Abstract
This study was performed to investigate the role of PTK7 in esophageal squamous cell carcinoma (ESCC) stem-like cells (CSCs). PTK7 expression in ESCCs identified by RT-qPCR, and CSC-like cells were isolated from populations of NEC and TE-1 cells. The CSC-like cells were verified by flow cytometric analyses performed using CD34 and CD133 antibodies, and RT-qPCR and western blot assays were used to examine the self-renewal capability of CSC-like cells. CSC-like cells treated with PTK7 siRNA or a P53-specific inhibitor (PFTα) were analyzed for their sphere formation capacity and their apoptosis and migration/invasion capabilities by sphere formation, flow cytometry, and transwell assay, respectively. Their levels of P53, MKK3, and cleaved caspase 3 expression were examined by western blot analysis. Our results revealed that a majority of the isolated CSC-like cells were CD34+/CD133+ double positive cells. Nango, Sox2, and OCT4 were dramatically increased in the separated CSC-like cells, which had the pluripotency and self-renewal properties of stem cells. Additional, PTK7 was dramatically upregulated in the ESCC tissues and CSC-like cells. An investigation of the function of CSC-like cells revealed that knockdown of PTK7 reduced their sphere formation, promoted apoptosis, and suppressed their migration and invasion abilities, all of which could be significantly reversed by PFTα. Mechanistic studies showed that PFTα could attenuate the upregulation of P53, MKK3, and cleaved caspase 3 expression that was induced by PTK7 knockdown in CSC-like cells. PTK7 increased the malignant behaviors of CSC-like cells derived from ESCC cells by regulating p53. Therefore, this study suggests PTK7 as an underlying target for therapy against ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is one of the common malignant tumors worldwide, and its tumor-related mortality rate is the sixth highest among all tumors types [1]. The development and occurrence of esophageal cancer are closely related to a person’s lifestyle and dietary habits, and changes in those risk factors have increased the incidence of esophageal cancer in recent years [2]. Esophageal squamous cell carcinoma (ESCC) is one of the most common types of esophageal cancer and has a high mortality rate [3]. Due to a lack of effective early diagnostic methods, most patients with ESCC are initially diagnosed at an advanced stage of their disease [4]. Furthermore, the risks for ESCC metastasis and recurrence are high, and the 5-year survival rate is < 20% [5]. Therefore, there is an urgent need to further study the mechanism of ESCC progression, so as to provide a theoretical basis for the development of new methods that can aid in the early diagnosis, prognosis, and effective treatment of ESCC. The discovery of cancer stem cells (CSCs) might suggest new methods for treating ESCC [6, 7]. CSCs comprise a small population of cells with the characteristics of stem cells, which are capable of self-renewal, multi-directional differentiation, and high rates of proliferation [8]. CSCs play decisive roles in the occurrence, invasion, metastasis, recurrence, and drug resistance of cancers [9]; therefore, a therapeutic strategy that targets CSCs might represent a new direction in cancer therapy. However, due to the different subsets of CSCs and their strong plasticity and dynamics, there is still no effective target or intervention method for eliminating CSCs.
Receptor protein tyrosine kinases (RTKs) comprise the largest class of transmembrane receptors, and a vital role in cell signal transduction [9]. RTKs not only play decisive roles in cell survival, proliferation, differentiation, migration, and apoptosis, but are also closely related to the development of cancers [10, 11]. Among those kinases, protein tyrosine kinase 7 (PTK7), a newly discovered member of the RTK family, is also known as colon carcinoma kinase 4 (CCK-4) because of its high expression in colon cancer tissue. PTK7 catalyzes the transfer of γ-phosphate from ATP onto the tyrosine residues of various protein substrates [12]. Multiple studies have found that PTK7 is highly expressed in many human malignancies, including colorectal cancer [13, 14], cervical cancer [15], lung cancer [16], breast cancer [17], gastric cancer [18], and acute myeloid leukemia [19]. Recent studies have also suggested PTK7 as a new target for use in cancer therapy and that PTK7 expression is upregulated in ESCC [20,21,22,23,24] Although the involvement of PTK7 in ESCC has been previously reported, its specific functions and mechanisms of action in CSC-like cells derived from ESCC cells remain unclear.
