Abstract
Objective
This multicenter study focuses on the use of a film-forming wound dressing in the form of a gel that can be applied directly to the area affected by radiation dermatitis, especially after skin breakdown. The primary objective of the study was to validate the efficacy of an innovative film-forming wound dressing used as monotherapy in the treatment of radiation dermatitis in patients with RTOG score 2.5 (± 0.5) confirmed by the investigator.
Methods
Fifty-four patients undergoing radiation therapy for different cancer types and developing radiation dermatitis were recruited in the study; they were treated with the film-forming wound dressing when reaching an RTOG score of 2.5 (± 0.5). The evaluation of radiation dermatitis during ongoing radiation therapy was performed using the RISRAS, which includes investigator-assessed items (erythema, dry desquamation, moist desquamation, necrosis) and patient-assessed items (pain, itch, burning sensation, affection of daily activities).
Results
The following study shows a statistically significant clinical improvement (p < 0.05) of the RISRAS score (− 16.9%), as well as of specific clinical outcomes, such as erythema (− 20.6%), pain (− 20.5%), itch (− 22.2%), and burning sensation (−24.7%), after the treatment with the film-forming wound dressing during ongoing radiation therapy. Other radiation dermatitis markers, such as inflammation (− 28.9%) and hydration (26.0%), appeared to be significantly influenced.
Conclusion
The use of the innovative film-forming wound dressing for radiation dermatitis treatment shows first time evidence of improving the RISRAS score during ongoing radiation therapy, showing major improvements in patients’ quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute radiation-induced skin reactions are an inevitable consequence of radiation therapy and occur in up to 95% of patients receiving treatment to the breast, groins, or perineum [1]. Commonly, radiation dermatitis develops briefly after (2 to 4 weeks) the start of radiation therapy. Its onset can differ conditionally to the magnitude of the radiation dose and the subjective tissue sensitivity of the patient [2], but its severity escalates over time and is proportionally associated with the accruing radiation on the tissues.
The severity of radiation dermatitis is mainly measured via two main scales, Radiation Therapy Oncology Group (RTOG) and Radiation-Induced Skin Reaction Assessment Scale (RISRAS). The RTOG is a clinical scale which objectively measures the severity of skin toxicity, the evaluation of which is performed by a clinician [3]. The RISRAS incorporates both clinically objective signs of skin toxicity as well as patient-reported symptoms and quality of life measures [4].
With current treatment protocols, the level of radiation dermatitis worsens as radiation therapy continues, the skin condition deteriorates with a measured progression along the severity scales (RTOG, RISRAS). Therefore, treatment is successful if the score of the recognized scales is reduced or maintained at the same level during the ongoing radiation therapy doses over time, as the untreated toxicity has been demonstrated to increase with daily cumulative doses of radiation, frequently peaking after 2 weeks after radiation therapy plan completion [2].
Erythema (RTOG 1 through 2) is the first stage of acute radiation skin reaction, presenting as reddening of the skin. It may occur at around 2 to 3 weeks of standard fractionated radiotherapy. A thickening of the stratum corneum is seen in the subsequent phase of dry desquamation (RTOG 2). It is manifested as flaky skin and may occur from about week three of treatment. The next stage of skin reaction seen in some patients is moist desquamation (RTOG 3).The skin will look bright red and inflamed and may have blisters or ulcers and may be at risk of infection. The main difference between diverse stages of moist desquamation resides in the surface of skin affected. The RTOG scale does not account for this difference, and that is the reason why it was subsequently adapted to increase specificity and ease of use by some authors including a 2.5 step to account for patchy moist desquamation [2, 5, 6]. RTOG 2.5 is reported in case of non-continuous moist desquamation, whereas RTOG 3 is instead reported in case of confluent moist desquamation[2]. Moist desquamation can occur from about week four onwards, but may peak 1–2 weeks after the treatment. Necrosis is the last stage of radiation dermatitis (RTOG 4). Radiation therapy is commonly terminated before reaching this stage [2, 7].
