strategy. It is known that some factors may affect embryonic development and grading of embryos, such as temperature and pH value. If the temperature drops and the pH value drifts (usually toward alkalinity), it may result in slow development of the embryo [19]. Similarly, if 20% oxygen concentration is used, it will delay embryonic development [20, 21]. Different culture media and incubators in different laboratories can lead to different rates of development. Therefore, each reproductive center should establish evaluation criteria and formulate appropriate transplantation strategies according to the actual situation.
The establishment of the classification decision tree takes into account the fact that singleton pregnancy is the best clinical outcome, so singleton pregnancy and twin pregnancy are treated as pregnancy classification dependent variables. The age, first transplant, cycle rank order, frozen–thawed embryo cell number, infertility type, and infertility years were used as independent variables, whereas the transformation-day intimal thickness as an influence variable. Using the growth method, the CHAID selected the independent variables that had the strongest interaction with the dependent variables in each step and merged these categories to establish a classification decision tree model if the categories of the independent variables were not significantly different from the dependent variables. The results revealed three independent variables to constitute the decision tree: age, the number of frozen–thawed embryo cells, and being the first embryo transfer, suggesting that the number of frozen–thawed embryo cells has an important predictive value for clinical outcome. The proportion of multiple births in the two branches of the decision tree is higher than that of the total number of multiple births (age ≤ 29 years old: 8C2/8C1– > 8C1, and 29 < age ≤ 36 years for the first transfer: 8C2/8C1– < 8C1/8C1– > 8C1), whereas one branch showed similar rates (age ≤ 29 years for the first transfer: 8C2/8C1– > 8C1), with the remaining four branches demonstrating significant lower rates.
In short, during the frozen–thawed cycles of day 3 double embryos, our study suggested the elective SET strategy below according to the results of classification decision tree: to choose an embryo ≥ 8 cells for women at age ≤ 29 years: 8C2/8C1– > 8C1, and to choose an embryo ≥ 8 cells for women at 29 < age ≤ 36 years for the first transfer: 8C2/8C1– < 8C1/8C1– > 8C1. The above strategy has been confirmed by the confirmatory analysis comparing the results before and after optimization, with the clinical pregnancy rate being 51.0% vs 50.5%, and the multiple pregnancy rate being 38.5% vs 16.9%, respectively. At present, the development of blastocyst culture technology and the application of time-lapses are beneficial to the SET strategy in the day-3 double-embryo frozen–thawed cycle, and it is not necessary to continue to culture and transfer two embryos directly.
Some limitations existed in this study including the retrospective nature, Chinese ethnicity enrolled only, and one center study, which may all affect the effectiveness of this study. Moreover, this study only provides a selection method under the situation that day 3 double embryos have already been frozen in one tube while more embryos cannot be further cultivated due to patient reasons or laboratory conditions.
In conclusion, the number of pre-frozen embryonic cells is an important factor affecting the clinical outcome of frozen–thawed embryo transfer in day 3 double good embryos frozen–thawed cycles. The age of patient, number of embryo cells, and the first time of transfer are the most valuable parameters for prediction.
References
Pinborg A, et al. Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Hum Reprod. 2003;18(6):1234–43.
Pinborg A, et al. Maternal risks and perinatal outcome in a Danish national cohort of 1005 twin pregnancies: the role of in vitro fertilization. Acta Obstet Gynecol Scand. 2004;83(1):75–84.
Johnston J, Gusmano MK, Patrizio P. Preterm births, multiples, and fertility treatment: recommendations for changes to policy and clinical practices. Fertil Steril. 2014;102(1):36–9.
Maheshwari A, Hamilton M, Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage? Reprod Biomed Online. 2016;32(2):142–6.
Vaegter KK, et al. Construction and validation of a prediction model to minimize twin rates at preserved high live birth rates after IVF. Reprod Biomed Online. 2019;38(1):22–9.
Racowsky C, et al. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95(6):1985–9.
Hill MJ, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99(5):1283 e1-1289 e1.
Giorgetti C, et al. Elective single embryo transfer: a justified policy for selected patients. Gynecol Obstet Fertil. 2006;34(4):317–22.
Racowsky C, et al. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73(3):558–64.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80.
Ohata K, Ezoe K, Miki T, Morita H, Tsuchiya R, Kaneko S, Okimura T, Uchiyama K, Yabuuchi A, Kobayashi T, Montag M, Kato K. Blastomere movement post first cell division correlates with embryonic compaction and subsequent blastocyst formation. Reprod Biol Endocrinol. 2019;17(1):1–6.
Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertil Steril. 2016;105(3):656–62.
Carrasco B, et al. Selecting embryos with the highest implantation potential using data mining and decision tree based on classical embryo morphology and morphokinetics. J Assist Reprod Genet. 2017;34(8):983–90.
Racowsky C, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6(3):323–31.
Zhao H, et al. Over ten-cell good embryo transfers on day three have equivalent clinical outcomes with those of eight-cell embryos in female patients aged ≤ 35 years: a retrospective cohort study. Gynecol Obstet Invest. 2019;84(3):298–304.
Stylianou C, et al. Embryo morphology as a predictor of IVF success: an evaluation of the proposed UK ACE grading scheme for cleavage stage embryos. Hum Fertil (Camb). 2012;15(1):11–7.
Desai NN, et al. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–6.
Kong X, et al. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS ONE. 2016;11(4):e0153697.
Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 2016;22(1):2–22.
Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 2013;99(3):738 e4-744e4.
Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online. 2010;21(3):402–10.
Funding
This work was partially funded by National Key Research and Development Project of China (Grand number 2018YFC1002104) and Natural Science Foundation of Hebei Province in 2019 (Grand number 19JCZDJC6500(Z)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest
Research involving Human Participants and/or Animals
This study was approved by the Ethics Committee of The Second Hospital of Hebei Medical University and we certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent has been obtained from every participant in this study. All potentially recognizable data including photos, figures, data, etc. have been given written informed consent to being published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, XL., Zhao, ZM., Du, YJ. et al. The optimal number of embryo cells for effective pregnancy and decrease of multiple pregnancy rate in frozen-thawed embryo transfer. Human Cell 34, 836–846 (2021). https://doi.org/10.1007/s13577-021-00516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00516-0