In this study, we extracted CSC-like cells from populations of ESCC cells and then analyzed various properties of those CSC-like cells. We conducted functional experiments that verified the effects of PTK7 knockdown on the biological processes and CSC-like phenotypes of ESCC cells. We found that PTK7 could help regulate the MAP kinase kinase 3 (MKK3)/p53 signaling pathway and that the effects of PTK7 knockdown on CSC-like cells derived from ESCC cells could be attenuated. Therefore, we hypothesize that PTK7 promotes CSC-like properties in ESCC cells and provide evidence for the roles played by PTK7 in those CSC-like properties.
Materials and methods
Tissue samples
Specimens of ESCC and normal tissue were collected from 15 ESCC patients who were diagnosed at the The First Affiliated Hospital of Chongqing Medical University during the time period of July 2016 to February 2018. After collection, the tissue samples were immediately frozen and stored in liquid nitrogen until further use. Each patient enrolled in the study provided their written informed consent, and the study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (Approval No. 2017-08-01). All study procedures were performed in accordance with ethical principles stated in the Declaration of Helsinki.
Cell lines
NEC cells (BNCC339909) and TE-1 cells (BNCC100151) were purchased from the BeNa Culture Collection, Beijing, China. NEC cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) and TE-1 cells were grown in RPMI-1640 (Gibco, Waltham, Ma, USA). The growth media for both cell lines contained 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin–streptomycin (Life Technologies, Carlsbad, CA, USA; cat. no. 15140-122). Both cell lines were cultured at 37 °C in an atmosphere containing 5% CO2 and 95% air.
Cell transfection
Two siRNAs against PTK7 (siRNA1 and siRNA2) were utilized to silence PTK7 expression; a negative control (NC) siRNA (si-NC) was used as a control. All 3 siRNAs were purchased from Hanbio Biotechnology Co., Ltd., China. Briefly, CSC-like cells derived from NEC and TE-1 cells were cultured in Neural Stem Cell Basal Medium (Cyagen Biosciences, Suzhou, P. R. China) including bFGF, and EGF, then cells were contained in 6-well plates, and transfected with 50 nM siRNA using Lipofectamine 3000 (Invitrogen) according to the instructions provided by the supplier. Further experiments were performed 72 h after transfection.
Separation of side population (SP) cells
NEC and TE-1 cells (5 × 106/mL) in their logarithmic growth phase were cultured in serum-free medium containing 20 ng/mL of epidermal growth factor (EGF, R&D, Minneapolis, MN, USA) and 20 ng/mL of basic fibroblast growth factor (bFGF, R&D,) at 37 °C in an incubator with 5% CO2. The medium was changed or added every 2 days. After a week of suspension culture, the cells were collected and digested with 0.05% trypsin (Biochrom, Cambridge, UK cat. no. L2143) and 0.53 mM EDTA (Invitrogen) at 37 °C for 10 min. Single-cell suspensions (1 × 106 cells/mL) were prepared in HBSS containing 3% FBS. The cells were then washed in water at 37 °C for 90 min and centrifuged. Side population (SP) cells were analyzed by flow cytometry (FACS Vantage SE, Becton–Dickinson, Franklin Lakes, NJ, USA).
SP cell phenotype analysis
Cancer cells (CCs) and SP cells (CSC-like cells) were suspended in 100 μL of PBS containing 2% FBS at a final concentration of 1 × 106/cells/mL. After blocking, the cells were treated with PE-labeled CD133 (Miltenyi Biotec, Germany) and FITC-labeled CD34 (Becton–Dickinson) at 4 °C for 30 min. Mouse IgG was used in the control group. The results were examined by flow cytometry (Becton–Dickinson).