The RISRAS is a comprehensive tool including symptomatic investigator-based assessments of erythema, dry desquamation, moist desquamation, and necrosis, in addition to quality of life-related patient-based assessments of pain, itch, burning sensation, and affection of daily activities [3]. Symptom-based items are assessed based on the severity of the condition and the extension of the affected skin area. Quality of life scale components are instead rated by the patient according to their perception of the item’s magnitude.
Due to the very special case of radiation dermatitis pathophysiology and its gradual deterioration, both in toxicity and symptoms over time, maintaining the same level or grade of deterioration during ongoing radiotherapy is considered as an effective prevention. Therefore, any therapy that stops or improves the exacerbation of the symptoms of radiation dermatitis can be considered prophylactic [2, 8].
As illustrated in Fig. 1, radiation dermatitis necessarily happens in a progressive way, as the pathophysiology happens due to continuous insult to the basal cell layer through fractionated radiation doses. A patient developing a RTOG grade 3 of skin toxicity necessarily needs to pass through RTOG stages 1 and 2 first. Those stages of toxicity are already expected from the planning phase within defined time frames during a standard radiation plan. As an example, RTOG stage 2.5–3 of toxicity is only expected after week 4 of radiation and not before [2, 9]. There are several confirmed risk factors (both treatment- and patient-related) contributing to the exacerbation of the skin reaction. Treatment-related risk factors encompass site and dimension of the treated area, as well as overlying irradiated fields, specific features of the utilized beam, and total radiation dose [7, 10]. Patient-related risk factors can range instead from obesity and skin folds, to inadequate nutritional conditions, smoking habits, simultaneous UV exposure, and comorbidities [10, 11].
Methods
Patient eligibility
Inclusion criteria for this investigation included patients to (1) be over 18 years of age, undergoing radiation therapy and developing radiation dermatitis to a RTOG score of 2.5 (± 0.5), (2) be able to assist to the radiation therapy on a daily basis until the finalization of the plan, up to 10 to 14 days after, and continue with the cures if necessary, (3) be able to administer the gel themselves and to understand the instructions, and (4) not to have any associated comorbid diseases (such as collagen diseases) and take any concomitant medication (e.g., corticosteroids) which could affect the results of the study.
Patient population and sample size
Patients undergoing radiation therapy for different cancer types were monitored until reaching a RTOG score of 2.5 ([± 0.5] dry desquamation in at least 25% of the irradiated area). Sample size could not be calculated prior to study initiation, as the authors could not use any published assumptions for sample size calculations. Fifty-four patients reached the mentioned RTOG score and therefore were treated with the film-forming wound dressing to observe the evolution of radiation dermatitis during the subsequent radiation plan doses. Twenty-six patients were excluded from statistical analyses, as treatment with the wound dressing was not performed in accordance with study protocol (e.g., applied as prevention or started after end of radiation). Twenty-eight patients were therefore included in the statistical analyses for study endpoints 1 and 2.
Product evaluation was carried out on all patients (n = 33) in whom the assessors had a direct experience vs. physical dressing, regardless of the inclusion of the patient in the statistical analysis for study endpoints 1 and 2.
Treatment delivery and planning
Patients were given the studied product in 50-g tubes (StrataXRT, Stratpharma AG, Basel, Switzerland). The dressing consists of a semi-occlusive, self-drying, and transparent gel. When used as directed, the gel dries to form a layer. Dressing application occurred as indicated by the product’s PIL, by applying a thin layer of gel directly to the affected area, once or twice daily, as advised by the investigator. Patient adherence to the treatment was assessed through the CRF.
Patients follow-up
The evolution of radiation dermatitis, as well as of any adverse event, was monitored by the clinical investigator on the CRF after each radiation therapy session. Photographs of the affected area were taken at day 0, i.e., when patients reach RTOG grade 2.5 (± 0.5) and before applying the product on the skin. Photographs of the affected area were then taken on the day in which radiation therapy finished and after 10 to 14 days after the end of radiation, in order to monitor the evolution of the skin condition through images.