Quantitative real-time PCR (RT-PCR)
Total RNA was extracted with TRIzol reagent (Life Technology, USA; cat. no. 15596026) and reverse transcribed to cDNA using a cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Next 2 × Taq PCR Master Mix (DBI Bioscience, Newark, Del, USA; cat. no. DBI-2028) was used to examine the cellular levels of Nango, Sox2, OCT4 and PTK7 expression on an ABI7500 RT-PCR system. The sequences of all primers used for RT-PCR are listed in Table 1.
Sphere formation assay
Cells were collected, centrifuged, and suspended in complete medium as previously described [25]. The treated cells (2 mL, 1 × 105 cells/mL) were plated into 6-well ultra-low attachment plates and cultured at 37 °C in a 5% CO2 atmosphere for 7 days. The formatting spheres were observed under a microscope and their diameters were measured and analyzed.
Western blot assays
RIPA buffer (Santa Cruz Biotechnology, Dallas, TX, USA; cat. no. sc-24948) was utilized to extract total cellular proteins, and protein concentrations were determined using a BCA protein quantitation kit (Genenode, China; cat. no. 7015A). Next, a 30 µg sample of protein from each treatment group was separated by 10% SDS-PAGE, and the protein bands were transferred onto PVDF membranes. The membranes were then blocked with 5% skim milk for 2 h and subsequently incubated overnight with primary antibodies at 4 °C. The following morning, the membranes were washed with PBS and then incubated with a secondary antibody for 2 h. Finally, the membranes were visualized by chemiluminescence. The primary antibodies used were Nango (Abcam, Cambridge, UK, cat. no. ab80892), Sox2 (Abcam, cat. no. ab97959), OCT4 (Abcam, cat. no. ab181557), PTK7 (Abbexa, Cambridge, UK, cat. no. abx011451), P53 (Abcam, cat. no. ab1101), MKK3 (Abcam, ab154913), and cleaved caspase 3 (Abcam, cat. no. ab214430).
Cell apoptosis assays
The treated CSC-like cells were harvested and their apoptosis was measured using annexin V-FITC/PI (Boster, Pleasanton, CA, USA; MK1028) according to the instructions provided by the manufacturer. The results were obtained by flow cytometry (Becton–Dickinson).
Transwell assay
The migration and invasion abilities of treated CSC-like cells were assessed using Transwell chambers (Corning Incorporated, Corning, NY, USA) with and without Matrigel matrix (BD Biosciences), respectively. In brief, CSC-like cells from each group were harvested and suspended in serum-free medium. After counting, 200 µL (1 × 105 cells/mL) of cells was added to the upper chamber of a Transwell plate and 500 µL of medium containing 15% FBS was added to the lower chamber. After incubation for 24 h, the migrated and invaded cells were fixed for 10 min and then stained with 0.5% crystal violet for 15 min. The results were visualized under a microscope (Olympus IX71, Japan).
Statistical analysis
All experiments were repeated three times and results are presented as the mean ± SD. The t test or one-way analysis of variance followed by Tukey’s test was used to analyze differences between the groups. The data were analyzed using GraphPad Prism Software (Ver. Prism 7), and a P value < 0.05 was considered to be statistically significant.
Results
PTK7 was upregulated in ESCC tissues
To investigate PTK7 expression in ESCC tissues, 15 pairs of ESCC tissue and adjacent normal tissue were collected and analyzed. RT-qPCR results revealed that PTK7 expression was significantly upregulated in the ESCC tissues relative to the non-tumor tissues (P < 0.001, Fig. 1a). These results were further confirmed by western blot analyses of PTK7 expression (Fig. 1b).
PTK7 was highly expressed in CSC-like cells derived from ESCC cells
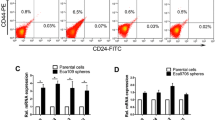
To explore whether PTK7 expression was related to CSC-like cells, we first separated and extracted CSC-like cells from cultures of NEC and TE-1 cells, respectively. Results from RT-qPCR analyses revealed that the levels of stem cell transcription factor-related genes (Nango, Sox2, and OCT4) were significantly higher in the CSC-like cells than in the cancer cells (CC, NEC, and TE-1 cells), and PTK7 was also upregulated in the CSC-like cells (P < 0.001, Fig. 2a). Furthermore, those trends in Nango, Sox2, OCT4, and PTK7 expression were further confirmed in western blot analyses (Fig. 2b). Results of flow cytometric analyses showed that the percentage of CD34+ and CD133+ cells was higher among the CSC-like cells than among the ESCC cells (Fig. 2c). These results indicated that CSC-like cells had been successfully extracted from populations of NEC and TE-1 cells and that PTK7 was highly expressed in the CSC-like cells.
PTK7 was highly expressed in CSC-like cells derived from ESCC cells. CSC-like cells were isolated from the populations of NEC and TE-1 cells, respectively. a The relative levels of Nango, Sox2, OCT4, and PTK7 in CC and CSC-like cells were confirmed by RT-qPCR assays, **P < 0.01 vs. CC group. b The levels of Nango, Sox2, OCT4, and PTK7 expression in CC and CSC-like cells were examined by western blotting. c The levels of CD34 and CD133 expression on the surface of CC and CSC-like cells, respectively, were determined by flow cytometric analysis. CC cancer cells
Knockdown of PTK7 inhibited sphere formation and self-renewal, induced apoptosis, and prevented the migration and invasion of CSC-like cells derived from ESCC cells
To examine the influence of PTK7 on ESCC CSC-like properties, we transfected CSC-like cells derived from NEC and TE-1 cells with PTK7 siRNA. Furthermore, the levels of PTK7 were dramatically decreased in CSC-like cells transfected with PTK7 siRNA, indicating the strong PTK7 silencing efficacy of the PTK7 siRNA. Knockdown of PTK7 markedly reduced the expression levels of transcription factors Nango, Sox2, and OCT4 along with the characteristics of self-renewal and differentiation (P < 0.05, P < 0.01, P < 0.001, Fig. 3a, b). Moreover, we demonstrated that the apoptosis rate of CSC-like cells was higher among the PTK7 siRNA groups than the NC group, suggesting that knockdown of PTK7 facilitated CSC apoptosis (P < 0.01, Fig. 3c). We further investigated the effects of PTK7 knockdown on the migration and invasion capabilities of CSC-like cells derived from ESCC cells by using the transwell assay. Those assay results showed that knockdown of PTK7 significantly reduced the numbers of migrated and invaded CSC-like cells derived from NEC and TE-1 cells (P < 0.01, Fig. 3d–g). With regard to mechanism, we further demonstrated that knockdown of PTK7 significantly increased P53, MKK3, and cleaved caspase 3 expression in CSC-like cells derived from NEC and TE-1 cells (Fig. 3h). These findings verified that the CSC-like phenotypes and functions of ESCC cells could be inhibited by PTK7 knockdown.
Knockdown of PTK7 inhibited sphere formation and self-renewal, induced apoptosis, and prevented the migration and invasion of CSC-like cells derived from ESCC cells. CSC-like cells derived from NEC and TE-1 cells were transfected with NC siRNA, PTK7 siRNA#1 or PTK7 siRNA#2 for 72 h, respectively. a, b After transfection with PTK7 siRNA, the levels of Nango, Sox2, OCT4, and PTK7 expression in CSC-like cells were evaluated by qRT-PCR (a) and western blot assays (b). c The apoptosis rates of CSC-like cells derived from NEC and TE-1 cells transfected with the NC or PTK7 siRNA were assessed by flow cytometry. d, e The influence of PTK7 knockdown on the migration and invasion of CSC-like cells was estimated by transwell assays. f, g The numbers of migrated and invaded cells were determined by transwell assays. h The effects of PTK7 knockdown on P53, MKK3, and cleaved caspase 3 expression were determined by western blot assays. i The sphere formation capacities of the transfected cells were examined by sphere formation assays, *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC group
To further investigate the stem-like property of the CSC-like cells, the sphere formation assay was used to examine the sphere formation ability of the transfected CSCs following transfection. The results showed that the sphere formation capacity of the transfected CSC-like cells was markedly decreased when compared to the sphere formation capacity of CSC-like cells transfected with the NC siRNA. Measurements showed that the diameters of the spheres formed by CSC-like cells transfected with PTK7 siRNA were significantly smaller than those of spheres formed by CSC cells transfected with the NC siRNA (P < 0.001, Fig. 3i).