Control
Since the level of radiation dermatitis is known to worsen throughout the course of radiation therapy, maintaining the same level of acute signs and symptoms can be already considered a successful treatment [4, 7]. As this trial was a first exploratory study regarding the treatment of radiation dermatitis during ongoing radiation therapy with an innovative film-forming wound dressing, the need of a control group was not considered as determinant. Each patient was considered their own control, with the baseline value corresponding to their initial inclusion score.
Assessment of radiation dermatitis
The severity of radiation dermatitis was measured via the Radiation-Induced Skin Reaction Assessment Scale (RISRAS). The RISRAS is a compound measure that incorporates both clinically objective signs of skin toxicity rated by the assessor (erythema, dry desquamation, moist desquamation, and necrosis), as well as patient-reported symptoms (pain, itch, burning sensation, and affection of daily activities), which are important quality of life measures [3].
Patients were rated by the assessor at every radiation assessment with the clinically objective section of the RISRAS (that includes erythema, dry desquamation, moist desquamation, and necrosis). Patients were then asked to fill out the subjective section of the RISRAS, related to patient-reported symptoms (that includes pain, itch, burning sensation, affection of daily activities).
Besides rating specific RISRAS items, the clinical investigators were also required to rate specific radiation dermatitis markers, such as size of open wound area, size of bleeding wound area, size of exudative area, inflammation, and hydration. Size of open wound area, size of bleeding wound area, size of exudative area, and inflammation were rated by using a 5-item Likert scale (ranging from 0 to 0% of irradiated skin affected, to 4 to 75–100% of irradiated skin affected). Hydration was rated according to a 4-item Likert scale (ranging from 1 – None, to 4 – A lot). As hydration was not reported for two of the included patients, the analysis was carried out on 26 patients for this specific outcome.
Product evaluation
Finally, the studied wound dressing was evaluated both from assessors and patients.
Assessors were asked to rate the investigated wound dressing vs. standard clinical practice, as well as to provide a product evaluation in terms of simplifying treatment, nursing time saved, and decreased amount of product use. Product’s global rating vs. standard clinical practice was rated with a 5-item Likert scale (ranging from 1 – Much worse, to 5 – Much better). Simplifying treatment, nursing time saved, and decreased amount of product use were instead rated by using a 4-item scale (ranging from 0 – Not at all, to 3 – Very much).
Patients were also required to provide an assessment of the applied dressing concerning its ease of use, comfort, and general rating. All items were rated with a 5-item scale (0 – Bad, to 4 Excellent).
Data correction
Due to nonconformities to the assessment protocol, two patients were subject to a subsequent blinded assessment, and data were corrected accordingly.
Statistical analysis
SPSS 24 (IBM, Chicago, IL) was used for all statistical analyses. Descriptive analysis was done using standard statistical procedures. To describe the improvement, p < 0.05 and power = 80 were used. The statistical significance in RISRAS scores and radiation dermatitis markers at endpoint 1 (end of radiation) was determined by non-parametric Wilcoxon’s signed-ranks test. In order to assess the change of RISRAS scores and radiation dermatitis markers for endpoint 2 (10 to 14 days after radiation), an analysis of variance (ANOVA) was implemented.
Results
No adverse events related to the device were reported throughout the study.
Demographic data and missing data
From the 54 total cases, 26 cases were excluded from statistical analysis (see Methods – Patient population).
Out of the 28 patients included in the analyses, 15 did not attend study follow up visits after radiation therapy completion, therefore only data coming from 13 patients were available for post-radiation analysis (10 to 14 days after radiation finished, endpoint 2) Table 1.
Radiation dermatitis evolution at end of radiation therapy (endpoint 1)
In the 28 patients presenting a clinically confirmed skin toxicity of RTOG 2.5 ± 0.5, the RISRAS score improved for 21 patients, got worse for 6 and stayed constant for 1 by using the innovative film-forming wound dressing. Therefore, it improved or stayed constant for 78.6% of the patients.
The measured outcome of the RISRAS score (− 16.9%) showed statistically significant clinical improvement (p = 0.004). It is therefore possible to demonstrate that the application of the film-forming wound dressing improves the RISRAS score during ongoing radiation (Table 2).