Knockdown of PTK7 suppressed the biological processes and CSC-like phenotypes of ESCC cells by regulating p53
To explore whether p53 was involved in the CSC-specific functions mediated by PTK7 knockdown, a p53-specific inhibitor (PFTα) was used to treat PTK7 siRNA (siRNA #1)-transfected CSC-like cells derived from NEC and TE-1 cells. We first examined whether knockdown of PTK7 would inhibit the tumor-sphere forming capability of CSC-like cells. As shown in Figs. 4a, the sphere forming capability of those cells was significantly reduced after transfection with PTK7 siRNA, while treatment with PFTα reversed the PTK7 siRNA-mediated reduction in sphere formation by CSC-like cells (P < 0.05, P < 0.01). Secondly, we demonstrated that the inhibitory effects of PTK7 knockdown on Nango, Sox2, OCT4, and PTK7 expression in CSC-like cells could be reversed by PFTα treatment (P < 0.05, P < 0.01, P < 0.001, Fig. 4b, c). Furthermore, a flow cytometric analysis suggested that PFTα treatment significantly attenuated the increase in CSC-like cell apoptosis that was induced by PTK7 knockdown (P < 0.05, P < 0.01, Fig. 4d). Studies of cell migration and invasion capabilities demonstrated that PFTα treatment markedly attenuated the inhibitory effects of PTK7 knockdown on the migration and invasion capabilities of CSC-like cells (P < 0.05, P < 0.01, Fig. 4e–h). We also verified that the upregulated levels of P53, MKK3, and cleaved caspase 3 expression caused by PTK7 knockdown in CSC-like cells derived from NEC and TE-1 cells could be reversed by PFTα treatment (Fig. 4i). In conclusion, we proved that knockdown of PTK7 could prevent the malignant behaviors of CSC-like cells derived from ESCC cells by downregulating p53.
A P53-specific inhibitor (PFTα) reversed the effect of PTK7 knockdown on apoptosis, metastasis, and the proportion of CSC-like cells among ESCC cells. After being transfected with PTK7 siRNA (siRNA #1), CSC-like cells derived from NEC and TE-1 cells were treated with PFTα. a Sphere formation assays were performed to assess the sphere formation capacity in each group of cells, and the diameters of tumor spheres were calculated based on the results of sphere formation assays. b, c The levels of Nango, Sox2, OCT4, and PTK7 in the treated CSC-like cells were analyzed qRT-PCR (b) and western blot assays (c). d A flow cytometric analysis verified that the effect of PTK7 knockdown on apoptosis could be attenuated by PFTα. f The rate of apoptosis in each group was calculated. e, f After treatment with PFTα, the migration and invasion capabilities of PFTα-silenced CSC-like cells were investigated using transwell assays. g, h The numbers of migrated and invaded cells were quantitatively analyzed. i The western blot analyses for P53, MKK3, and cleaved caspase 3. *P < 0.05, **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. PTK7 siRNAs group
Discussion
Similar to stem cells, CSCs possess the various properties of stem cells, can produce heterogeneous tumor cells, and cause the occurrence of cancer [26]. As is well known, CSCs have four crucial characteristics: a self-renewal ability, high proliferative capacity, a multi-differentiation potential, and drug resistance [12, 27]. While treatment with chemotherapy, radiation therapy, and biological immunotherapy will kill most tumor cells in a majority of cancer patients, their tumors are not fundamentally cured [28]. This is because only ordinary cancer cells can be eradicated by conventional cancer therapies, and CSCs still remain in the tumor tissues; those CSCs which will lead to tumor recurrence [29]. Therefore, it is critically important to investigate the key pathways for CSCs and non-CSCs when developing a cancer therapy that targets CSCs.
Previous studies proved that the newly discovered kinase PTK7 has multiple biological functions, and is involved in the formation of embryonic tubes and the functions of pluripotent stem cells [30, 31]. In addition, the PTK7 gene is associated with the occurrence, development, and invasion of various tumors, and its abnormally high expression often indicates a poor prognosis [13, 20, 32, 33] For example, PTK7 is highly expressed in CD44-high glioma cells [34]; upregulated PTK7 expression was identified as a malignant factor in cervical cancer [15]; PTK7 is associated with the radio-resistance of ESCC [35]. In addition, another study confirmed that human colon stem cells (hCoSCs) that highly express PTK7 possess high re-seeding and self-renewal capabilities [36]. In our study, we further confirmed that PTK7 was significantly upregulated in ESCC cells, which was consistent with the findings in previous studies [22, 25].
Oct4, Sox2, and Nanog are crucial transcription factors that significantly contribute to maintaining the pluripotency and self-renewal characteristics of embryonic stem cells [37]. Our results revealed that Nango, Sox2, and OCT4 were upregulated in the isolated CSC-like cells, indicating that those cells were capable of self-renewal and differentiation. We utilized flow cytometry to observe the subpopulation of CD34+CD133+ cells and determined that the percentage of CD34+CD133+ cells was higher among the population of CSC-like cells than among the cancer cells, which also suggested the successful separation of CSC cells. Moreover, we proved that PTK7 expression was also increased in the isolated CSC-like cells derived from ESCC cells, suggesting that cells with high levels of PTK7 expression were capable of self-renewal. Studies have demonstrated that the characteristics of CSCs are associated with multiple biological processes, such as sphere formation, self-renewal, apoptosis, migration, and invasion [38, 39]. In our study, a large number of functional experiments verified that knockdown of PTK7 expression could suppress the sphere formation, self-renewal, migration, and invasion capabilities of CSC-like cells derived from ESCC cells, and accelerate their apoptosis. Therefore, we demonstrated that knockdown of PTK7 might be novel therapeutic strategy for ESCC, because it alters the biological properties of CSC-like cells.
Mitogen-activated protein kinase kinase 3 (MAP2K3, MKK3) is a member of the dual specificity kinase group (MKK) and is formed by the action of mitogen-activated protein kinase (MAPK) [40]. MKK3 can be activated by MKKK proteins via phosphorylation of its Ser-189 and Thr-193 sites; after which, MKK3 specifically phosphorylates p38MAPK [40]. Studies have shown that MKK3 is closely associated with various cellular processes, including cell differentiation, movement, division, apoptosis, and death [41, 42]. Literature reports have stated that upregulation of MKK3 is involved in the carcinogenesis of various cancers [41, 42], Previous studies demonstrated that an upregulation of MKK3 could be induced by mutant p53 [43]. P53, as a tumor suppressor, can significantly regulate cell cycle arrest and apoptosis [44, 45]. In our study, we found that knockdown of PTK7 could upregulate P53, MKK3, and cleaved caspase 3 (apoptosis-associated protein) expression. Meanwhile, we further demonstrated that a p53-specific inhibitor (PFTα) could reverse the inhibitory effects of PTK7 knockdown on the malignant behaviors of CSC-like cells in ESCC tumors.
Conclusion
In conclusion, our study results suggest that PTK7 promotes the formation of ESCC tumors by activating malignant properties that are mediated by the p53/MKK3 signaling pathway in CSC-like cells. Our results also suggest PTK7 as a promising new target for treating ESCC.
Abbreviations
- CSC:
-
Cancer stem cell
- ESCC:
-
Esophageal squamous cell carcinoma
- OCT4:
-
POU class 5 homeobox 1
- PTK7:
-
Protein tyrosine kinase 7
- SOX2:
-
SRY-box transcription factor 2
References
Short MW, Burgers KG, Fry VTJAFP. Esophageal Cancer. Am Fam Physician. 2017;95:22.