Figure 2 shows the statistically significant improvement (p = 0.004) of the RISRAS score measured between the start of treatment with the studied dressing and the endpoint 1 (end of radiation therapy).
Specific items of the RISRAS (such as pain, itch, burning sensation, affection of daily activities, erythema, dry desquamation, moist desquamation, and necrosis) were also measured at endpoint 1. With a decrease in pain (− 20.5%), itch (− 22.2%), and burning sensation (− 24.7%), the subjective perception improved strongly despite ongoing radiation. Erythema in the irradiated zone (− 20.6%; p = 0.015) also appeared to be considerably lower (Table 2).
During ongoing radiation, hydration of the skin in the irradiated zone was considerably increased (26.0%; p = 0.021).The inflammation level also appeared to be significantly lower (− 28.9%; p = 0.011). For hydration and inflammation, it is possible to demonstrate that they are indeed influenced by the use of the wound dressing (Table 2).
The results shown in Table 2 demonstrate the overall change of all measured outcomes during ongoing radiation (endpoint 1).
Radiation dermatitis evolution after 10 to 14 days post-radiation therapy (endpoint 2)
Although radiation dermatitis should worsen until ~2 weeks after end of radiation therapy [4, 7] (Fig. 1), a statistically significant decrease vs. baseline (start of treatment with the wound dressing) of the RISRAS score (− 37.6%; p = 0.001) was measured 10 to 14 days (average 11.7 days) after end of radiation therapy (Fig. 3).
Changes in RISRAS items were measured at endpoint 2, as well. A statistical significant clinical improvement (p < 0.05) vs. baseline was shown in critical outcomes, such as pain (− 34.2%; p = 0.014), itch (− 40.0%; p = 0.004), burning sensation (− 47.5%; p = 0.000), erythema (− 47.2%; p = 0.001), and dry desquamation (− 16.7%; p = 0.003), as shown in Table 3.
Radiation dermatitis markers after end of radiation therapy were also analyzed: skin hydration in the radiation site was significantly improved (41.7%; p = 0.005) 10 to 14 days after the end of radiation therapy than at the beginning of treatment with the dressing (Table 3).
The results shown in Table 3 demonstrate the overall change of all measured outcomes after 10 to 14 days follow-up post-radiotherapy (endpoint 2).
Evaluation of the product
Assessor evaluation
Following an evaluation on 33 patients, the investigators provided a general rating of the wound dressing’s performance vs. standard of care treatment, as well as the wound dressing simplifying treatment, nursing time saved, and decreased amount of product used.
In 85% of patients (n = 28), the use of the film-forming wound dressing was considered by the assessors to be an improvement compared to standard clinical practice in treatment of radiation dermatitis during ongoing radiation; in 67% of patients (n = 22), the assessors rated it as “better” and in 18% (n = 6) as “much better” than the current treatments, as shown in Fig. 4. These data demonstrate that the assessors’ perception of treating radiation dermatitis during ongoing radiation is much improved with the use of the studied dressing vs. standard clinical practice.
A supplementary series of factors was also tested, in order to assess an improvement of the treatment of radiation dermatitis during ongoing radiation. As to the studied dressing’s investigator evaluation (Fig. 5), it was stated to have a “moderate” impact in simplifying treatment and in the amount of nursing time saved in the majority of patients (55% [n = 18] and 53% [n = 17] respectively). On the other hand, in 64% of patients (n = 21), it was stated that the innovative film-forming wound dressing “very much” decreases the amount of product used.
Overall, these data support the abovementioned results.
Patient evaluation
Sixteen patients compiled a product evaluation sheet, accounting for ease of use and comfort (Fig. 6). The wound dressing’s ease of use was rated as “excellent” by 50% of patients (n = 8). The dressing’s comfort was rated as “very good” by the majority of patients (n = 7; 44%).
In order to have a more complete product evaluation from a patient’s perspective, patients’ general rating of the product was also measured. It was found “excellent” for most of patients (n = 7; 44%).
These data reveal an extremely positive patient perception of the application of the film-forming wound dressing.
Discussion
To the authors’ knowledge, the stand-alone use of the innovative film-forming wound dressing, as described in this exploratory study, shows first time evidence of reducing the severity of radiation dermatitis during ongoing radiation therapy.
Several parameters assessing the severity of radiation dermatitis were taken into account (RISRAS score and radiation dermatitis markers) and measured at the end of radiation dermatitis therapy (endpoint 1), as well as 10 to 14 days after the end of radiation therapy (endpoint 2).
At endpoint 1, the measured outcome of the RISRAS score (Fig. 2) showed statistically significant clinical improvement. It is therefore possible to confirm that the studied dressing improves the RISRAS score during ongoing radiation, comparing the differential change of the RISRAS (− 16.9%). Based on clinical practice and literature vs. a hypothetical control showing a linear evolution of radiation dermatitis (i.e., no improvement, nor deterioration), it is possible to reveal a statistically significant benefit in the use of the film-forming wound dressing.
As to the specific items of the RISRAS score, besides measuring a significantly reduced erythema in the site of radiation, major improvements in the subjective patient assessment part were highlighted (pain, itch, and burning sensation), resulting in a big relief for the radiation patient increasing its overall quality of life, by soothing the skin areas exposed to the radiation (Table 2).
Radiation dermatitis markers also improved at the end of radiation therapy. Hydration of the skin in the irradiated zone was considerably increased. The inflammation level also appeared to be significantly lower (Table 2). It was therefore possible to conclude that they are indeed influenced by the use of the studied wound dressing.
Reducing all the above mentioned factors allows the radiation to be continued, instead of being interrupted due to an increase of severity of radiation dermatitis. It has been demonstrated that not complaining with radiotherapy plans correlates with poor clinical outcomes [12]. Avoiding interruptions translates in turn into a higher efficacy of the therapy, with direct benefits for the patient, the radiation unit planning and, indirectly, the use of resources. Furthermore, a reduction of patients with radiation-related skin toxicity will achieve better health outcomes while reducing costs associated with radiation therapy discontinuation.
Continuation of the use of the dressing until 10 to14 days after the end of radiation therapy appears to be significantly beneficial and significantly improving the clinical condition of the patient.
At endpoint 2, although the overall radiation dermatitis condition should get worse until 2 weeks after radiation (Fig. 1), a statistically significant decrease of the RISRAS score vs. baseline (start of treatment with the dressing) was measured 10 to 14 days after end of radiation therapy (Fig. 3).
Regarding the specific items of the RISRAS score, a statistical significant clinical improvement vs. start of treatment with the dressing was shown in erythema and dry desquamation, as well as in subjectively perceived (and assessed) parameters, such as pain, itch, and burning sensation (Table 3).
Among the measured radiation dermatitis markers, hydration increased significantly at endpoint 2, as reported in Table 3.
The described results allow to hypothesize that the studied dressing acted as a self-leveling and film-forming film normalizing trans-epidermal water loss and created a moist wound healing environment that helped promoting faster re-epithelialization and significantly reduced the skin’s acute inflammatory response.
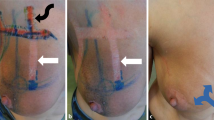
A particularly important observation to be made is that the film-forming dressing was used across several types of cancer localizations: the flexible nature of the wound dressing allows it to adapt to all body surfaces where traditional physical sheeting treatments are ineffective or impossible to use, such as those with high mobility (joints), high friction (axilla), wet skin (mucosa), and higher hygienic requirements (perineum). The gel nature of the wound dressing is able to completely resurface any kind of wound or damaged surface: irregular or under skin folds (Figs. 7, 8, and 9). This provides a nice envelope to protect any wound or damaged surface at its earliest, most delicate phase of repair. It builds a protected environment for healing to begin.
Improper wound care prolongs healing time and extends the inflammatory period, which is especially exacerbating in case of radiation dermatitis [7]. The use of the innovative film-forming wound dressing demonstrates that a prompt treatment of radiation dermatitis during ongoing radiotherapy is indeed preventive of a deterioration of radiation-dependent skin toxicity.