Arnal MJD, Arenas ÁF, Arbeloa ÁL. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933.
Ohashi S, Miyamoto SI, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700–15.
Saeki H, Tsutsumi S, Tajiri H, et al. Prognostic significance of postoperative complications after curative resection for patients with esophageal squamous cell carcinoma. Ann Surg. 2017;265:527–33.
Ninomiya I, Okamoto K, Tsukada T, et al. Recurrence patterns and risk factors following thoracoscopic esophagectomy with radical lymph node dissection for thoracic esophageal squamous cell carcinoma. Mol Clin Oncol. 2016;4:278–84.
Liu Q, Cui X, Yu X, et al. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol Cancer. 2017;16:81.
Kojima H, Okumura T, Yamaguchi T, Miwa T, Shimada Y, Nagata T. Enhanced cancer stem cell properties of a mitotically quiescent subpopulation of p75NTR-positive cells in esophageal squamous cell carcinoma. Int J Oncol. 2017;51:49–62.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124.
Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107:5–11.
Rekhtman N, Leighl NB. Somerfield MR Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: american society of clinical oncology endorsement of the college of american pathologists/international association for the study of lung cancer/association for molecular pathology guideline. J Oncol Pract. 2015;11:135.
Jiao Y, Yin J, He H, Peng X, Gao Q, Duan CJ. Conformationally induced off–on cell membrane chemosensor targeting receptor protein-tyrosine kinases for in vivo and in vitro fluorescence imaging of cancers. J Am Chem Soc. 2018;140:5882–5.
Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75.
Lhoumeau AC, Martinez S, Boher JM, et al. Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PLoS One. 2015;10:e0123768.
Tian X, Liang Y, Zhang D, et al. PTK7 overexpression in colorectal tumors: clinicopathological correlation and prognosis relevance. Oncol Rep. 2016;36:1829–36.
Sun J-J, Li H-L, Guo S-J, et al. The increased PTK7 expression is a malignant factor in cervical cancer. Dis markers. 2019;2019:5380197.
Chen R, Khatri P, Mazur PK, et al. A meta-analysis of lung cancer gene expression identifies PTK7 as a survival gene in lung adenocarcinoma. Cancer Res. 2014;74:2892–902.
Beyhan A, Regina A, Ronald K, et al. PTK7 expression in triple-negative breast cancer. Anticancer Res. 2013;33:3759–63.
Lin Y, Zhang LH, Wang XH, et al. PTK7 as a novel marker for favorable gastric cancer patient survival. J Surg Oncol. 2012;106:880–6.
Prebet T, Lhoumeau AC, Arnoulet C, et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood. 2010;116:2315.
Liu K, Song G, Zhang X, et al. PTK7 is a novel oncogenic target for esophageal squamous cell carcinoma. World J Surg Oncol. 2017;15:105.
Park M, Yoon HJ, Kang MC, Kwon J, Lee HW. PTK7 regulates radioresistance through nuclear factor-kappa B in esophageal squamous cell carcinoma. Tumour Biol. 2016;37:14217–24.
Shin WS, Gim J, Won S, Lee ST. Biphasic regulation of tumorigenesis by PTK7 expression level in esophageal squamous cell carcinoma. Sci Rep. 2018;8:8519.
Shin WS, Hong Y, Lee HW, Lee ST. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-kappaB in esophageal squamous cell carcinoma cells. Oncotarget. 2016;7:73242–56.
Shin WS, Kwon J, Lee HW, et al. Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1120–6.
Park M, Yoon HJ, Kang MC, Kwon J, Lee HW. PTK7 regulates radioresistance through nuclear factor-kappa B in esophageal squamous cell carcinoma. Tumor Biol. 2016;37:1–8.
Phi L, Sari IN, Yang YG, et al. Cancer stem cells (CSCs) in Drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:1–16.
Butler SJ, Richardson L, Farias N, Morrison J, Coomber BL. Characterization of cancer stem cell drug resistance in the human colorectal cancer cell lines HCT116 and SW480. Biochem Biophys Res Commun. 2017;490:29–35.