As for the investigator evaluation of the innovative wound dressing, it was considered to be an improvement compared to the standard clinical practice in treatment of radiation dermatitis during ongoing radiation in the majority of patients (Fig. 4). Moreover, investigators reported the dressing to have a “moderate” impact in simplifying treatment and in the amount of nursing time saved in the majority of patients, as well as stating that the product “very much” decreased the amount of product used (Fig. 5).
Also, patients positively perceived treatment with the wound dressing: the majority of them stated that its ease of use was “excellent,” whereas comfort was rated as “very good.” Patients’ general rating of the product was also measured and was found to be “excellent” for most of them (Fig. 6).
It can also be traumatic for patients when dressings need to be removed and reapplied. This is an important consideration in the case of home care, where patients are responsible for changing their own dressings. Same accounts for the nursing time that can be potentially saved for ambulatory patients when complex physical dressing applications are implemented. Application of the product is intuitive and familiar: a few drops of the inert gel are spread over the affected area. The need for complex tapes or advanced dressings is avoided and patients can see the progression of healing underneath the transparent film.
A trial against a control group during ongoing radiation therapy should be implemented, in order to exactly quantify the benefits of the film-forming wound dressing’s use.
Conclusion
When applied during ongoing radiation therapy, the innovative film-forming wound dressing reduces the severity of existing radiation-dependent skin reactions and significantly decreases the RISRAS score, until 10–14 days post-radiation therapy.
Limitations
The limitation to this trial was the missing prospective comparison group. The adherence to the CIP was partly difficult, therefore 26 subjects had to be excluded from statistical analysis. These cases will still be used for case studies.
Abbreviations
- CRF:
-
Case report form
- GCP:
-
Good clinical practice
- PIL:
-
Patient information leaflet
- RISRAS:
-
Radiation-Induced Skin Reaction Assessment Scale
- RTOG:
-
Radiation Therapy Oncology Group
- SD:
-
Standard deviation
- SEM:
-
Standard error mean
References
Naylor W, Mallett J (2001) Management of acute radiotherapy induced skin reactions: a literature review. Eur J Oncol Nurs 5(4):221–233
Porock D, Kristjanson L (1999) Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. Eur J Cancer Care 8(3):143–153
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Noble-Adams R (1999) Radiation-induced skin reactions 3: evaluating the RISRAS. Br J Nurs 8(19):1305–1312
Harris R. (2011) Summary of interventions for acute radiotherapy-induced skin reactions in cancer patients: a clinical guideline recommended for use by The Society and College of Radiographers. Society of Radiographers (http://www.sor.org). 1–5
Trueman E (2015 Apr 1) Management of radiotherapy-induced skin reactions. Int J Palliat Nurs 21(4):187–192
Bernier J, Bonner J, Vermorken JB, Bensadoun RJ, Dummer R, Giralt J, Kornek G, Hartley A, Mesia R, Robert C, Segaert S, Ang KK (2008) Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol 19(1):142–149
Herst PM, Bennett NC, Sutherland AE, Peszynski RI, Paterson DB, Jasperse ML (2014) Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother Oncol 110(1):137–143
Kedge EM (2009) A systematic review to investigate the effectiveness and acceptability of interventions for moist desquamation in radiotherapy patients. Radiography 15(3):247–257
Hymes SR, Strom EA, Fife C (2006) Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol 54(1):28–46
Yarbro CH, Wujcik D, Gobel BH.(2011) Cancer nursing: principles and practice. Jones & Bartlett Publishers
Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M (2016) Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys 95(2):563–570
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was supported by Laboratorios LETI S.L.U. Dressings were supplied by Stratpharma AG, Switzerland free of charge.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Quilis, A., Martín, J., Rodríguez, C. et al. Reducing radiation dermatitis during ongoing radiation therapy: an innovative film-forming wound dressing. J Radiat Oncol 7, 255–264 (2018). https://doi.org/10.1007/s13566-018-0356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-018-0356-5