Chalbatani GM, Gharaghouzlo E, Fard AAJ. Analysis of Cancer stem cells (CSCs) in the prevention and treatment of cancer. J Cell Immunother. 2017;3:11.
Mashima TJYZ. Cancer stem cells (CSCs) as a rational therapeutic cancer target, and screening for CSC-targeting drugs. Yakugaku Zasshi. 2017;137:129–32.
Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne MJN. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature (London). 2004;430:93.
Kim Y-S, Yi B-R, Kim N-H. Choi K-C Role of the epithelial–mesenchymal transition and its effects on embryonic stem cells. Exp Mol Med. 2014;46:e108.
Damelin M, Bankovich A, Bernstein J, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017;9:eaag2611.
Messerli SM, Hoffman MM, Gnimpieba EZ, Bhardwaj RD. Therapeutic targeting of PTK7 is cytotoxic in atypical teratoid rhabdoid tumors. Mol Cancer Res. 2017;15:973–83.
Liu Q, Zhang C, Yuan J, et al. PTK7 regulates Id1 expression in CD44-high glioma cells. Neuro-Oncology. 2014;17:505–15.
Park M, Yoon H-J, Kang MC, Kwon J, Lee HW. PTK7 regulates radioresistance through nuclear factor-kappa B in esophageal squamous cell carcinoma. Tumor Biol. 2016;37:14217–24.
Jung P, Sommer C, Barriga F, et al. Isolation of human colon stem cells using surface expression of PTK7. Stem Cell Repts. 2015;5:979–87.
van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang TJ. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol. 2018;71:88–91.
Kim MJ, Lee YS, Han GY, Lee HN, Ahn C, Kim CWJ. Profilin 2 promotes migration, invasion, and stemness of HT29 human colorectal cancer stem cells. Biosci Biotechnol Biochem. 2015;79:1438–46.
Zhou Y, Kipps TJ, Zhang S. Wnt5a Signaling in normal and cancer stem cells. Stem Cells Int. 2017;2017:5295286.
Derijard B, Raingeaud J, Barrett T, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–5.
Demuth T, Reavie LB, Rennert JL, et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6:1212–22.
Jia M, Souchelnytskyi N, Hellman U, O’Hare M, Jat PS, Souchelnytskyi S. Proteome profiling of immortalization-to-senescence transition of human breast epithelial cells identified MAP2K3 as a senescence-promoting protein which is downregulated in human breast cancer. Proteomics Clin Appl. 2010;4:816–28.
Gurtner A, Starace G, Norelli G, Piaggio G, Sacchi A, Bossi GJ. Mutant p53-induced Up-regulation of mitogen-activated protein kinase kinase 3 contributes to gain of function. J Biol Chem. 2010;285:14160.
Li G, Niu H, Zhang Y, et al. Haemophilus parasuis cytolethal distending toxin induces cell cycle arrest and p53-dependent apoptosis. PLoS One. 2017;12:e0177199.
Blatter S, Stokar-Regenscheit N, Kersbergen A, Guyader C, Rottenberg SJ. Chemotherapy induces an immunosuppressive gene expression signature in residual BRCA1/p53-deficient mouse mammary tumors. J Mol Clin Med. 2018;1:7–17.
Acknowledgements
This work was supported by the National Science Foundation for Distinguished Young Scholars of China (No. 81903026).
Author information
Authors and Affiliations
Contributions
JB and ZWW contributed to the research design. JB performed the experiments. JB and XH collected the data and analyzed the data. MY and XWS validated the data and analysis. XPZ contributed to results description and data visualization. JB drafted the manuscript and ZWW revised the manuscript. All authors approved the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial or other associations that might pose a conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. All study procedures were performed in accordance with the ethical principles stated in the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bie, J., Hu, X., Yang, M. et al. PTK7 promotes the malignant properties of cancer stem-like cells in esophageal squamous cell lines. Human Cell 33, 356–365 (2020). https://doi.org/10.1007/s13577-019-00309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00309-6